Vincenzo De Sanctis1, Ashraf T. Soliman2, Shahina Daar3, Niveen Alansary4, Antonis Kattamis5, Myrto Skafida5, Maria Concetta Galati6, Soteroula Christou7, Saveria Campisi8, Giuseppe Messina9, Mohamed A. Yassin10, Duran Canatan11, Salvatore Di Maio12, Soad Al Jaouni13, Giuseppe Raiola14, Mehran Karimi15, Valeria Kaleva16, Shruti Kakkar17, Demetris Mariannis18 and Christos Kattamis19.
1 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
2 Department of Pediatrics, Division of Endocrinology, Hamad General Hospital, Doha, Qatar.
3
Department of Haemtology, College of Medicine & Health Sciences
Sultan Qaboos University, Muscat, Oman & Stellenbosch Institute for
Advanced Study (STIAS), Wallenberg Research Centre at Stellenbosch
University, Stellenbosch, South Africa.
4 Department of Haematology, Sultan Qaboos University Hospital, Sultanate of Oman.
5
Thalassemia Unit, Division of Pediatric Hematology-Oncology. First
Department of Pediatrics, University of Athens, “Agia Sofia” Children's
Hospital, Athens, Greece.
6 Department of Pediatric
Haematoncology, Thalassaemia and Prenatal Diagnosis Regional Center,
Pugliese-Ciaccio Hospital, Catanzaro, Italy.
7 Archbishop Makarios III, Thalassemia Center, Nicosia, Cyprus.
8 U.O.S.D. Thalassemia, Umberto 1° Hospital, Siracusa, Italy.
9 SSD Centro per le Microcitemie- Azienda Grande Ospedale Metropolitano, Reggio Calabria,Italy.
10 National Center for Cancer Care and Research, Medical Oncology Hematology Section HMC, Doha, Qatar.
11 Director of Thalassemia Diagnosis Center of Mediterranean Blood Diseases Foundation, Antalya, Turkey.
12 Emeritus Director in Pediatrics, Children’s Hospital “Santobono-Pausilipon”, Naples, Italy.
13 Hematology Department, King Abdulaziz University Hospital, Faculty of Medicine
King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia.
14 Department of Paediatrics, Pugliese-Ciaccio Hospital,Catanzaro, Italy.
15 Hematology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
16 Center for Coagulopathies and Rare Anemias,Varna, Bulgaria.
17 Department of Pediatrics, Dayanand Medical College & Hospital, Ludhiana, India.
18 Royal Lancaster Infirmary, United Kingdom.
19 First Department of Pediatrics, Aghia Sophia Children Hospital, National Kapodistrian University of Athens, Athens, Greece.
Corresponding
author: Vincenzo De Sanctis MD, Pediatric and Adolescent Outpatient
Clinic, Quisisana Hospital, 44100 Ferrara, Italy; Tel.: +39 0532
770243; E-mail:
vdesanctis@libero.it
Published: January 1, 2020
Received: October 13, 2019
Accepted: December 3, 2019
Mediterr J Hematol Infect Dis 2020, 12(1): e2020006 DOI
10.4084/MJHID.2020.006
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Due
to the recent alarming increase in the incidence of hepatocellular
carcinoma (HCC) in thalassemias, the present report reviews briefly the
frequency, the major risk factors, and the surveillance of HCC in
β-thalassemias. Over the past 33 years, 153 cases of HCC were reported
in patients with thalassemia, mainly in Italy and Greece. Among
HCV-infected patients, additional factors promoting the development of
HCC included: advanced age, male sex, chronic hepatitis B (CHB)
co-infection, and iron overload. For early diagnosis of HCC, sequential
ultrasound screening is recommended especially for thalassemia patients
with chronic hepatitis C (CHC), which coincides with (one or more)
additional risk factors for HCC. Here we report also the preliminary
data from thalassemic patients, above the age of 30 years, followed in
13 ICET-A centers. The total number of enrolled patients was 1,327
(males: 624 and 703 females). The prevalence of HCC in thalassemia
major patients [characterized by transfusion-dependency (TDT)] and
thalassemia intermedia [characterized by nontransfusion dependency
(NTDT)] was 1.66 % and 1.96 % , respectively. The lowest age at
diagnosis of HCC was 36 years for TDT and 47 years for NTDT patients.
We hope that this review can be used to develop more refined and
prospective analyses of HCC magnitude and risk in patients with
thalassemia and to define specific international guidelines to support
clinicians for early diagnosis and treatment of HCC in thalassemic
patients.
|
Introduction
Hepatocellular
carcinoma (HCC) is one of the most common malignant tumors worldwide,
representing approximately 90% of all cases of primary liver cancer
occuring usually in the setting of chronic liver disease and cirrhosis.
Chronic hepatitis C (CHC), chronic hepatitis B (CHB) and hereditary
hemochromatosis may directly lead to HCC, while HCC related to other
underlying liver diseases is linked to the development of cirrhosis.[1]
The global age distribution of HCC varies by region, sex, and
aetiology. Rates of liver cancer among persons of the same ethnicity
may vary by geographic location.[2-5]
Environmental and acquired
individual factors, such as excessive alcohol consumption, fatty liver
disease, non-alcoholic steatohepatitis, cigarette smoking, occupational
exposure to chemicals such as pesticides, and contamination of
foodstuff with aflatoxins (a group of mycotoxins produced by the fungi
Aspergillus flavus and Aspergillus parasiticus), can increase the risk
of developing liver cancer.[1]
HCC incidence rises after the age
of 45 to 50 and becomes almost consistently high after 65 years.[3] HCC
is rare among adolescents and accounts for less than 1% of all
malignant neoplasms in this age group.[4] The male-to-female ratio in
HCC incidence is lower than 2 below 25 years, increases from 25 to 29
years, and peaks at the age of 50-54 years up to 5.40 (95% CI: 5.02,
5.82).[5]
The guidelines for the management of HCC suggested by
the American Association for the Study of Liver (AASLD) and the
European Association for the Study of Liver (EASL), recommend the
implementation of surveillance by a combination of abdominal ultrasound
(US), computed tomography (CT) and/or magnetic resonance imaging (MRI).
These can improve the prognosis thanks to the detection of smaller
tumors.[6,7] Guidelines for HCC in thalassemias are lacking.
The
treatment of HCC requires a multidisciplinary approach involving
hepatologists, surgeons, oncologists, radiologists, and other
disciplines. Multiple options for treatment are available, depending on
the tumor stage, patient's performance status and liver function
reserve; they include radiofrequency or microwave ablation, liver
resection, or transplantation. For patients who are not candidates of
curative treatments, locoregional therapies such as trans-arterial
chemoembolization (TACE), trans-arterial radio-embolization (TARE), and
stereotactic body radiation (SBRT) can improve survival and quality of
life. Liver transplantation is another radical therapy for selected
patients with HCC.[8]
Sorafenib, a multi-kinase vascular
endothelial growth factor (VEGF) inhibitor, is the most widely used
systemic chemotherapy approved as a first-line agent for unresectable
or advanced HCC.[8]
However, patients with HCC are often diagnosed
in advanced stages, and even after complete HCC tumor resection or
ablation, the carcinogenic tissue microenvironment in the remnant liver
can give rise to recurrent de novo HCC tumors, which progress into
incurable, advanced-stage disease in the majority of patients.[9]
The
recent alarming increase in the incidence of HCC in thalassemias,
prompted us to overview the most common risk factors involved in the
development of HCC, to briefly describe the frequency and the clinical
and diagnostic characteristics, and to discuss the recommendations for
HCC surveillance in high-risk patients with β-thalassemias. The
preliminary data of the International Network of Clinicians for
Endocrinopathies in Thalassemia and Adolescent Medicine (ICET-A) on HCC
survey in thalassemia major (transfusion-dependent thalassemia:TDT) and
intermedia patients (transfusion-dependent thalassemia: NTDT) are also
presented.
Frequency of HCC in the General Population and in Thalassemias
The
global distribution of HCC varies by region, gender, and aetiology. The
disease burden is highest in areas with endemic HBV infection (where
HBsAg prevalence is 8% or more), such as in sub-Saharan Africa and
Eastern Asia, with an HCC incidence rate of over 20 per 100,000
individuals. In Egypt, hepatocellular carcinoma (HCC) is the second
most common cancer in men and the 6th
most common cancer in women. Italy, Spain, and Greece have intermediate
incidence rates of 10-20 per 100,000 individuals, while North and South
America have a relatively low incidence (< 5 per 100,000
individuals).[10]
Kourakli et al.[11] analyzed all cases of
malignant neoplastic disorders, occurring in 3,652 Greek thalassemic
patients, diagnosed between 1985 and 2018. A total of 165 cases of
malignant disorders were identified, (84 males and 81 females) with a
median age at diagnosis of 45 years (range 9-73 years). The dominant
malignancy was hepatocellular carcinoma, diagnosed in 63 patients,
followed by thyroid cancer (17 cases), non-Hodgkin's lymphoma (13
cases), and renal cell carcinoma (10 cases). There was a strong
positive association between HCV infection and hepatocellular carcinoma.
The
literature review showed that HCC in β-thalassemias had been reported
in Italy (85 cases), UK (2 cases), Lebanon (2 cases), and Iran (1
case), in addition to Greece (63 cases).[12-23]
In a prospective
study, based on ultrasound screening of 105 Italian thalassemic
patients, over 18 years of age, Mancuso et al.[16] found a 2% incidence
of HCC during a 1-year observation period. Risk factors were present in
seventy-two patients: iron overload in 72, HCV infection in 46, HBV
infection in 2 and cirrhosis in 10 patients.
Ten years later,
Borgna-Pignatti et al. performed a survey of patients followed in 55
Italian centers. The authors collected data from 4,248 patients with
thalassemia major and 1,607 patients with thalassemia intermedia. Since
their last report,[14] 62 new cases have been identified, of whom 52%
with thalassemia major and 45% with thalassemia intermedia and 2 (3%)
with compound sickle cell /β- thalassaemia. The cumulative incidence
reported at diagnosis of HCC was 1.02% and 1.74%, respectively.[22] The
mean age at diagnosis of HCC in patients with TDT was 48 years (33–56
years) and in patients with thalassemia intermedia 47 years (36–72
years); 37 patients (60%) were males and 25 (40%) females.[22]
The ICET-A Experience
In
June 2019, to ascertain the frequency of HCC in patients with
thalassemias, the Coordinator (VDS) of ICET-A invited 13 centers of the
network to take part in a study and collected information on all
patients they followed for HCC. An ad hoc questionnaire, prepared by VDS in accordance with the Declaration of Helsinki (http://www.wma.net),
was distributed by mail to participating centers. The deadline for
sending the requested data was 2 months. The exclusion criteria were:
patients with sickle cell disease, and patients already included in
other previous publications.
Considering that the youngest
patient reported in the literature was 36 years old, we included in the
study, only the patients with β-thalassemia above the age of 30 years
with β-thalassemias, followed in the participating centers. In detail,
the required data were: date of birth, type of haemoglobinopathy,
serology for HBV, HCV, detection of HCV-RNA, levels of serum ferritin
at diagnosis and chelation therapy, the presence of obesity, alcohol
abuse, smoking, and associated clinical complications were also
included. In addition, symptoms at onset and clinical course of
patients with HCC were reported. Liver iron concentration, measured by
magnetic resonance imaging (MRI), was also included.
The
demographic details of TDT and NTDT patients, above the age of 30
years, who developed HCC in 13 thalassemia centers from 10 different
countries, are presented in table 1.
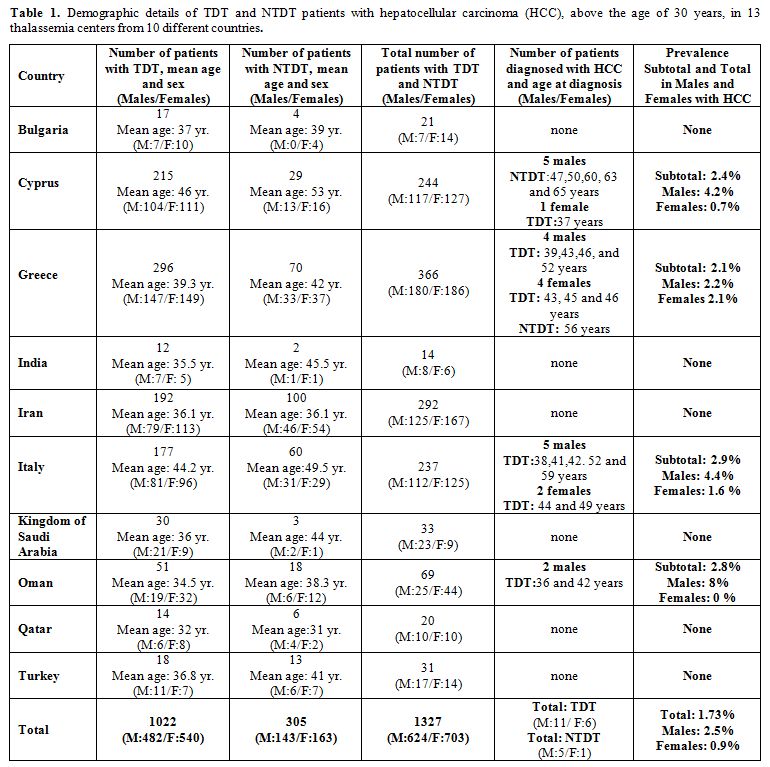 |
Table 1. Demographic
details of TDT and NTDT patients with hepatocellular carcinoma (HCC),
above the age of 30 years, in 13 thalassemia centers from 10 different
countries. |
The total number of enrolled patients with TDT and NTDT was 1,327 (males: 624 and 703 females).
17
patients (mean age: 44.3 years) diagnosed with HCC had TDT (11 males
and 6 females), and 6 patients (mean age:56.8 years) had NTDT (5 males
and 1 female). The total prevalence of HCC in this subgroup of patients
( > 30 year) was 1.66 % for TDT group and 1.96 % for NTDT. The
lowest age at diagnosis of HCC in TDT patients was 36 years and 47
years in NTDT patients, and the highest was 59 and 65, respectively.
The
mean age in the group of patients with HCC was higher compared to the
patients' group without HCC (TDT and NTDT: 41.0 and 45.7 years vs. 35.5
and 39.2 years, respectively).
Full data was available in 15 out of 23 patients with thalassemias and HCC (Tables 2-4).
The presence of obesity, alcohol abuse, or smoking, was absent or very
uncommon. The value of αFP at diagnosis was raised ( > 10 ng/mL) in
9 out of 12 patients for whom it was available; 8 out of 14 patients
(57.1%) were clinically symptomatic (Tables 2-4).
None was positive for HBV surface antigen (HBsAg), while one patient
showed evidence of past HBV infection, being HBsAg negative but
positive for the antibodies against the HBV core antigen (HBcAb).
Interestingly, all Italian patients diagnosed with HCC had genotype 1b.
At the time of survey 6 out of 23 patients with thalassemias are alive.
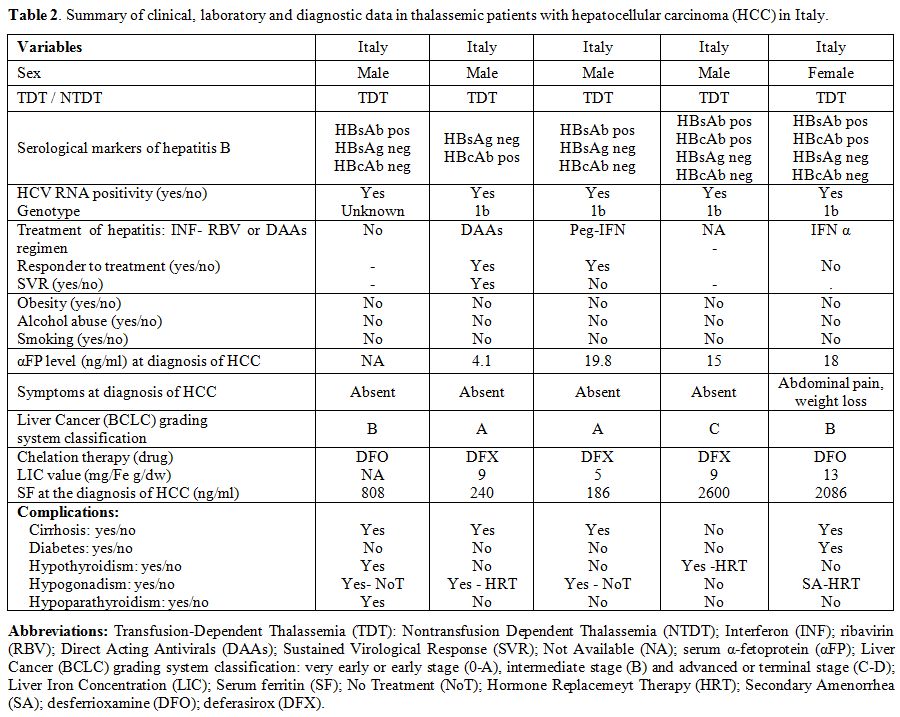 |
Table 2.
Summary of clinical, laboratory and diagnostic data in thalassemic patients with hepatocellular carcinoma (HCC) in Italy. |
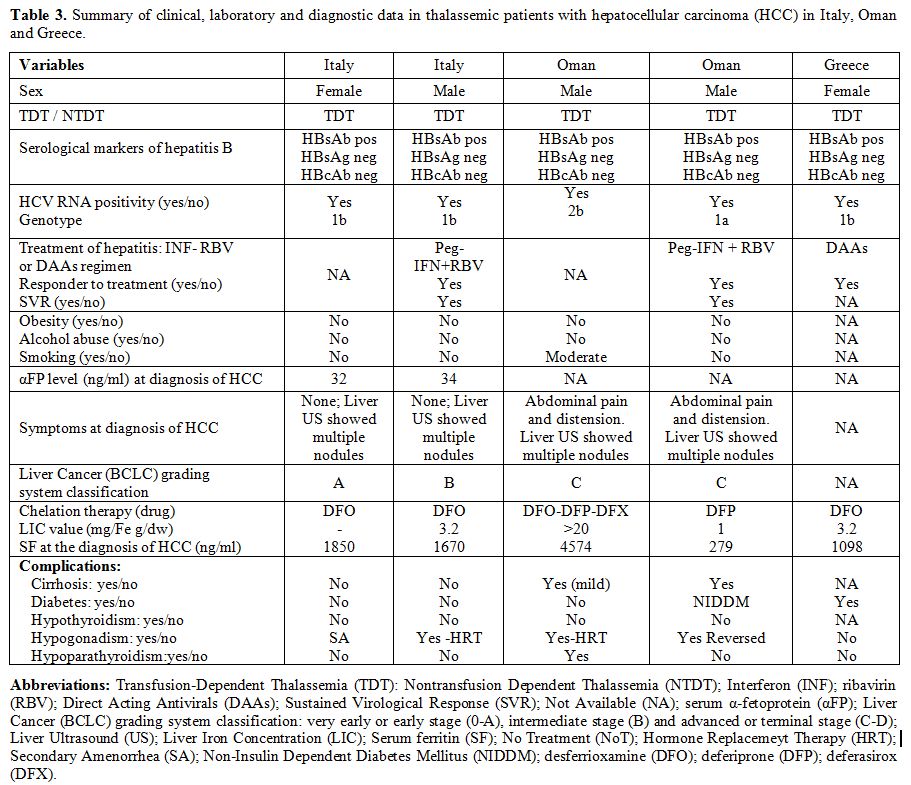 |
Table 3. Summary of
clinical, laboratory and diagnostic data in thalassemic patients with
hepatocellular carcinoma (HCC) in Italy, Oman and Greece. |
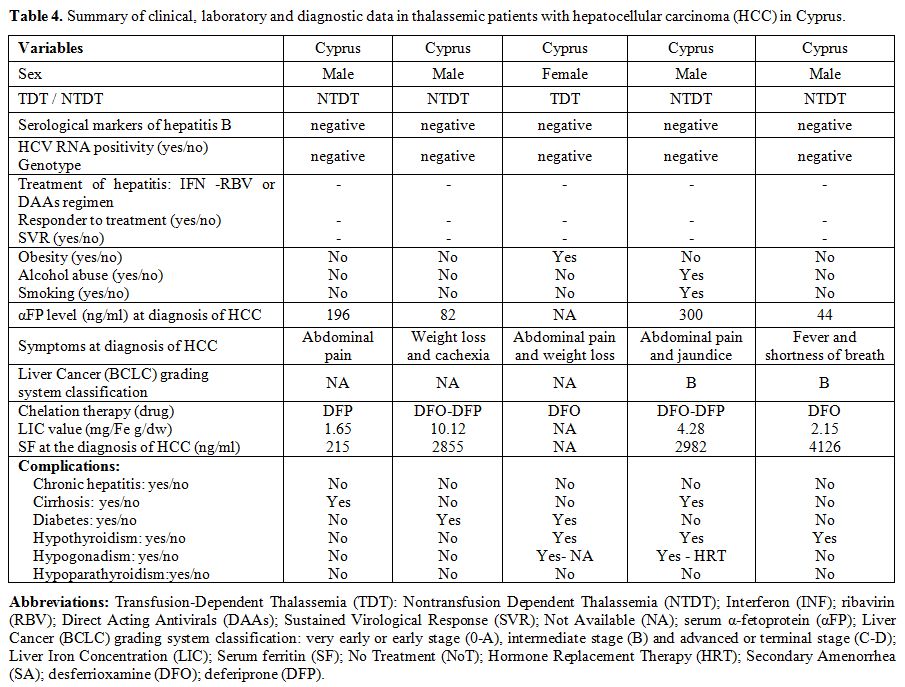 |
Table 4. Summary of clinical, laboratory
and diagnostic data in thalassemic patients with hepatocellular
carcinoma (HCC) in Cyprus. |
Comments.
In the general population, the risk for HCC is known to be age
dependent; HCC is rarely seen during the first 4 decades of life,
except in populations where HBV infection is hyperendemic. The age
range at diagnosis of HCC was 63–65 years in Europe and North America,
with rare occurrence before the age of 40 years.[3-5]
In
our patients with TDT, the mean age at diagnosis of HCC was 44.3 years,
younger than that reported in patients without thalassaemia, suggesting
the concomitance of other risk factors, namely hemosiderosis,
implicated in the development of HCC. Similar results were also
reported in 63 Greek thalassemia patients with HCC (median age: 45
years).[11]
Comparing our data with the results of a previous publication,[22]
it emerges that our subgroup of NTDT patients with HCC were older (56.8
vs.47 years). However, our series consisted of 6 NTDT patients vs. 28
reported in the multicentre Italian study.[22]
The
distribution of HCC varies significantly according to geographic
location, and it is more common in middle and low income countries than
in developed ones.
It is also interesting to note that no case
of HCC in patients with thalassemias was reported in Bulgaria, India,
Kingdom of Saudi Arabia, Qatar, (total number of patients:105
patients), and in a large group of patients (292 patients; 125 males
and 167 females) followed in a single center in Iran, although the
prevalence of HCV Ab and HCV RNA positivity reported in a previous
study was 42.8% and 29.9%, respectively.[24] However,
the mean age of patients followed in these centers was lower compared
to patients with HCC followed in Cyprus, Greece, Italy, and Oman.
In
this group of patients with HCC, obesity, NAFLD (assessed by liver
ultrasound), or alcoholic abuse was not reported, and NIDDM was
diagnosed only in a male TDT patient from Oman (Tables 2-4).
Globally,
the rate of males with HCC was higher than of females. This is probably
due to two main reasons: (a) the effects of estrogen may suppress the
inflammatory process mediated by interleukin-6 (IL-6) in women,
reducing hepatic injury and the compensatory proliferation of
hepatocytes;[25,26] and (b) the effects of testosterone may increase signalling androgen receptor in men, promoting proliferation of hepatocytes.[27-29]
However,
no HCCs have been reported up to now in association with currently used
transdermal, subcutaneous, or intramuscular testosterone (T)
formulations in hypogonadal thalassemic patients.[30,31] Nevertheless, because of the virtual "hepatotoxicity” of T preparations, monitoring of liver function is necessary.
On
the other hand, the presence of hypogonadism in female thalassemic
patients could be a potential additional risk factor of HCC. This
hypothesis is supported by a pooled analysis of data from 11
prospective US studies.[32] The authors found that oophorectomy was a significant risk factor for HCC.[32]
However,
the role of estrogen-progesterone replacement therapy (HRT) in
hypogonadal patients remains controversial. The present evidence
suggests both a carcinogenic and protective effect of HRT on liver
function.[32-34] Further studies on the indications,
duration, and safety of different HRT formulations used in hypogonadal
females with thalassemia are required to solve this intriguing puzzle.
In
summary, the pivotal role for the evaluation of risk factors of HCC in
patients with thalassemias seems to be advanced age, male sex, viral
liver infections, and prolonged iron overload.
Further studies on
the indications, duration, and safety of different HRT formulations
used in hypogonadal patients with thalassemias are required.
Finally,
HCC seems to be more common in patients with TDT than NTDT. One
possible explanation is that patients with NTDT have a milder
progression of iron overloading and a lower incidence of chronic viral
liver infections.
Major Risk Factors in Thalassemic Patients with HCC
HCV and HBV.
In patients with thalassemias, the predominant role of chronic
infection with HBV, and mainly with HCV, in the etiology of HCC is well
documented.[12,14,17]
A prospective study to evaluate the incidence and aetiology of transfusion-related hepatitis was reported by Lai et al.[35]
Seventy-three of 135 thalassemic patients (62.2%) acquired HCV. An
extended follow-up (22 to 30 yr) with HCV RNA assessment was available
in 52 patients. Of them, 23 (44.2%) cleared the virus. Fibrosis
progression was similar in HCV RNA-positive and HCV RNA-negative
patients. Liver iron was the only factor associated with fibrosis.
In
another series of 233 HCV positive thalassemic patients, 30% were RNA
negative. In these patients, from 2 to 20 years after HCV infection,
liver fibrosis was minimal, if any. (Kattamis C et al. unpublished
data).
Generally, chronic hepatitis C (CHC) is a slowly
progressive disease characterized by persistent hepatic inflammation,
leading to the development of cirrhosis in approximately 10-20% of
patients over 20-30 years of HCV infection. Overall, once cirrhosis has
developed, there is a 1-5% annual risk of HCC and a 3-6% annual risk of
hepatic decompensation. The clinical and histopathological disease
progress is influenced by several factors, mainly the level of HCV
viremia, the virus genotype, the duration of infection, the age at
infection, the sex, and the co-existence of active HBV.[36,37]
HCV
is classified into 6 major genotypes. Some genotypes have a restricted
geographical distribution (genotypes 4-6), while others (genotypes 1-3)
are more broadly disseminated. Genotype 1 (subtypes 1a and 1b) is the
most prevalent genotype in the world. Genotype 2 is found in clusters
in the Mediterranean region; genotype 3 is most prevalent among
intravenous drug users and genotype 4 is found mostly in Egypt, while
genotypes 5 and 6 are less frequent.[38] The HCV genotypes strongly affect the response to antiviral treatment.
HCV infection is the main risk factor for liver fibrosis in TDT.[39]
An accelerated hepatic fibrosis was observed by Kamal et al. in
patients with thalassemias and was attributed to the cumulative effect
of HCV induced liver injury and the increased iron overload,
particularly in inadequately chelated patients.[40]
Efficient chelation therapy may prevent the development of liver fibrosis in thalassemic patients with CHC.[41]
Hepatic siderosis and fibrosis were assessed in 99 TDT patients using
transient elastography (TE) and liver iron concentration (LIC) at
baseline and after 4 years. At baseline, the overall mean liver
stiffness measurement (LSM) was 7.4 ± 3.2 kPa, and the mean LIC,
assessed by T2*MRI, was 4.81 ± 3.82 mg/Fe g/dw. At 4 ± 1.5 years, a
significant reduction in LSM (6.6 ± 3.2 kPa; p: 0.017) and LIC (3.65 ±
3.45 mg/Fe g/dw; p: 0.001) was observed.[42]
Comments.
Due to frequent blood transfusion treatment, many patients with
thalassemias were infected with HCV, HBV, or both. This applies mostly
to patients in their 30s or older, as the risk of viral transmission
through blood transfusion was minimized after the 1990s by
implementation of blood donor screening in developed and developing
countries.
Preventive measures include early immunisation
against the hepatitis B virus and prevention of iron accumulation by
intensive use of iron chelation therapy. In non-immune thalassemia
patients, the implementation of HBV vaccination started in the early
80s. The development of a vaccine for HCV remains an important goal for
global control and eradication of infection.
Globally, chronic
hepatitis C progresses to liver fibrosis in 60%-70% of patients,
cirrhosis in 10%-20%, and eventually HCC in 1%-5% within two decades of
harbouring the virus. The ability of HCV to promote cirrhosis is 10- to
20-fold higher than HBV.[3,6-8]
Therefore,
patients in the high-risk category should be offered antiviral therapy,
as well as appropriate HCC surveillance. Effective suppression of HBV
and HCV replication by antiviral therapy can reduce the risk of HCC
development. However, antiviral therapy does not eliminate the HCC risk
because of the presence of virus integrated into the host genome.
Interferon-based
regimens (Peg-IFN) have been the mainstay of anti-HCV therapy, yielding
HCV cure, or sustained virologic response (SVR), in approximately 50%
of patients, followed by pegylated interferon and ribavirin
(Peg-IFN/RBV) for 24 weeks or 48 weeks, the use of which was limited by
adverse effects.[43] The host factors influencing
response to treatment include: genetics, particularly interleukin
(IL)-28B polymorphisms, race, obesity, insulin resistance, severity of
hepatic fibrosis, and the related viral genotype and viral load at
initiation of therapy.[44]
The recently
developed direct-acting antivirals (DAAs), directly targeting the viral
protease, polymerase, or non-structural proteins, enabled
interferon-free anti-HCV therapies with a revolutionary improvement of
SVR rate, approaching or surpassing 90%.[45,46]
The
host factors influencing response to treatment include genetics,
particularly interleukin (IL)-28B polymorphisms, race, obesity, insulin
resistance, the severity of hepatic fibrosis, and the related viral
genotype and viral load at initiation of therapy.[46]
The
first generation of DAAs, including boceprevir (BOC) and telaprevir
(TVR), was introduced in 2011 and added to the previous regimen of
pegylated interferon/RBV (Peg IFN/RBV). In October 2014, the FDA
approved the use of ledipasvir (LDV) in combination with sofosbuvir
(SOF) for the treatment of chronic HCV genotype 1 hepatitis, which had
an efficacy of more than 95%. Both drugs are NS3/4A protease inhibitors
and were used in combination with peg-interferons and ribavirin to
avoid the emergence of resistant variants. These agents improved SVR
rates but did not improve the side-effect profile.[47]
Thus,
the use of triple therapy with ombitasvir/paritaprevir/dasabuvir, for
the treatment of HCV genotype 1 hepatitis, was introduced in December
2014. In the next two years, several other regimes as DAA - sofosbuvir
in combination with ledipasvir (NS5A inhibitor), and combination of
ombitasvir (NS5A inhibitor), paritaprevir (NS3/4A protease inhibitor),
ritonavir (CYP3A inhibitor) and dasabuvir (non-nucleoside NS5B palm
polymerase inhibitor), and daclatasvir (NS5A inhibitor) were
introduced. The latter was approved for the treatment of HCV genotype 3
infection in combination with sofosbuvir.[47]
In January 2016, the FDA approved the combination treatment of grazoprevir/elbasvir (GZR/EBR) with a 95% SVR.[47]
for naïve patients or those who failed treatment with genotype 1 or 4,
with or without cirrhosis who failed treatment. The approval of
sofosbuvir and velpatasvir (NS5A inhibitor) as a fixed dose combination
initiated the third generation of DAAs. However, access to therapies
remains limited due to the high costs.[47]
The
most extensive observational study on DAAs, in patients with
hemoglobinopathies and HCV infection, published to date has been
reported by Origa et al.[48]
The majority of
patients had TDT (114 patients, 82%), 7 patients (5%) had sickle-cell
thalassemia, 3 patients (2%) NTDT and 1 patient (0.7%) sickle-cell
anemia. The most common HCV genotype was 1b (91, 65.5%). DAA therapy
included different basic and in combination treatment regimens. The
mean level of serum ferritin was 1,450 ng/mL (range: 103-11,190 ng/mL)
and liver iron overload was observed in 51 (37%) patients. 136 patients
(97.8%) achieved a response at the end of treatment, and 130 (93.5%)
achieved an SVR. Treatment regimens were well tolerated, and no major
adverse events or drug-drug interactions were observed. Treatment with
DAAs was also associated with a significant reduction of liver enzymes
and serum ferritin.[48]
Supportive evidence for
the use of DAAs was reported in 61 thalassemic patients (age range:
40–48 years), including some who previously did not respond to
antiviral treatment with pegylated IFN and RBV or/and with advanced
liver disease due to CHC. The choice of treatment regimen was mainly
based on HCV genotype according to published guidelines and also on the
chronological availability of HCV agents. The treatment was highly
effective (SVR: 90%) and safe.[49]
In another
study, the SVR rate to DAAs in patients with chronic HCV and
thalassemia was 82%, while in chronic HCV patients without thalassemia
it was higher (94.7%), but not statistically significant.[40] Furthermore, SVR rates were significantly higher in patients who were treated with DAAs compared with PEG-IFN based therapy.[40]
Early diagnosis and treatment with DAAs were recommended to prevent
cirrhosis and HCC, which are relevant causes of mortality in this
patients population.[40]
Iron Overload in TDT and NTDT.
The current treatment of TDT consists of regular transfusions to
maintain hemoglobin (Hb) levels of 9 to 10 g/dL. Chronic transfusions
lead to iron overload and, then, eventual multiorgan damage if not
conveniently treated. Therefore, iron chelation therapy is mandatory to
remove iron in excess. Regular subcutaneous (SC) desferrioxamine
mesylate (DFO) infusion for the treatment of iron overload was started
in 1978 in patients older than 2 years, and since 1995 and 2007, two
oral chelators, deferiprone (DFP) and deferasirox (DFX) were
respectively available and used.
Iron overload is known to cause cancer in animal models,[50] and conversely, iron depletion has been reported to suppress tumor growth.[51]
The
first mechanism by which free iron is believed to trigger malignant
transformation is the generation of reactive oxygen species (ROS),
which causes peroxidation of membrane fatty acids and subsequent
formation of toxic by-products that impair protein synthesis and
disrupt DNA, leading to mutations in tumor suppressor genes (such as
p53) and DNA repair genes.[52,53]
Iron overload
may also promote malignant transformation in the liver through the
acceleration of fibrosis to cirrhosis by activation of stellate cells
and the profibrogenic effects of lipid peroxidation.[54,55]
It
also has been suggested that iron overload induces immunologic
aberrancies, which may contribute to cancer formation. Growing evidence
highlights the negative effect of iron and ferritin on the tumoricidal
function of macrophages in mice,[56] in which it was
specifically demonstrated that iron overload decreased
antibody-mediated and mitogen-stimulated phagocytosis by monocytes and
macrophages.[57]
Patients with genetic
hemochromatosis were found to be 23 times more likely to have liver
cancer than healthy people, with an annual incidence rate of
HCC-related cirrhosis of 3%–4%.[58]
Furthermore,
a number of case reports and case series have described the development
of HCC in patients with thalassemia who were negative for hepatitis B
and C testing but had significant hepatic iron overload.[17,21-23]
One
NTDT male patient, anti-HCV and HbsAg negative and never transfused,
with a previous histological diagnosis of cirrhosis related to
secondary hepatic iron overload developed HCC. He was diagnosed at the
age of 34 years and was chelated first with deferoxamine and later with
deferiprone. Before the diagnosis of HCC, at the age of 73 years, the
serum ferritin level was 222 ng/mL.[17] Similar findings were reported by Borgna Pignatti et al.[22] and Maakaron et al.[21,23] in patients with negative serology for HBV and HCV infection.
In
a Greek study, HCC was reported in 3 out of 9 NTDT patients with liver
fibrosis and siderosis due to the late introduction of iron chelation
therapy, and negative history for viral hepatitis.[18]
Notably, the incidence of HCC was higher in NTDT compared to TDT (p:
0.032). These findings suggest that the duration of exposure to toxic
iron levels may be more important for the development of HCC than the
amount of liver iron content.
Comments.
Due to frequent and regular blood transfusions, many patients with
thalassemia are infected with either HCV or HBV. This occurrence
applies mostly to patients in their 30s or older because the risk of
viral transmission through blood transfusion was minimized after the
1990s (even annihilated) through blood donor screening.
Preliminary data seem to indicate that NTDT patients might be at a higher risk for developing HCC than TDT patients.[17,20,22]
One possible explanation is that patients with NTDT have much longer
survival compared to TDT patients, which enables them to live long
enough to develop HCC. The state of chronic anemia and hypoxia in NTDT
patients, resulting from ineffective erythropoiesis and hemolysis,
leads to the expansion of the erythroid marrow and extramedullary
hematopoiesis. The chronic ineffective erythropoiesis also triggers
increased intestinal iron absorption and deposition in the liver and
endocrine glands despite the lack of transfusional iron.[59] Therefore, close surveillance of iron overload via non-invasive quantification of LIC with R2 or T2* MRI is recommended.
Pathophysiology Past and Recent Issues
Transfusion-Associated Immunomodulation.
Increasing evidence suggests that blood transfusions, in addition to
iron overload, by inducing chronic antigenic stimulation, have adverse
effects on the recipient’s immune system, which is an essential
mechanism for antiviral immunity and anticancer immune surveillance.[54,55,58]
However, to date, the precise role of transfusion-related
immunomodulation in the development of HCC in thalassemia has not yet
been elucidated, and needs further studies.[60]Host genetics factors associated with HCC.
Genetic variability has been discussed as a risk factor for the
development of HCC since many patients exposed to known environmental
risk factors never develop cirrhosis or HCC.[61]
Furthermore, family clustering and incidence differences among
different ancestry groups suggest that inherited genetic factors may
contribute to HCC risk.[62]From a search of 1,668 publications, Walker et al.[63]
identified 166 relevant studies evaluating the associations of HCC with
cirrhosis or fibrosis in HCV-infected patients in 137 different genes.
IFNL3/4, TNF-α, and PNPLA3 genes had the most probable evidence of
association. In
summary, host genetics could add discriminatory value to risk
prediction tools, allowing better stratification and personalized
assessment of optimal long-term management, thereby increasing the
efficacy of surveillance programmes.[63]Insulin resistance. Chronic hepatitis C is associated with an increased risk of diabetes mellitus (DM) or insulin resistance (IR).[64,65] IR is associated more frequent in patients with chronic hepatitis C with hepatic steatosis, advanced fibrosis, and HCC.[64]
IR may induce the release of free fatty acids (FFA) towards hepatocytes
and may cause oxidative stress through the overproduction of ROS,
cellular inflammation, and carcinogenesis.Disturbances
of glucose homeostasis, ranging from mild glucose intolerance to overt
diabetes mellitus, and hyperinsulinism were reported in young adult
patients with thalassemia and have been attributed to iron overload,
HCV infection, anemia, and chronic liver disease.[66,67]An
acute effect of blood transfusion on insulin sensitivity and β-cell
function in patients with thalassemia has been reported by Wankanit et
al.[68]Alcohol and Tobacco.
Alcohol and iron are known prooxidants, and oxidative stress is known
to play an essential role in the development of several diseases,
including cancer. The metabolism of alcohol, especially through CYP2E1,
can lead to the generation of superoxide and hydrogen peroxide.
Moreover, hydrogen peroxide can react with ferrous iron (Fe2+) through the Fenton reaction, and generate highly reactive hydroxyl radicals.[69]Hydroxyl
radicals can react with lipid molecules, initiating chain reactions
that lead to lipid peroxidation and generation of products, such as
acrolein, crotonaldehyde, MDA and 4-HNE; the latter is known to cause
mutations of p53 gene (a tumor suppressor gene), which may initiate the development of HCC.[70]Tobacco
exposure is also a risk factor for HCC. Tobacco smoking is associated
with increased plasma levels of inflammatory cytokines such as
TNF-alpha and IL-1beta[71,72] and markers of oxidative stress.[72,73] These mediators can contribute to necro-inflammatory changes in the liver, which in turn may promote the development of HCC.[74]In
brief, prolonged exposure to alcohol and tobacco is expected to promote
the development of HCC in an additive and/or synergistic manner.
Tobacco smoking may contribute to the initiation and promotion of HCC
due to the presence of mutagenic and carcinogenic compounds as well as
by promoting oxidative stress via the generation of ROS and depletion
of endogenous antioxidants.Therefore,
thalassemic patients should be discouraged from alcohol consumption and
tobacco exposure, regardless of the severity of their disease.Impact of direct-acting antiviral agents in treated patients.
Several retrospective uncontrolled studies in 2016 reported an
increased incidence of de novo HCC among patients treated with DAA for
HCV infection.[75-77] In a multicentre study of 58
patients in Spain and a single center study of 59 patients in Italy,
occurrence or recurrences of HCC after curative therapies in DAA
treated patients appeared unusually high (28% and 29%, respectively).[75-77]
Although the mechanism underlying this unexpected early HCC recurrence
is unknown, it has been reported that HCV eradication by DAA therapy
might enhance HCC development or recurrence in patients who have
elevated risk for HCC.[78]In
contrast, some other retrospective and prospective cohort studies
suggested no significant difference in liver cancer development
following DAA therapy.[78-80]In
conclusion, the risk for HCC development is modulated by host features
such as gender (male), advanced age, metabolic syndrome, and the
severity of the underlying liver disease. The risk for HCC is
substantially reduced in patients with CHC who achieve virologic cure,
but the risk is not eliminated, and patients should continue
surveillance for HCC. Before attempting any interpretation of the
discrepancies between the above mentioned DAA reports, long term
studies are needed to elucidate this controversial issue.Vitamin D deficiency. To date, clinical studies have demonstrated that vitamin D deficiency is common, not only in patients with thalassemias,[81] but also in those with HCC. The potential role of vitamin D in HCC has been recently reviewed by Wu et al.[82] and Chiang et al.[83]Vitamin
D is involved in cell proliferation, apoptosis, differentiation,
inflammation, invasion and metastasis, angiogenesis and micro-RNA
modulation. Although the epidemiologic evidence regarding the
association of vitamin D and HCC is still inconclusive, biochemical
evidence clearly indicates that HCC cells are responsive to the
inhibitory effect of vitamin D and its analogs.[83]Nonalcoholic fatty liver disease (NAFLD).
Metabolic risk factors commonly associated with NAFLD or NASH,
including diabetes mellitus type II, obesity, and metabolic syndrome,
are becoming emerging risk factors for HCC. The pathophysiology of
hepatic carcinogenesis in patients with NAFLD-NASH has not been
completely elucidated, but initial research suggests that excess fatty
acid supply and hepatocellular steatosis elicit increased fatty acid
oxidation with subsequent enhanced reactive oxidative stress. This
process further promotes the release of proinflammatory cytokines,
prooncogenic signals, and epigenetic changes.[84]
Genetic and environmental factors, as well as the interaction between
them, may be responsible for both the individual susceptibility and the
clinical course of NAFLD. HCC Surveillance
The
increasing incidence of HCC in advanced age thalassemic populations led
to the need to identify early patients at risk for HCC through an
efficient program of screening. Nowadays, almost 50% of HCC are
diagnosed at an advanced stage with a poor prognosis and a 5-year
overall survival rate lower than 10%. In contrast, in patients with
early diagnosis, the 5-year survival rate increases to 70%.[85]Surveillance
consists of the periodic application of a diagnostic test to subjects
at risk for developing a specific disease. In one study, the risk
factors for HCC in thalassemias were defined as follows: increased
serum ferritin (at least 2 determinations > 1,000 ng/ml) or
increased LIC >4 mgFe/g dry weight or both; HCV-RNA positivity by
polymerase chain reaction and anti-HCV positivity; hepatitis B surface
antigen (HBsAg) positivity, or histological diagnosis of cirrhosis.[17]Currently, the surveillance of HCC in β-thalassemias
is achieved by analyzing imaging techniques, abdominal US at 6-12
months frequency, combined with serum α‑fetoprotein (AFP) levels.[15-17,22]The
early detection of HCC by the US is highly dependent on the expertise
of the operator and the quality of the equipment. Thus, specialized
training for ultrasonographers is recommended. The levels of
sensitivity and specificity are also dependent on the size of the
tumor, with ultrasound able to detect 80%–95% of tumors 3–5 cm in
diameter and 60%–80% of tumors < 1 cm in diameter.[86]Combining ultrasound and AFP appears to improve the detection rates.[87-89] AFP values may be influenced by non-neoplastic factors, such as viral infections and cirrhosis.[90]
At diagnosis of HCC, in thalassemic patients, AFP was within the normal
range in 20 out of 45 patients (44.4%) for whom it was available. The
median value in TDT patients with HCC was 9.8 µg/L (range 1,1–2,000
µg/L) while in NTDT it was 12.3 µg/L (range:1,5–3,000 µg/L)[22]
while in a previous study AFP, at the time of diagnosis was 2,103 ±
2,067 kU/L (normal value <16 kU/L); it was within normal levels in 2
out of 22 patients (9.0%).[14]The
recent introduction of US contrast-enhanced agents (CEUS) has not
proven to increase the ability of the US to detect small HCC tumors.[91]
However, CEUS provides an accurate differentiation between benign and
malignant liver nodules, which is critical for adequate management of
HCC and is also useful for guidance of local percutaneous therapy of
HCC and post procedure monitoring of the therapeutic response.[92]Liver
fibrosis is a risk factor for HCC, but at what fibrotic stage, the risk
for HCC is increased has been poorly investigated. Nakayama et al.
reported that a liver stiffness of more than 12.0 kPa was an
independent risk factor for HCC development.[93]
Therefore, it could be an additional surveillance factor to be included
in the surveillance of patients with chronic liver disease and iron
overload.In
conclusion, although the optimal methods of screening and the
cost-effectiveness of surveillance for HCC remain to be defined,
systematic screening still offers the best hope for early diagnosis,
treatment eligibility, and improved survival. Current guidelines
advocate the use of abdominal US at 6 months frequency to screen for
HCC in high-risk patients because the median doubling time of an HCC
lesion is 117 days (range 29 to 398 days).[94,95]
Conclusions
A
large number of studies have provided evidence that thalassemic
patients have a high risk of developing HCC in advanced age.
Multicenter international studies for a better evaluation of HCC
incidence in different countries are urgently needed. Factors that
predispose to HCC, among chronically HCV-infected patients, include
advanced age, male sex, viral liver infections, and iron overload (Figure 1). However, several aspects remain to be explored further.
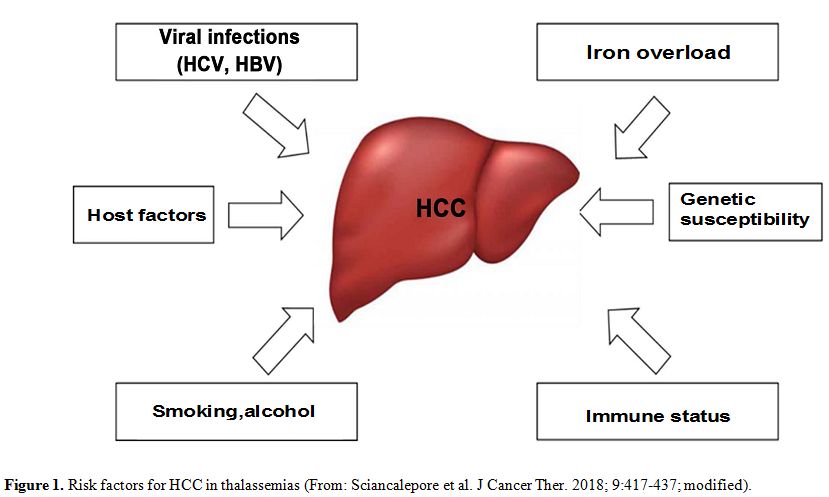 |
Figure 1. Risk factors for HCC in thalassemias (From: Sciancalepore et al. J Cancer Ther. 2018; 9:417-437; modified). |
HCC is more frequently diagnosed (at present) in men with TDT in their fourth - fifth decade and older in NTDT[11,22]
with long-standing, untreated chronic hepatitis C (76%), irrespective
of the content of hepatic iron load, estimated with MRI T2*.[11]
One possible explanation is that patients with NTDT may have a milder
process of iron overloading at late age and a lower incidence of
chronic viral liver infections, mainly HCV.HCV
is characterized by genetic heterogeneity. Based on the sequence
divergence rate, six HCV genotypes and more than 50 subtypes have been
identified.[96] The most prevalent HCV subtypes are
1a, 1b, 2a, 2b, and 3a, which are widely distributed globally and
account for most HCV infections worldwide.[97] After
HCV acute infection an average 50-85% of patients will not clear the
virus, and HCV 1b appears to have a high prevalence of HCC.[98]Interestingly,
all 1b HCV genotype-associated neoplasms, reported in the literature,
in Greek thalassemic patients were hepatocellular carcinoma (7 cases):[11] a similar pattern was observed in the Italian patients enrolled in our survey. The
youngest thalassemic patient with HCC was 33 years old. Thus at
present, we recommend an abdominal US screening associated with AFP
levels estimation in all patients over the age of 30 years with an
interval of 6 months. For younger patients there are no specific
recommendations. However,
we believe that in these patients, surveillance should be based on the
evidence of active HBV/HCV infections, iron overload, and advanced
liver fibrosis (Figure 2).
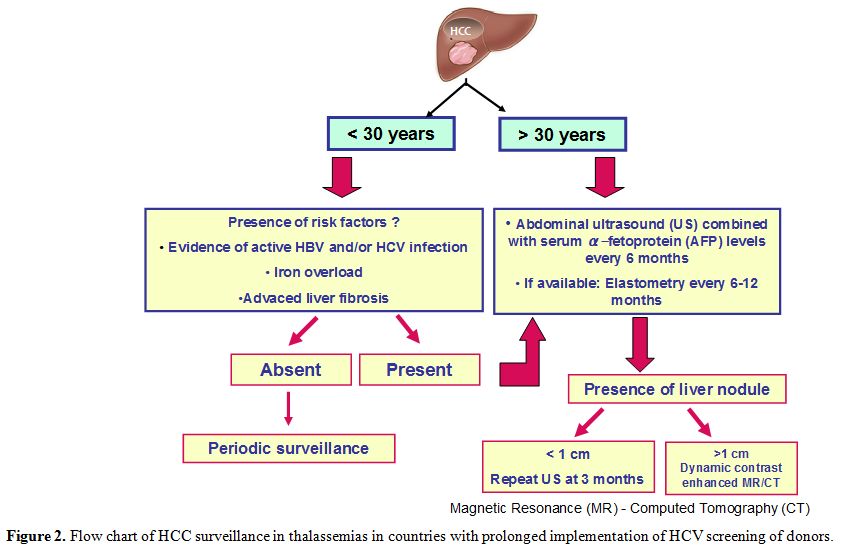 |
Figure 2. Flow chart of HCC surveillance in thalassemias in countries with prolonged implementation of HCV screening of donors. |
Liver biopsy has been considered the gold standard of staging liver disease in patients chronically infected with HCV.[99]
However, due to its invasive nature, sampling error, the potential for
adverse events, and high intra- and interobserver variability,[100] noninvasive diagnostic methods have begun to replace the biopsy in many settings.[7]Transient
elastography (TE; e.g., Fibroscan®), is an accurate and reproducible
method to detect liver fibrosis using ultrasound. It is a promising
alternative due to its high accuracy in detecting severe fibrosis and
cirrhosis.[101]
According
to the TE values, patients are grouped into 3 categories: those with
elastography values of ≤7.0 kPa corresponding to METAVIR stages F0 or
F1 (F0: no fibrosis; F1: portal fibrosis without septa); those with
elastography values > 7kPa-≤ 15kPa who have moderate to severe
fibrosis (stages F2 and F3) and are at risk for fibrosis progression
and the third group includes patients with high elastography values
> 15.0 kPa (METAVIR stage of F4 or some cases of F3) who have a high
likelihood of cirrhosis.[102]Noninvasive,
serum biomarker panels, which have also been validated against biopsy,
are an attractive alternative for staging patients with chronic HCV
infection in low- and middle-income countries.[103]We
hope that this review will stimulate the development of more refined,
prospective analyses of HCC magnitude and of the risk factors in
patients with thalassemia. Finally, we believe that specific
international guidelines are urgently needed to support clinicians in
the early diagnosis and treatment of thalassemic patients at risk of
HCC development. References
- Shiani A, Narayanan S, Pena L, Friedman M. The Role
of Diagnosis and Treatment of Underlying Liver Disease for the
Prognosis of Primary Liver Cancer. Cancer Control. 2017 Jul-Sep;24 (3):
10732748 177 29240. https://doi.org/10.1177/1073274817729240 PMid:28975833 PMCid:PMC5937237
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69-90. https://doi.org/10.3322/caac.20107 PMid:21296855
- Ferenci
P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, Heathcote J,
Piratsivuth T, Kew M, Otegbayo JA, Zheng SS, Sarin S, Hamid SS, Modawi
SB, Fleig W, Fedail S, Thomson A, Khan A, Malfertheiner P, Lau G,
Carillo FJ, Krabshuis J, Le Mair A; World Gastroenterology
Organization. World Gastroenterology Organisation guideline.
Hepatocellular carcinoma (HCC): a global perspective. J Gastrointestin
Liver Dis. 2010;19:311-317. https://doi.org/10.1097/MCG.0b013e3181d46ef2
- Darbari
A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic
malignancies in U.S. children. Hepatology. 2003;38:560-566. https://doi.org/10.1053/jhep.2003.50375 PMid:12939582
- Liu
P, Xie SH, Hu S, Cheng X, Gao T, Zhang C, Song Z. Age-specific sex
difference in the incidence of hepatocellular carcinoma in the United
States, Oncotarget. 2017 Jul 12;8(40):68131-68137. https://doi.org/10.18632/oncotarget.19245
- Bruix,
J, Sherman M, American Association for the Study of Liver Diseases.
Management of Hepatocellular Carcinoma: An Update.
Hepatology.2011;53:1020-1022. https://doi.org/10.1002/hep.24199 PMid:21374666 PMCid:PMC3084991
- European
Association for the Study of The Liver, European Organisation for
Research and Treatment of Cancer. EASL-EORTC Clinical Practice
Guidelines: Management of Hepatocellular Carcinoma. J Hepatol.
2012;56:908-943. https://doi.org/10.1016/j.jhep.2011.12.001 PMid:22424438
- Mokdad
AA, Hester CA, Singal AG, Yopp AC. Management of hepatocellular in the
United States. Chin Clin Oncol. 2017 Apr; 6(2):21. https://doi.org/10.21037/cco.2017.04.04 PMid:28482674
- Bettinger
D, Spode R, Glaser N, Buettner N, Boettler T, Neumann-Haefelin C,
Brunner TB, Gkika E, Maruschke L, Thimme R, Schultheiss M. Survival
benefit of transarterial chemoembolization in patients with metastatic
hepatocellular carcinoma: a single center experience. BMC
Gastroenterol. 2017 Aug 10;17(1):98. https://doi.org/10.1186/s12876-017-0656-z PMid:28797231 PMCid:PMC5553671
- Mittal
S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the
population, J Clin Gastroenterol. 2013;47 (Suppl):S2-6. https://doi.org/10.1097/MCG.0b013e3182872f29 PMid:23632345 PMCid:PMC3683119
- Kourakli
A, Diamantidis MD, Skafidas ME, Delicou S, Pantelidou D, Fragodimitri
C, Vlachaki E, Lafioniatis S, Petropoulou F, Eftychiadis E, Kapsali E,
Kalpaka A, Zissis C, Koutsouka F, Vasileiadi A, Goula A, Giasari P,
Katsatou M, Lafiatis I, Klironomos E, Kaiafas P, Kyriacopoulou D,
Lazaris V, Maragkos K, Fotiou P, Chalkia P, Schiza V, Kattamis A,
Symeonidis A. Hepatitis C Virus Infection, but Not Hepatic Iron
Overload Is the Dominant Risk Factor for the Manifestation of
Hepatocellular Carcinoma Among Greek Thalassemic Patients. Blood. 2018
132:2347; https://doi.org/10.1182/blood-2018-99-119731
- Borgna-Pignatti
C, De Stefano P, Sessa F, Avato F. Hepatocellular carcinoma in
thalassemia major. Med Pediatr Oncol.1986;14:327-328. https://doi.org/10.1002/mpo.2950140610 PMid:2431257
- Zurlo
M, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, Di
Gregorio F, Burattini MG, Terzoli S. Survival and causes of death in
thalassaemia major. Lancet. 1989;334:27-30. https://doi.org/10.1016/S0140-6736(89)90264-X
- Borgna-Pignatti
C, Vergine G, Lombardo T, Cappellini MD, Cianciulli P, Maggio A, Renda
D, Lai ME, Mandas A, Forni G, Piga A, Bisconte MG. Hepatocellular
carcinoma in the thalassaemia syndromes. Br J Haematol.
2004;124:114-117. https://doi.org/10.1046/j.1365-2141.2003.04732.x PMid:14675416
- Mancuso
A, Rigano P, Renda D, Maggio A. Hepatocellular carcinoma on
cirrhosis-free liver in a HCV-infected thalassemic. Am J
Hematol.2005;78:158-159. https://doi.org/10.1002/ajh.20289 PMid:15682406
- Mancuso
A, Sciarrino E, Renda MC, Maggio A. A prospective study of
hepatocellular carcinoma incidence in thalassemia. Hemoglobin.
2006:30:119-124. https://doi.org/10.1080/03630260500455565 PMid:16540424
- Restivo
Pantalone G, Renda D, Valenza F, D'Amato F,Vitrano A, Cassarà F, Rigano
P, Di Salvo V, Giangreco A, Bevacqua E, Maggio A. Hepatocellular
carcinoma in patients with thalassaemia syndromes: Clinical
characteristics and outcome in a long term single centre experience. Br
J Haematol. 2010;150:245-247. https://doi.org/10.1111/j.1365-2141.2010.08180.x PMid:20433678
- Fragatou
S, Tsourveloudis I, Manesis G. Incidence of hepatocellular carcinoma in
a thalassemia unit. Hemoglobin. 2010: 34:221-226. https://doi.org/10.3109/03630269.2010.485071 PMid:20524812
- Ansari
S, Azarkivan A, Halagi F. Incidence of hepatocellular carcinoma in
patients with thalassemia who had hepatitis C. Acta Med Iran.
2013;51:404-407.
- Maakaron JE, Cappellini
MD, Graziadei G, Ayache JB, Taher AT. Hepatocellular carcinoma in
hepatitis-negative patients with thalassemia intermedia: A closer look
at the role of siderosis. Ann Hepatol. 2013; 12: 142-146. https://doi.org/10.1016/S1665-2681(19)31397-3
- Modell
B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved
survival of thalassaemia major in the UK and relation to T2*
cardiovascular magnetic resonance [serial online]. J Cardiovasc Magn
Reson. 2008;10:42. https://doi.org/10.1186/1532-429X-10-42 PMid:18817553 PMCid:PMC2563008
- Borgna-Pignatti
C, Garani MC, Forni GL, Cappellini MD, Cassinerio E, Fidone C, Spadola
V, Maggio A, Restivo Pantalone G, Piga A, Longo F, Gamberini MR, Ricchi
P, Costantini S, D'Ascola D, Cianciulli P, Lai ME, Carta MP, Ciancio A,
Cavalli P, Putti MC, Barella S, Amendola G, Campisi S, Capra M, Caruso
V, Colletta G, Volpato S.Hepatocellular carcinoma in thalassaemia: An
update of the Italian Registry. Br J Haematol. 2014;167:121-126. https://doi.org/10.1111/bjh.13009 PMid:24992281
- Moukhadder
HM, Roumi JE, Bou-Fakhredin R, Taher AT. Hepatocellular Carcinoma in a
β- Thalassemia Intermedia Patient: Yet Another Case in the Expanding
Epidemic. Hemoglobin. 2018; 42:58-60. https://doi.org/10.1080/03630269.2018.1434197 PMid:29493312
- Haghpanah
S, Jelodari S, Karamifar H, Saki F, Rahimi R, De Sanctis V,
Dehbozorgian J, Karimi M. The frequency of hypothyroidism and its
relationship with HCV positivity in patients with thalassemia major in
southern Iran. Acta Biomed. 2018;89:55-60.
- Shi
L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular
carcinoma: is inflammation the key? J Transl Med. 2014 Apr 8;12:93. https://doi.org/10.1186/1479-5876-12-93 PMid:24708807 PMCid:PMC3992128
- Nagasue N, Ito A, Yukaya H, Ogawa Y. Estrogen receptors in hepatocellular carcinoma. Cancer. 1986;57:87-91. https://doi.org/10.1002/1097-0142(19860101)57:1<87::AID-CNCR2820570118>3.0.CO;2-K
- Nagasue
N, Ito A, Yukaya H, Ogawa Y. Androgen receptors in hepatocellular
carcinoma and surrounding parenchyma. Gastroenterology.
1985;89:643-647. https://doi.org/10.1016/0016-5085(85)90463-9
- Pok
S, Barn VA, Wong HJ, Blackburn AC, Board P, Farell GC, Teoh NC.
Testosterone regulation of cyclin E kinase: a key factor in determining
gender differences in hepatocarcinogenesis. J Gastroenterol
Hepatol.2016;31:1210-1219. https://doi.org/10.1111/jgh.13232 PMid:26574916
- Chen
PJ, Yeh SH, Liu WH, Lin CC, Huang HC, Chen CL, Chen DS, Chen PJ.
Androgen pathway stimulates microRNA-216a transcription to suppress the
tumor suppressor in lung cancer-1 gene in early hepatocarcinogenesis.
Hepatology. 2012;56: 632-643. https://doi.org/10.1002/hep.25695 PMid:22392644
- De
Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ, Pepe A, Kattamis C,
Soliman NA, Elalaily R, El Kholy M, Yassin M. Acquired Hypogonadotropic
Hypogonadism (AHH) in Thalassaemia Major Patients: An Underdiagnosed
Condition? Mediterr J Hematol Infect Dis. 2016 Jan 1;8(1):e2016001. https://doi.org/10.4084/mjhid.2016.001 PMid:26740862 PMCid:PMC4696472
- De
Sanctis V, Soliman AT, Daar S, Di Maio S. Adverse events during
testosterone replacement therapy in 95 young hypogonadal thalassemic
men. Acta Biomed. 2019;90:228-232.
- Maheshwari
S, Sarraj A, Kramer J, El-Serag HB. Oral contraception and the risk of
hepatocellular carcinoma. J Hepatol. 2007;47:506-513. https://doi.org/10.1016/j.jhep.2007.03.015 PMid:17462781
- McGlynn
KA, Sahasrabuddhe VV, Campbell PT, Graubard BI, Chen J, Schwartz LM,
Petrick JL, Alavanja MC, Andreotti G, Boggs DA, Buring JE, Chan AT,
Freedman ND, Gapstur SM, Hollenbeck AR, Hou L, King LY, Koshiol J,
Linet M, Palmer JR, Poynter JN, Purdue M, Robien K, Schairer C, Sesso
HD, Sigurdson A, Wactawski-Wende J, Zeleniuch-Jacquotte A. Reproductive
factors, exogenous hormone use and risk of hepatocellular carcinoma
among US women: results from the Liver Cancer Pooling Project. Br J
Cancer. 2015;112:1266-1272. https://doi.org/10.1038/bjc.2015.58 PMid:25742475 PMCid:PMC4385955
- De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol. 2002;193:59-63. https://doi.org/10.1016/S0303-7207(02)00096-5
- Lai
ME, Origa R, Danjou F, Leoni GB, Vacquer S, Anni F, Corrias C, Farci P,
Congiu G, Galanello R. Natural history of hepatitis C in thalassemia
major: a long-term prospective study. Eur J Haematol. 2013;90:501-507. https://doi.org/10.1111/ejh.12086 PMid:23414443
- Cho
LY, Yang JJ, Ko KP, Park B, Shin A, Lim MK, Oh JK, Park S, Kim YJ, Shin
HR, Yoo KY, Park SK. Coinfection of hepatitis B and C viruses and risk
of hepatocellular carcinoma: systematic review and meta-analysis. Int J
Cancer. 2011;128:176-184. https://doi.org/10.1002/ijc.25321 PMid:20232388
- El-Serag HB. Hepatocellular Carcinoma and Hepatitis C in the United States. Hepatology. 2002;36:S74-83. https://doi.org/10.1053/jhep.2002.36807 PMid:12407579
- Raimondi
S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b
as a risk factor for hepatocellular carcinoma development: a
meta-analysis. J Hepatol. 2009;501142-1154. https://doi.org/10.1016/j.jhep.2009.01.019 PMid:19395111
- Di
Marco V, Capra M, Gagliardotto F, Borsellino Z, Cabibi D, Barbaria F,
Ferraro D, Cuccia L, Ruffo GB, Bronte F, Di Stefano R, Almasio PL,
Craxì A. et al. Liver disease in chelated transfusion-dependent
thalassemics: The role of iron overload and chronic hepatitis C.
Hematologica. 2008;93:1243-1246. https://doi.org/10.3324/haematol.12554 PMid:18556410
- Kamal
S, Abdelhakam S, Ghoraba D, Mohsen MA, Salam AA, Hassan H, Nabeigh L.
The Course of Hepatitis C Infection and Response to Anti-viral Therapy
in Patients with Thalassemia major and Hepatitis C Infection: A
Longitudinal, Prospective Study. Mediterr J Hematol Infect Dis. 2019
Nov 1;11(1): e2019060. https://doi.org/10.4084/mjhid.2019.060 PMid:31700585 PMCid:PMC6827603
- Di
Marco V, Capra M, Gagliardotto F, Borsellino Z, Cabibi D, Barbaria F,
Ferraro D, Cuccia L, Ruffo GB, Bronte F, Di Stefano R, Almasio PL,
Craxì A. Liver disease in chelated transfusion-dependent thalassemics:
The role of iron overload and chronic hepatitis C. Hematologica.
2008;93:1243-1246. https://doi.org/10.3324/haematol.12554 PMid:18556410
- Maira
D, Cassinerio E, Marcon A, Mancarella M, Fraquelli M, Pedrotti P,
Cappellini MD. Progression of liver fibrosis can be controlled by
adequate chelation in transfusion-dependent thalassemia (TDT). Ann
Hematol. 2017;96:1931-1936. https://doi.org/10.1007/s00277-017-3120-9 PMid:28875336
- Zachou
K, Arvaniti P,Gatselis NK, Azariadis K, Papadamou G, Rigopoulou E,
Dalekos GN. Patients with haemoglobinopathies and chronic hepatitis C:
a real difficult to treat population in 2016? Mediterr J Hematol Infect
Dis. 2017, 9(1): e2017003, https://doi.org/10.4084/mjhid.2017.003 PMid:28101309 PMCid:PMC5224816
- Swain
MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, Abergel
A, Pessôa MG, Lin A, Tietz A, Connell EV, Diago M. A sustained
virologic response is durable in patients with chronic hepatitis C
treated with peg-interferon alfa-2a and ribavirin. Gastroenterology.
2010; 139: 1593-1601. https://doi.org/10.1053/j.gastro.2010.07.009 PMid:20637202
- Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. J Am Med Assoc 2014;312:631-640. https://doi.org/10.1001/jama.2014.7085 PMid:25117132
- Geddawy
A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct acting anti-hepatitis C
virus drugs: Clinical pharmacology and future direction. J Transl
Intern Med 2017;5:8-17. https://doi.org/10.1515/jtim-2017-0007 PMid:28680834 PMCid:PMC5490957
- Zamani
F, Ajdarkosh H, Safarnezhad-Tameshkel F, Azarkeivan A, Keyvani H,
Naserifar F, Vafaeimanesh J. The effectiveness of sofosbuvir and
daclatasvir in the treatment of hepatitis C in thalassaemia major
patients and their effect on haematological factors. Indian J Med
Microbiol. 2018;36:224-229. https://doi.org/10.4103/ijmm.IJMM_18_90 PMid:30084415
- Origa
R, Ponti ML, Filosa A, Galeota Lanza A, Piga A, Saracco GM, Pinto V,
Picciotto A, Rigano P, Madonia S, Rosso R, D'Ascola D, Cappellini MD,
D'Ambrosio R, Tartaglione I, De Franceschi L, Gianesin B, Di Marco V,
Forni GL; Italy for THAlassemia and hepatitis C Advance - Società
Italiana Talassemie ed Emoglobinopatie (ITHACA-SITE). Treatment of
hepatitis C virus infection with direct-acting antiviral drugs is safe
and effective in patients with hemoglobinopathies. Am J Hematol.
2017;92:1349-1355. https://doi.org/10.1002/ajh.24911 PMid:28929515
- Sinakos
E, Kountouras D, Koskinas J, Zachou K, Karatapanis S, Triantos C,
Vassiliadis T, Goulis I, Kourakli A, Vlachaki E, Toli B, Tampaki M,
Arvaniti P, Tsiaoussis G, Bellou A, Kattamis A, Maragkos K, Petropoulou
F, Dalekos GN, Akriviadis E, Papatheodoridis GV. Treatment of chronic
hepatitis C with direct-acting antivirals in patients with
β-thalassaemia major and advanced liver disease.Br J Haematol.
2017;178:130-136. https://doi.org/10.1111/bjh.14640 PMid:28439915
- Okada
S, Hamazaki S, Toyokuni S, Midorikawa O. Induction of mesothelioma by
intraperitoneal injections of ferric saccharate in male Wistar rats.
British J Cancer. 1989; 60:708-711. https://doi.org/10.1038/bjc.1989.344 PMid:2803947 PMCid:PMC2247310
- Yu
Y, Suryo Rahmanto Y, Richardson DR. Bp44mT: an orally active iron
chelator of the thiosemicarbazone class with potent anti-tumour
efficacy. British J Pharmacol. 2012; 165:148-166. https://doi.org/10.1111/j.1476-5381.2011.01526.x PMid:21658021 PMCid:PMC3252974
- Kew MC. Hepatic iron overload and hepatocellular carcinoma. Cancer Lett.2009;286:38-43. https://doi.org/10.1016/j.canlet.2008.11.001 PMid:19081672
- Tirnitz-Parker JE, Glanfield A, Olynyk JK, Ramm GA. Iron and hepatic carcinogenesis. Crit Rev Oncog. 2013; 18:391-407. https://doi.org/10.1615/CritRevOncog.2013007759 PMid:23879586
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127 (5 suppl 1):S79-S86. https://doi.org/10.1016/j.gastro.2004.09.019 PMid:15508107
- Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer 2014; 3:31-40. https://doi.org/10.1159/000343856 PMid:24804175 PMCid:PMC3995380
- Matzner
Y, Hershko C, Polliack A, Konijn AM, Izak G. Suppressive effect of
ferritin on in vitro lymphocyte function. Br J
Haematol.1979;42:345-353. https://doi.org/10.1111/j.1365-2141.1979.tb01142.x PMid:157770
- Walker EM, Walker SM. Effects of iron overload on the immune system. Ann Clin Lab Sci. 2000; 30:354-365. https://doi.org/10.1023/A:1003905109007
- Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011; 53:1020-1022. https://doi.org/10.1002/hep.24199 PMid:21374666 PMCid:PMC3084991
- Sleiman
J, Tarhini A, Bou-Fakhredin R, Saliba AN, Cappellini MD, Taher
AT.Non-Transfusion-Dependent Thalassemia: An Update on Complications
and Management. Int J Mol Sci. 2018 Jan 8;19(1). pii: E182. https://doi.org/10.3390/ijms19010182 PMid:29316681 PMCid:PMC5796131
- Finianos
A, Matar CF, Taher A. Hepatocellular Carcinoma in β-Thalassemia
Patients: Review of the Literature with Molecular Insight into Liver
Carcinogenesis. Int J Mol Sci. 2018 Dec 17;19(12). pii: E4070. doi:
10.3390/ijms19124070 https://doi.org/10.3390/ijms19124070 PMid:30562917 PMCid:PMC6321074
- El-Serag
HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular
carcinogenesis. Gastroenterology 2007;132:2557-2576. https://doi.org/10.1053/j.gastro.2007.04.061 PMid:17570226
- Shen
FM, Lee MK, Gong HM, Cai XQ, King MC. Complex segregation analysis of
primary hepatocellular carcinoma in Chinese families: interaction of
inherited susceptibility and hepatitis B viral infection. Am J Hum
Genet.1991;49:88-93.
- Walker AJ, Peacock
CJ, Pedergnana V; STOP-HCV Consortium, Irving WL. Host genetic factors
associated with hepatocellular carcinoma in patients with hepatitis C
virus infection: A systematic review. J Viral Hepat. 2018;25:442-456. https://doi.org/10.1111/jvh.12871 PMid:29397014 PMCid:PMC6321980
- Raff
EJ, Kakati D, Bloomer JR, Shoreibah M, Rasheed K, Singal AK. Diabetes
mellitus predicts occurrence of cirrhosis and hepatocellular cancer in
alcoholic liver and nonalcoholic fatty liver diseases. J Clin Transl
Hepatol. 2015;3:9-16. https://doi.org/10.14218/JCTH.2015.00001 PMid:26356325 PMCid:PMC4542082
- Mowla
A, Karimi M, Afrasiabi A, De Sanctis V. Prevalence of diabetes mellitus
and impaired glucose tolerance in beta-thalassemia patients with and
without hepatitis C virus infection.Pediatr Endocrinol Rev. 2004;2
(Suppl 2):282-824.
- De Sanctis V, Soliman
AT, Elsedfy H, Yaarubi SA, Skordis N, Khater D, El Kholy M, Stoeva I,
Fiscina B, Angastiniotis M, Daar S, Kattamis C. The ICET-A
Recommendations for the Diagnosis and Management of Disturbances of
Glucose Homeostasis in Thalassemia Major Patients Mediterr J Hematol
Infect Dis. 2016 Oct 28;8(1):e2016058. https://doi.org/10.4084/mjhid.2016.058 PMid:27872738 PMCid:PMC5111521
- Noetzli
LJ, Mittelman SD, Watanabe RM, Coates TD, Wood JC. Pancreatic iron and
glucose dysregulation in thalassemia major. Am J Hematol
2012;87:155-160. https://doi.org/10.1002/ajh.22223 PMid:22120775
- Wankanit
S, Chuansumrit A, Poomthavorn P, Khlairit P, Pongratanakul S,
Mahachoklertwattana P. Acute Effects of Blood Transfusion on Insulin
Sensitivity and Pancreatic β-Cell Function in Children with
β-Thalassemia/Hemoglobin E Disease. J Clin Res Pediatr Endocrinol.
2018;10:1-7. https://doi.org/10.4274/jcrpe.4774 PMid:28739553 PMCid:PMC5838366
- Petersen DR. Alcohol, iron-associated oxidative stress, and cancer. Alcohol. 2005;35:243-249. https://doi.org/10.1016/j.alcohol.2005.03.013 PMid:16054986
- Hu
W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The
major lipid peroxidation product, trans-4-hydroxy-2-nonenal,
preferentially forms DNA adducts at codon 249 of human p53 gene, a
unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis.
2002;23:1781-1789. https://doi.org/10.1093/carcin/23.11.1781 PMid:12419825
- Barbieri
SS, Zacchi E, Amadio P, Gianellini S, Mussoni L, Weksler BB, Tremoli E.
Cytokines present in smokers' serum interact with smoke components to
enhance endothelial dysfunction. Cardiovasc Res. 2011;90:475-483. https://doi.org/10.1093/cvr/cvr032 PMid:21285293
- Díez
Piña JM, Fernández Aceñero MJ, Llorente Alonso MJ, Díaz Lobato S,
Mayoralas Alises S, Pérez Rodríguez E, Alvaro Álvarez D, Flórez
Horcajada A, Pérez Rojo R. Tumor necrosis factor as an early marker of
inflammation in healthy smokers. Med Clin (Barc). 2012;139:47-53. https://doi.org/10.1016/j.medcli.2011.11.032 PMid:22401725
- Valenca
SS, Silva Bezerra F, Lopes AA, Romana-Souza B, Marinho Cavalcante MC,
Lima AB, Gonçalves Koatz VL, Porto LC. Oxidative stress in mouse plasma
and lungs induced by cigarette smoke and lipopolysaccharide. Environ
Res. 2008;108:199-204. https://doi.org/10.1016/j.envres.2008.07.001 PMid:18721919
- Purohit
V, Rapaka R, Kwon OS, Song BJ. Roles of alcohol and tobacco exposure in
the development of hepatocellular carcinoma. Life Sci. 2013;92:3-9. https://doi.org/10.1016/j.lfs.2012.10.009 PMid:23123447 PMCid:PMC3822918
- Conti
F, Buonfiglioli F, Scuteri A , Crespi C, Bolondi L, Caraceni P, Foschi
FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early
occurrence and recurrence of hepatocellular carcinoma in HCV related
cirrhosis treated with direct-acting antivirals. J Hepatol.
2016;65:727-733. https://doi.org/10.1016/j.jhep.2016.06.015 PMid:27349488
- Kozbial
K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, Stättermayer
AF, Beinhardt S, Graziadei I, Freissmuth C, Maieron A, Gschwantler M,
Strasser M, Peck-Radosalvjevic M, Trauner M, Hofer H, Ferenci P.
Unexpected high incidence of hepatocellular carcinoma in cirrhotic
patients with sustained virologic response following interferon-free
direct-acting antiviral treatment. J Hepatol. 2016; 65:856-858. https://doi.org/10.1016/j.jhep.2016.06.009 PMid:27318327
- Cardoso
H, Vale AM, Rodrigues S, Gonçalves R, Albuquerque A, Pereira P, Lopes
S, Silva M, Andrade P, Morais R, Coelho R, Macedo G. High incidence of
hepatocellular carcinoma following successful interferonfree antiviral
therapy for hepatitis C associated cirrhosis. J Hepatol. 2016;
65:1070-1071. https://doi.org/10.1016/j.jhep.2016.07.027 PMid:27476768
- Reig
M, Mariño Z, Perello C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A,
Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix
J.Unexpected high rate of early tumor recurrence in patients with
HCV-related HCC undergoing interferon-free therapy. J Hepatol.
2016;65:719-726. https://doi.org/10.1016/j.jhep.2016.04.008 PMid:27084592
- ANRS
collaborative study group on hepatocellular carcinoma (ANRS CO22
HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an
effect of direct-acting antivirals on the recurrence of hepatocellular
carcinoma: Data from three ANRS cohorts. J Hepatol.2016;65:734-740. https://doi.org/10.1016/j.jhep.2016.05.045 PMid:27288051
- Adhoute
X, Castellani P, Bourlière M. Impact of direct-acting antiviral agents
on the risk for hepatocellular carcinoma. Transl Gastroenterol Hepatol
2017;2:110. https://doi.org/10.21037/tgh.2017.12.04 PMid:29354767 PMCid:PMC5762991
- Soliman
A, De Sanctis V, Yassin M. Vitamin d status in thalassemia major: an
update. Mediterr J Hematol Infect Dis. 2013 Sep 2;5(1):e2013057. https://doi.org/10.4084/mjhid.2013.057 PMid:24106607 PMCid:PMC3787712
- Wu
DB, Wang ML, Chen EQ, Tang H. New insights into the role of vitamin D
in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol.
2018;12:287-294. https://doi.org/10.1080/17474124.2018.1406307 PMid:29140126
- Chiang
KC, Yeh CN, Chen MF, Chen TC. Hepatocellular carcinoma and vitamin D: a
review. J Gastroenterol Hepatol. 2011;26:1597-603. https://doi.org/10.1111/j.1440-1746.2011.06892.x PMid:21880026
- Omar
A, Abou-Alfa GK, Khairy A, Omar H. Risk factors for developing
hepatocellular carcinoma in Egypt. Chin Clin Oncol. 2013 Dec;2(4):43. https://doi.org/10.3978/j.issn.2304-3865.2013.11.07.
- Tsuchiya
N, Sawada Y, Endo I, Saito K, Uemura Y, NakatsuraT. Biomarkers for the
Early Diagnosis of Hepatocellular Carcinoma. World J Gastroenterol.
2015; 21:10573-10583. https://doi.org/10.3748/wjg.v21.i37.10573 PMid:26457017 PMCid:PMC4588079
- Makiyama
A, Itoh Y, Kasahara A, Imai Y, Kawata S, Yoshioka K, Tsubouchi H,
Kiyosawa K, Kakumu S, Okita K, Hayashi N, Okanoue T. Characteristics of
patients with chronic hepatitis C who develop hepatocellular carcinoma
after a sustained response to interferon therapy. Cancer.
2004;101:1616-1622. https://doi.org/10.1002/cncr.20537 PMid:15378504
- Ryder
SD; British Society of Gastroenterology. Guidelines for the diagnosis
and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;
52 (Suppl 3):1-8. https://doi.org/10.1136/gut.52.suppl_3.iii1 PMid:12692148 PMCid:PMC1867754
- Sanyal
AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and
consequences for treatment. Oncologist. 2010;15 (Suppl 4):14-22. https://doi.org/10.1634/theoncologist.2010-S4-14 PMid:21115577
- Singal
A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero
JA.Meta-analysis: surveillance with ultrasound for early-stage
hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol
Ther. 2009;30:37-47. https://doi.org/10.1111/j.1365-2036.2009.04014.x PMid:19392863 PMCid:PMC6871653
- Sciancalepore
D, Zingaro MT, Luglio CV, Sabba C, Napoli N. Hepatocellular Carcinoma:
Known and Emerging Risk Factors. J Cancer Ther. 2018;9:417-437. https://doi.org/10.4236/jct.2018.95037
- Lencioni
R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the
diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48:848-857. https://doi.org/10.1016/j.jhep.2008.02.005 PMid:18328590
- Jo
PC, Jang HJ, Burns PN, Burak KW, Kim TK, Wilson SR. Integration of
contrast-enhanced US into a multimodality approach to imaging of
nodules in a cirrhotic liver: how i do it. Radiology 2017;282:317- 331.
https://doi.org/10.1148/radiol.2016151732 PMid:28099108
- Nakayama
Y, Inoue T, Sakamoto M, Enomoto N. Liver stiffness measurement for risk
assessment of hepatocellular carcinoma. Hepatol Res. 2015;45:523-532. https://doi.org/10.1111/hepr.12377 PMid:24961848
- Marrero
JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR,
Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular
Carcinoma: 2018 Practice Guidance by the American Association for the
Study of Liver Diseases. Hepatology. 2018; 68:723-750. https://doi.org/10.1002/hep.29913 PMid:29624699
- Santi
V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P,
Di Nolfo MA, Benvegnù L, Farinati F, Zoli M, Giannini EG, Borzio F,
Caturelli E, Chiaramonte M, Bernardi M; Italian Liver Cancer
(ITA.LI.CA) Group. Semiannual surveillance is superior to annual
surveillance for the detection of early hepatocellular carcinoma and
patient survival. J Hepatol. 2010; 53:291-297. https://doi.org/10.1016/j.jhep.2010.03.010 PMid:20483497
- Simmonds
P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P,
Inchauspé G, Kuiken C, Maertens G, Mizokami M, Murphy DG, Okamoto H,
Pawlotsky JM, Penin F, Sablon E, Shin-I T, Stuyver LJ, Thiel HJ, Viazov
S, Weiner AJ, Widell A. Consensus proposals for a unified system of
nomenclature of hepatitis C virus genotypes.
Hepatology.2005;42:962-973. https://doi.org/10.1002/hep.20819 PMid:16149085
- Mondelli MU, Silini E. Clinical significance of hepatitis C virus genotypes. J Hepatol. 1999;31 (Suppl 1):65-70. https://doi.org/10.1016/S0168-8278(99)80377-8
- Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. https://doi.org/10.1038/nrgastro.2010.100 PMid:20628345 PMCid:PMC3926946
- Bedossa
P. Intraobserver and interobserver variations in liver biopsy
interpretation in patients with chronic hepatitis C. Hepatology. 1994;
20:15-20. https://doi.org/10.1002/hep.1840200104
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003; 38:1449-1457. https://doi.org/10.1053/jhep.2003.09022 PMid:14647056
- Mikolasevic
I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient
elastography [FibroScan ®] with controlled attenuation parameter in the
assessment of liver steatosis and fibrosis in patients with
nonalcoholic fatty liver disease - Where do we stand? World J
Gastroenterol. 2016;22:7236-7251. https://doi.org/10.3748/wjg.v22.i32.7236 PMid:27621571 PMCid:PMC4997649
- Bedossa
P, Poynard T. An algorithm for the grading of activity in chronic
hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996; 24,
289- 293. https://doi.org/10.1002/hep.510240201 PMid:8690394
- Castera
L. Noninvasive methods to assess liver disease in patients with
hepatitis B or C. Gastroenterology. 2012; 142:1293-1302. https://doi.org/10.1053/j.gastro.2012.02.017 PMid:22537436
[TOP]





