Vincenzo Accurso1, Marco Santoro2*, Salvatrice Mancuso1, Angelo Davide Contrino1, Paolo Casimiro1, Mariano Sardo1, Simona Raso2, Florinda Di Piazza3, Alessandro Perez3, Marco Bono3, Antonio Russo3 and Sergio Siragusa1.
1
Hematology Division University Hospital Policlinico "Paolo Giaccone",
Via del Vespro 129, 90127, Palermo, Italy, Via del Vespro 129, 90127,
Palermo, Italy.
2 Dept. of Surgical, Oncological and
Stomatological Disciplines, University of Palermo, Via del Vespro 129,
90127, Palermo, Italy.
3 Department of Surgical,
Oncological and Stomatological Disciplines, Section of Medical
Oncology, University of Palermo, Via del Vespro 129, 90127, Palermo,
Italy.
Corresponding
author: Vincenzo Accurso. Hematology Division University of
Palermo, Via del Vespro 129, 90127, Palermo, Italy, +393338963096.
E-mail:
casteldaccia@tiscali.it
Published: January 1, 2020
Received: September 8, 2019
Accepted: December 16, 2019
Mediterr J Hematol Infect Dis 2020, 12(1): e2020008 DOI
10.4084/MJHID.2020.008
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Thromboembolic
and bleeding events pose a severe risk for patients with Polycythemia
Vera (PV) and Essential Thrombocythemia (ET). Many factors can
contribute to promoting the thrombotic event due to the interaction
between platelets, leukocytes, and endothelium alterations. Moreover, a
significant role can be played by cardiovascular risk factors (CV.R)
such as cigarette smoking habits, hypertension, diabetes, obesity and
dyslipidemia. In this study, we evaluated the impact that CV.R plays on
thrombotic risk and survival in patients with PV and ET.
|
Introduction
Diagnosis
of PV requires as major criteria, the increase of hemoglobin/hematocrit
ratio, whose threshold levels have been established by 2016 World
Health Organization (WHO) revised criteria (>16.5 g/dL or >49%
for males and >16 g/dL or >48% for females), the presence of JAK2
mutation, the bone marrow tri‐lineage proliferation with Pleomorphic
mature megakaryocytes, and in the absence of a major criterium the
presence of one minor.[1,2] Almost all patients with
PV harbor a JAK2 mutation; approximately 96% and 3% of them display
somatic activating mutations in exon 14 (JAK2-V617F) and JAK2 exon 12,
respectively. Essential thrombocythemia (ET) is a chronic
myeloproliferative neoplasm (MPN) characterized by persistently high
platelet count and overall favorable prognosis with respect to the
other MPNs (but Life-expectancy in ET is inferior to the control
population).[2] Approximately 90% of patients with ET
show a mutually exclusive JAK2, CALR, or myeloproliferative leukemia
(MPL) mutation. In a recently published study conducted on 826 Mayo
Clinic patients with ET, PV, or PMF, the respective median survivals
were approximately 20 years for ET, 14 years for PV, and 6 years for
primary myelofibrosis.[3] The increased risk of
vascular complications over time is the main clinical feature of PV and
ET. Concerning PV, two risk categories are defined. In particular, a
low and high risk has been considered for patients without a history of
thrombosis and younger than 60 years, and patients aged older than 60
or with a history of thrombosis. In ET, four risk categories are
considered: very low (age ≤ 60 years, no thrombosis history, JAK2
wild-type), low (same as very low but presence of a JAK2 mutation),
intermediate (age > 60 years, no thrombosis history, JAK2 wild-type)
and high (thrombosis history present or age > 60 years with JAK2
mutation).[4] Current classifications of the
thrombotic risk in patients with PV, as well as in those with ET do not
predict cardiovascular risk (CVR), and this condition does not
currently influence the choice of cytoreductive therapy. In this study,
we considered evaluating the frequency of CVR in a cohort of patients
with ET and with PV and the possible impact on the thrombotic risk on
survival. This study was approved by our hospital's ethics committee.
Methods
From
January 1997 to May 2019, 403 consecutive patients were followed with a
median follow-up of 48,43 months (0.3 – 316 months). In particular, PV
patients (n.165) had a median follow-up of 58,18 months (0.3 – 289.30
months), while the ET patients (n. 238) had a median follow-up of 44.60
months (0.4 - 316 months). We evaluated, with retrospective analysis,
at diagnosis, the main characteristics of the study population such as
gender, age, and mutational status along with CVR factors frequency
such as cigarette smoking habits, hypertension, diabetes, obesity and
dyslipidemia. In particular, patients with only one of these conditions
were distinguished by those with more than one cardiovascular risk
factor or without CVR factors. Moreover, the correlation of these
cardiovascular risk conditions with the onset of thrombosis has been
evaluated. The frequencies were calculated by using the chi-square
method, and the comparison between medians was evaluated through the
Kruskal-Wallis test. Furthermore, the survival has been evaluated by
the Kaplan and Meyer method and the comparison between survival curves
with the log-rank test.
Results
The
main features, including sex, median age, and the mutational status,
along with the cardiovascular risk factors of the cohort under
examination (n. 403) were, respectively, summarized in Table 1 and Table 2.
In particular, 59 patients with ET (24.79%) and 37 PV patients (22.42)
have no CVR, while 93 ET patients (39.07%) show only one CVR factor and
85 (35.71%) have more than one. Furthermore, 66 PV patients (49%) have
only one cardiovascular risk factor, while 62 (37.57%) have more than
one CVR.
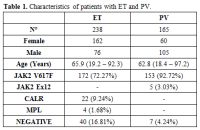 |
Table 1. Characteristics of patients with ET and PV. |
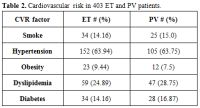 |
Table 2.Cardiovascular risk in 403 ET and PV patients. |
In
patients with PV, we highlighted 49 (29.69%) cases of thrombosis at
diagnosis or before diagnosis and 16 (9.69%) cases of thrombosis after
diagnosis, while in patients with ET, respectively 49 (20.61%) and 17
cases (6.72%). Overall, PV patients show a thrombosis frequency of
39.39% if compared to 27.39% of ET patients (p = 0.014).
In
patients with PV and ET, the frequency of thrombotic episodes is
strictly correlated with cardiovascular risk factors; in fact, the
frequency of thrombosis is much lower in patients without CVR. In ET
patients without CVR, the thrombotic event is present in 10/59 cases if
compared to patients with only one CVR factor (18/93) and to patients
with more than one CVR factor (37/85). In PV patients, thrombosis is
present, respectively, in 11/37 cases, 20/66 cases and 24/62 cases. Our
data also show a significant correlation between cardiovascular risk
factors and survival both in the cohort of patients with PV and ET,
distinguished by the number of cardiovascular risk factors (see Figure 1).
 |
Figure 1. Survival and CVR factors in PV (A) and ET (B) patients. |
Discussion
Patients
with myeloproliferative neoplasms (MPNs) have an increased risk of
thrombotic events if compared with the general population, adding the
latter a higher risk of morbidity and mortality MPNs-associated.[5,6]
A recent single-center study conducted on 526 patients with MPNs with
an overall study period of 3497.4 years, reported an incidence rate of
1.7% of venous thrombosis per patient/year.[7]
Overall, 38.4% of all venous thrombosis occurred before or at diagnosis
of MPNs, with 55.6% occurring at uncommon sites such as splanchnic or
cerebral veins. Polycythemia Vera (PV) and Essential Thrombocythemia
(ET) are myeloproliferative neoplasms respectively characterized by
erythrocytosis and thrombocytosis; other clinical features include
leukocytosis, splenomegaly, thrombosis, bleeding, microcirculatory
symptoms, pruritus, and risk of leukemic or fibrotic transformation;
moreover, thrombosis and cardiovascular disease are more prevalent in
PV than in the other MPNs. It has been estimated that 30% to 50% of PV
patients have minor and major thrombotic complications, and vascular
mortality accounts for 35% to 45% of all deaths.[8]
Also, in ET, thrombotic complications and cardiovascular events are
very frequent. In a recent study, thrombotic events before or at the
time of ET diagnosis were reported in 231 (17.8%) of 1,297 patients.[9]
Determining the thrombotic risk in the PV and ET is pivotal for the
proper therapeutic choice. In PV, two risk categories are generally
considered: high risk (age > 60 years or thrombosis history present)
and low risk (absence of both risk factors). The IPSETt classification
of the thrombotic risk contemplates the assignment of 2 points for
previous thrombotic events in patients aged greater than 60 years and
the presence of JAK2-V617F mutation, 1 point for age greater than 60
years and a point for the presence of CVR factors. The low-risk is
defined by a score lower than 2, the intermediate-risk by a score equal
to 2 and the high-risk by a score greater than 2.[10]
A new classification for the thrombotic risk describes four risk
categories: very low, low, intermediate and high (as described in the
introduction) but, unfortunately, cardiovascular risk factors are not
yet considered. In a previously proposed thrombotic risk classification
model, at the traditional high and low risk category was added an
intermediate risk category specific for all the patients aged under 60
years, with no history of thrombosis but with the presence of
cardiovascular risk factors.[11] This thrombotic risk
classification model was not followed. Cerquozzi et al. explored the
association of cardiovascular risk factors with the occurrence of
arterial or venous events at or following diagnosis; they found that
older age (≥ 60 years), hypertension, diabetes, hyperlipidemia, and
normal karyotype were associated with arterial events, whereas younger
age (< 60 years), female sex, palpable splenomegaly, and history of
major hemorrhage were associated with venous events.[12]
Today for patients with PV or ET with less than 60 years and with one
or more cardiovascular risk factors, there is no indication for
cytoreductive therapy, but only for antiplatelet drugs prophylaxis.
Only for ET, the IPSET-thrombosis system that includes age, previous
thrombosis, cardiovascular risk factors, and JAK2-V617F mutation is the
recommended prognostic system, and it should be scored in all patients
at diagnosis. This implies that general risk factors for thrombosis,
including smoking habits, diabetes mellitus, arterial hypertension, and
hypercholesterolemia, should also be considered even if the absence of
specific therapeutic indications remains.[13]
According to our experience, it should be useful to carry out
prospective studies for the characterization of the influence of
cardiovascular risk factors in the thrombotic event and on survival in
order to evaluate the opportunity to develop specific therapeutic
recommendations for patients with PV and ET with less than 60 years and
cardiovascular risk factors.
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016
revision to the World Health Organization classification of myeloid
neoplasms and acute leukemia. Blood. 2016;127:2391-2405. https://doi.org/10.1182/blood-2016-03-643544 PMid:27069254
- Tefferi
A, Barbui T. Polycytemia vera and essential thrombocythemia:2019 update
on diagnosis,risk stratification and management. Am J Hematol. 2019
Jan;94(1): 133-143. https://doi.org/10.1002/ajh.25303 PMid:30281843
- Tefferi
A, Guglielmelli P, Larson DR, et al. Long-term survival and blast
transformation in molecularly annotated essential thrombocythemia,
polycythemia vera, and myelofibrosis. Blood 2014;124:2507-2513; https://doi.org/10.1182/blood-2014-05-579136 PMid:25037629 PMCid:PMC4199952
- Mahnur
Haider, Naseema Gangat, Terra Lasho, Ahmed K. Abou Hussein, Yoseph C.
Elala, Curtis Hanson,and Ayalew Tefferi .Validation of the revised
international prognostic score of thrombosis for essential
thrombocythemia (IPSET- thrombosis) in 585 Mayo clinic patients.
American Journal of Hematology, Vol. 91, No. 4, April 2016. https://doi.org/10.1002/ajh.24293 PMid:26799697
- Barbui T, Finazzi G, Falanga A (2013) Myeloproliferative neoplasms and thrombosis. Blood 122:2176-2284. https://doi.org/10.1182/blood-2013-03-460154 PMid:23823316
- Marchioli
R.,Finazzi G,Landolfi R.,et al.Vascular and neoplastic risk in a large
cohort of patients with polycythemia vera. J Clin Oncol 2005; 23 (10):
2224-2232 https://doi.org/10.1200/JCO.2005.07.062 PMid:15710945
- Wille
K, Sadjadian P, Becker T, Kolatzki V, Horstmann A, Fuchs C,Griesshammer
M (2018) High risk of recurrent venous thromboembolism in
BCR-ABL-negative myeloproli ferative neoplasms after termination of
anticoagulation. Ann Hematol 98:93-100. https://doi.org/10.1007/s00277-018-3483-6 PMid:30155552
- Vannucchi
AM (2010) Insights into the pathogenesis and management of thrombosis
in polycythemia vera and essential thrombocythemia. Intern Emerg Med
5:177-184 https://doi.org/10.1007/s11739-009-0319-3 PMid:19789961
- Andriani
A., Latagliata R.,et al.Spleen enlargement is a risk factor for
thrombosis in essential thrombocythemia: Evaluation on 1,297 patients.
American Journal of Hematology, Vol. 91, No. 3, March 2016 https://doi.org/10.1002/ajh.24269 PMid:26748894
- Barbui
T, Finazzi G, Carobbio A,et al. Development and validation a
international Prognostic Score of thrombosis in World Health
Organization essential thrombocythemia Blood 2012 120: 5128-5133 https://doi.org/10.1182/blood-2012-07-444067 PMid:23033268
- Finazzi G.Barbui T. Risk-adapted therapy in essential thrombocythemia and polycythemia vera.Blood Reviews (2005) 19, 243-252 https://doi.org/10.1016/j.blre.2005.01.001 PMid:15963833
- Cerquozzi
S, Barraco D,et al (2017) Risk factors for arterial versus venous
thrombosis in polycythemia vera: a single center experience in 587
patients. Blood Cancer J 7:662 https://doi.org/10.1038/s41408-017-0035-6 PMid:29282357 PMCid:PMC5802551
- Barbui
T.,Tefferi A.Vannucchi A.M.,et al Philadelphia chromosome-negative
classical myeloproliferative neoplasms: revised management
recommendations from European LeukemiaNet Leukemia. 2018 May; 32(5):
1057-1069 https://doi.org/10.1038/s41375-018-0077-1 PMid:29515238 PMCid:PMC5986069
[TOP]


