Laura Jorge1, Diego Torres1, Agustín Languasco2, Pablo Rodríguez3, Pablo Bonvehí1, Elena Temporiti1, Silvia Relloso4, Cristina Videla5 and Fabián Herrera1.
1
Division of Infectious Diseases, Centro de Educación Médica e
Investigaciones Clínicas “Norberto Quirno” (CEMIC), Buenos Aires,
Argentina.
2 Department of
Medicine, Centro de Educación Médica e Investigaciones Clínicas
“Norberto Quirno” (CEMIC), Buenos Aires, Argentina.
3
Pulmonary Medicine Section, Department of Medicine, Centro de Educación
Médica e Investigaciones Clínicas “Norberto Quirno” (CEMIC), Buenos
Aires, Argentina.
4 Department
of Microbiology, Centro de Educación Médica e Investigaciones Clínicas
“Norberto Quirno” (CEMIC), Buenos Aires, Argentina.
5
Clinical Virology Laboratory, Centro de Educación Médica e
Investigaciones Clínicas “Norberto Quirno” (CEMIC), Buenos Aires,
Argentina.
Corresponding
author: Laura Jorge. Division of Infectious Diseases, Department of
Medicine, Centro de Educación Médica e Investigaciones Clínicas
(CEMIC), Av. E. Galván 4102, C1431FWO, Buenos Aires, Argentina. E-mail:
laurajorge1981@gmail.com.
Published: May 1, 2020
Received: February 5, 2020
Accepted: April 4, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e20200
25 DOI
10.4084/MJHID.2020.025
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
Pulmonary complications are frequent in patients with hematologic
malignancies and stem cell transplantation. Regardless of the
microbiological usefulness of bronchoalveolar lavage (BAL), little
information exists on both its benefits as a guide for therapeutic
decisions and its impact on patients’ clinical outcome.
Methods:
A prospective observational single-center study was performed between
July 2011 and July 2016. Consecutive episodes of pulmonary infiltrates
were analyzed in subjects over 18 years of age who presented
hematologic malignancies and underwent chemotherapy or stem cell
transplantation.
Results:
Ninety-six episodes of pulmonary infiltrates were analyzed. Acute
leukemia was the most frequent underlying condition. Thirty-seven
patients (38.5%) received a stem cell transplant. Sixty-one (62.9%)
were neutropenic at the moment of inclusion in the study. A definitive
etiologic diagnosis was obtained in 41 cases (42.7%), where infection
accounted for the vast majority of cases (33 cases, 80.5%). Definitive
diagnosis was reached by non-invasive methods in 13 cases (13.5%). BAL
was performed in 47 cases and led to a diagnosis in 40.4% of the cases.
BAL results led to therapeutic changes in 27 cases (57.4%), including
the addition of new antimicrobials to empiric treatments in 10.
Regarding BAL’s safety, two patients experienced minor adverse events
and one a severe adverse event; no procedure-related deaths were
observed.
Conclusions:
Infection was the leading cause of pulmonary infiltrates in patients
with hematologic malignancies and stem cell transplantation. BAL was a
useful decision-making diagnostic tool, with minor adverse events.
|
Introduction
Pulmonary complications are frequent in patients with hematological malignancies and stem cell transplant recipients.[1-4]
Infections account for the vast majority of these complications,
followed by alveolar hemorrhage, drug toxicity, graft-versus-host
disease, pulmonary edema, and transfusion-related injury.[5] Early diagnosis and appropriate antimicrobial treatment are crucial in reducing morbidity and mortality.[6]
In addition to invasive methods,[7,8]
microbiological non-invasive diagnostic tests, such as sputum culture,
nasopharyngeal swab for respiratory virus detection and a serologic
test for fungal and viral detection, have shown diagnostic usefulness,
especially in patients with respiratory failure where bronchoalveolar
lavage (BAL) performance may be harmful due to unstable clinical
setting.[9,10]
Bronchoalveolar lavage fluid
analysis has been widely used as a diagnostic tool for the diagnosis of
pulmonary infiltrates (PI) in immunosuppressed patients, with a
variable diagnostic yield among different series (31-80%).[11-15]
However, there is scarce robust evidence for its usefulness as a tool
for therapeutic decision-making (even with positive microbiological
culture results) and its impact on patients’ outcome and continues to
be investigated.[6,12,16,17,18]
The
main purpose of the present study was to describe the etiology of PI in
patients with hematological malignancies under chemotherapy or stem
cell transplantation. Secondary objectives were to assess BAL
diagnostic yield and usefulness as a therapeutic decision approach, and
finally to describe BAL safety in this subset of patients.
Methods
A
prospective observational study was carried out at a university
hospital in Buenos Aires, Argentina, which is specialized in the
diagnosis and treatment of hematological malignancies and stem cell
transplant recipients. All subjects over 18 years of age with a
diagnosis of PI (defined as abnormal parenchymal finding in chest X-ray
or CT-scan) and hematological malignancies under chemotherapy treatment
or stem cell transplantation were consecutively included between July
2011 and July 2016. Multiple cases of PI in the same subject were
independently analyzed. Palliative-care patients were excluded.
According
to the treating physician´s best judgment, non-invasive (sputum
culture, nasopharyngeal swab for respiratory virus detection, urinary
pneumococcal antigen, serum galactomannan index, blood cultures,
cytomegalovirus (CMV) viral load) and/or invasive (BAL, transbronchial
lung biopsy, surgical lung biopsy) diagnostic methods were performed.
BAL was performed under propofol or remifentanil sedation through
laryngeal mask access with mechanical ventilation support. Lavage was
performed using six 20ml saline solutions syringes. Our routine
microbiological research for BAL fluid consists of culture for
bacteria, Nocardia, mycobacteria and fungi, galactomannan index, PCR,
and shell vial detection for CMV and Gram Weigert’s technique for Pneumocystis jirovecii
detection. Respiratory syncytial virus (RSV), adenovirus, Influenza A
and B, and parainfluenza (1-3) diagnosis by direct antigen detection by
immunofluorescent assay with monoclonal antibodies (Millipòre) in
smears of respiratory samples. RSV, human metapneumovirus, adenovirus,
rhinovirus, and Influenza A detection by real-time PCR: nucleic acid
was extracted by total nucleic acids from 200 ul of the original sample
using the automated MagNA Pure LC 2.0 with the MagNA Pure Compact
Nucleic Acid Isolation Kit I extraction kit from Roche. Real-time PCR
was performed with 5ul of nucleic acid eluate using for Influenza
A/H1N1, adenovirus, and human metapneumovirus commercial assay set
(TibMolbiol, Roche) and the enzyme LightCycler Multiplex RNA Master
Virus in the Light Cycler 2.0 device as manufacturer’s instruction. For
RSV and rhinovirus detection, a homebrew real-time PCR was used.[19,20]
The same viral detection techniques were used for nasopharyngeal swab
samples. The serum galactomannan index was performed by enzyme
immunoassay (PlateliaâAspergillus Bio-Rad, France) and considered
positive with two independent samples ≥0.5 optical density index value.
Instead, positive BAL fluid was considered positive, with one sample ≥1
optical density index value.[21] Early BAL was defined as the one performed within 4 days after the diagnosis of PI.[6,22] Patients were followed for 30 days after the diagnosis of PI.
Predominant
abnormal radiologic patterns were defined as uni or bilateral alveolar
consolidation, nodular, “ground glass” opacity, or “tree-in-bud”
pattern, according to radiologists’ descriptions. The presence or
absence of pleural effusion was also recorded.
Etiologies of PI
were classified as either infectious or non-infectious. Infectious
etiologies could be bacterial pneumonia (defined as a significant
positive culture in sputum or BAL fluid of pathogenic bacteria or blood
culture and sputum culture with the growth of the same microorganism),
invasive pulmonary mycosis according to EORTC/MSG 2008,[23]
a viral infection caused by RSV, Influenza, Parainfluenza, Adenovirus
or Rhinovirus detected by indirect immunofluorescence or PCR in
nasopharyngeal swabs or BAL, and CMV pneumonia defined by shell vial
detection or identification of intranuclear/intracytoplasmatic
inclusion bodies.[23,24] Non-infectious etiologies
could be alveolar hemorrhage (defined as more than 20%
hemosiderin-loaded macrophages or bloody tube progression in BAL fluid
in the absence of infection), congestive heart failure (defined as
abnormal radiological images and clinical signs ameliorated by diuretic
therapy), a pulmonary manifestation of hematological cancer
(biopsy-proven), transfusion-related acute lung injury, engraftment
syndrome (noncardiogenic pulmonary edema during neutrophil recovery)
and graft-versus-host disease (clinical and laboratory or
biopsy-proven).[26,27]
Complications during or
following BAL were classified as minor (mild hypoxemia enhancement and
self-limited hemorrhage) or major (severe hypoxemia, hemorrhage
requiring specific intervention, arterial hypotension requiring
vasopressor therapy or death). According to institutional
protocols, patients with a platelet count lower than 50,000/mm3 received platelet infusion during the procedure.
Therapeutic
modifications were defined as any change, addition or discontinuation
of antibiotics, antifungal, or antiviral therapy according to BAL fluid
findings.
Data were analyzed using descriptive statistics.
Continuous variables were described according to their median, whereas
categorical variables were described according to their number and
percentage. For statistical analysis, Mann-Whitney U-test (for continuous variables) and Fischer’s exact test (for categorical variables) were used.
The Ethics Committee at our hospital approved the study. An informed consent form was obtained from each patient.
Results
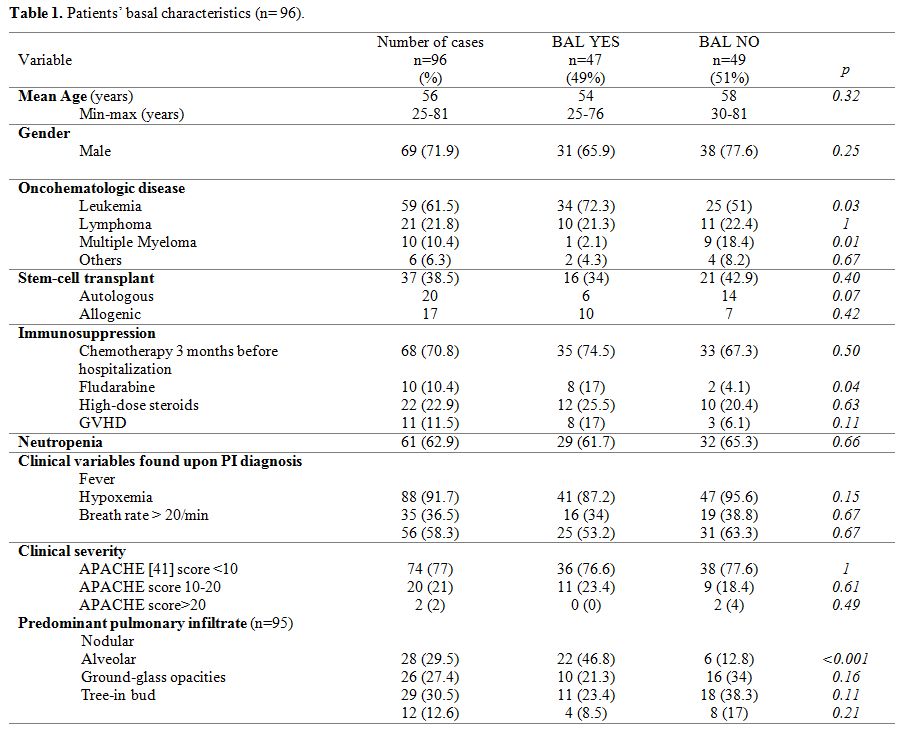
A total of 96 episodes of PI were analyzed in 77 patients. Table 1
shows demographic characteristics. The mean age was 56.4 years (range
25-81). Acute leukemia was the most frequent hematological cancer,
accounting for 59 cases (61.5%). Thirty-seven patients (38.5%) were
stem-cell transplant recipients (20 autologous and 17 allogenic).
Sixty-one patients (62.9%) were neutropenic upon inclusion.
 |
Table
1. Patients’ basal characteristics (n= 96). |
PIs
were diagnosed by CT-scan in 94 episodes (97.9%) and by X-ray in 2.
Sixty-eight (72.3%) had bilateral infiltrates. Alveolar pattern was
present in 36 cases (37.5%), ground-glass opacities in 42 (43.8%),
nodular in 37 (38.5%) and “tree-in-bud” pattern in 21 (22.3%). There
was more than one abnormal CT pattern in 42 cases (43.8%).
Results from non-invasive diagnostic methods are shown in Table 2.
Sputum culture had the highest diagnostic yield (32%), followed by
nasopharyngeal swab (18.8%), blood culture (7.3%), and serum
galactomannan (5.8%). All urinary pneumococcal antigen tests performed
were negative. Differences between positive tests and definitive
diagnosis are explained by co-infection (for the case of nasopharyngeal
swabs), the clinical relevance of microbiological findings (for sputum
cultures), or false-positive results according to clinical and
radiological criteria (for serum galactomannan).
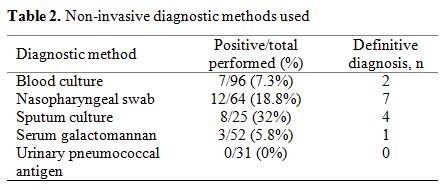 |
Table 2. Non-invasive diagnostic methods used. |
As far as CMV infection is concerned, serum viral load was performed in 8 patients with risk factors for CMV infection,[28-30] 2 of whom were positive. No BAL Shell vial or pathology sample yielded positive results for CMV infection.
BAL
was performed in 47 (49%) episodes, 40 of which (85%) occurred in a new
fashion, and 38 of which (81%) were under an empirical antimicrobial
therapy. There was a microbiological diagnostic yield of 40.4% (19
positive cultures, 5 of which with more than one pathogen identified).
The results are shown in Table 3.
All positive BAL results were considered diagnostic. BAL was not
performed in 49 cases for the following reasons: good response to
empirical treatment started at 48h (51%), a serious clinical condition
making procedure unsafe (18.4%), non-invasive diagnosis obtained
(22.4%) and non-infectious alternative diagnosis (8.2%). BAL was more
frequently performed in patients with acute leukemias and under
fludarabine regimes.
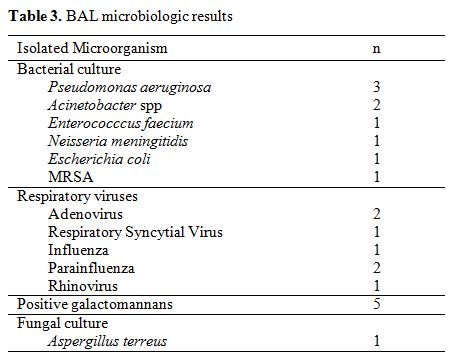 |
Table 3. BAL microbiologic results. |
We did not find a significant correlation between pulmonary imaging abnormalities and etiologic diagnostic yield (Table 4);
however, noteworthy is the fact that the majority of BAL performed was
in cases presenting with nodular lesions. BAL fluid results led to
therapeutic changes in antimicrobial drug and its duration in 27
episodes (Table 5). In those
cases evidencing negative BAL, and based on the BAL fluid galactomannan
negative results, 4 antifungal treatments could be discontinued. Seven
wide-spectrum antibiotic treatments against multi-resistant organisms
were also discontinued. All patients (n=11) in which treatment was
discontinued had a favourable outcome with the resolution of pulmonary
infection. As far as outcome is concerned, 16 (34%) patients had oxygen
saturation lower than 90%, and 25 (53.2%) had a platelet count
<50,000/mm3 (3 of which had <10,000/mm3) at the time of bronchoscopy. Breath rate and oxygen saturation were 20 (12-26) vs 20 (12-26), (p = 0.41) and 95 (64-100) vs 95 (86-98), (p
= 0.74) before and after bronchoscopy, respectively. BAL-related
complications consisted of two minor (mild hypoxemia) and one major
(respiratory failure requiring mechanical ventilation assistance)
events, with no procedure-related deaths.
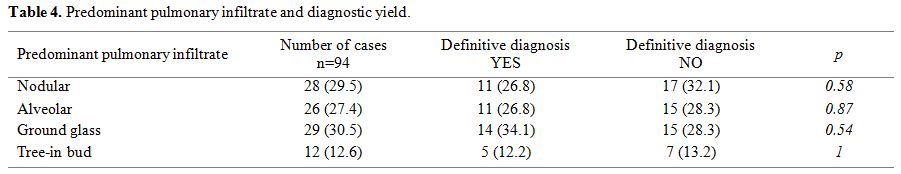 |
Table 4.
Predominant pulmonary infiltrate and diagnostic yield. |
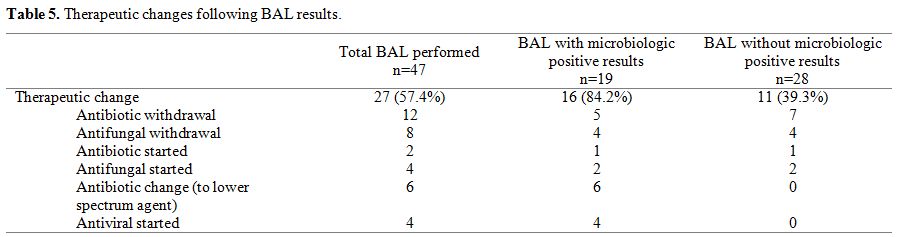 |
Table 5. Therapeutic changes following BAL results. |
Of all the 97 PIs herein included, a definitive etiology was obtained in 41 (42.7%) cases (Table 6).
The following results were obtained: infection in 33 cases (80.5%),
fluid overload in 4 (9.7%), alveolar hemorrhage in 2 (4.8%), underlying
hematological malignancy in 1 (2.5%) and graft-versus-host-disease in 1
(2.5%). Fourteen patients required ICU admission, and thirteen (13.5%)
died during the 30 days following the initial respiratory event, 6 of
them being directly related to PI event.
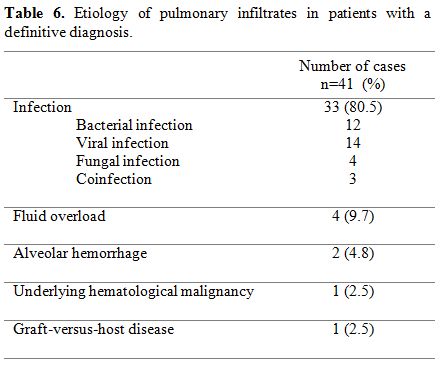 |
Table 6. Etiology of pulmonary infiltrates in patients with a definitive diagnosis. |
Discussion
This
study evidences the results of a local series of PI. As in previous
reports, we found a diagnostic yield of around 40% with BAL, which
allowed the identification of bacterial, viral, and also fungal
infections. However, 80.6% of BAL was performed under empirical
antimicrobial treatment. In almost half of the cases, BAL results,
either positive or negative, determined a modification in therapeutic
regimes, including the addition of an antimicrobial agent not included
in the empirical treatment in 22.7% of the cases. Since patients’
survival in this selected population is significantly determined by
early and appropriate treatment,[6] this finding is
highly relevant. Furthermore, negative cultures in BAL fluids allowed
antimicrobial de-escalation or even withdrawal in 11 cases.
Broad-spectrum antibiotics such as colistin, tigecycline, daptomycin,
and linezolid were part of empiric treatments accounting for
multidrug-resistant organisms and local microbiology; negative BAL
analysis allowed their discontinuation, therefore minimizing both
adverse events and probably the occurrence of multidrug-resistant
bacteria outbreaks..[31]
In four cases, Aspergillus galactomannan in BAL was negative, leading to discontinuation of antifungal treatment. Aspergillus galactomannan in BAL is known to have an excellent negative predictive value.[21,32] Locally, we have found that Aspergillus
spp was the most frequent mold isolate in immunosuppressed patients,
whereas Zygomycetes accounted for less than 6 % of the cases.[33]
In contrast with other reports,[34,35]
we did not find significant differences in diagnostic yield according
to pulmonary imaging patterns. We believe this may be, in part,
explained by our low total cases number or by the lack of randomization
according to radiographic findings.
BAL appeared to be safe in our
cohort, with only 1 severe adverse event related to the procedure.
53.2% of cases had significant thrombocytopenia, and 34% had hypoxia at
the time of BAL, with no procedure-related mortality. These numbers are
lower than those reported in the literature.[12,17,36]
Non-invasive
diagnostic methods led to 30 positive microbiological results, mainly
respiratory virus-positive swabs or sputum culture. However, BAL was
performed in 9 of these cases, mainly due to the high possibility of
co-infections in this group of patients.[37] Only 14 out of 96 cases could be diagnosed through non-invasive methods.
In
the absence of serum galactomannan positive tests, positive
galactomannan in BAL let to the diagnosis of pulmonary aspergillosis in
a small number of patients. This finding is similar to previous reports
evidencing a sensitivity gap of over 60% when comparing BAL fluid with
positive serum tests.[38-40]
This study has many
limitations. Firstly, the lack of randomization of patients undergoing
BAL leads to probable selection bias towards the group of patients in
which BAL was performed. However, no significant clinical differences
were observed between patients. There was also a selection bias toward
more immunosuppressed patients and specific imaging patterns since BAL
was mainly performed in patients with a diagnosis of acute leukemia,
those treated with fludarabine, and patients presenting nodular
pulmonary lesions. Given the design of the study, the clinical outcome
and management changes between groups of patients could not be compared.
The
multidisciplinary approach to the management of these patients
(including internists, oncologists, pneumologists, and infectious
diseases specialists) leads to discrepancies in the usefulness of BAL
in this setting.[41] In a recent study, Marchesi et
al. observed that a BAL-driven antimicrobial approach has a positive
impact on clinical outcome and mortality.[18]
We
believe our findings enlarge the still scarce body of evidence that
will help determine more precise algorithms for the diagnosis and
treatment of pulmonary infiltrates in patients with hematological
malignancies. Future comparative randomized studies are required to
determine the actual impact of BAL and the timing of performance on the
management of this complex group of patients.
Acknowledgments
Authors
acknowledge Valeria Melia, a scientific translator at CEMIC Research
Unit, for English edition of the manuscript and Dr. Alex Kostianovsky
for their helpful comments on this manuscript.
References
- Sharma S, Nadrous HF, Peters SG, Tefferi A, Litzow
M, Aubry MC and Afesso B. Pulmonary complications in adult blood and
marrow transplant recipients: autopsy findings. Chest.
2005;128(3):1385-1392. https://doi.org/10.1378/chest.128.3.1385 PMid:16162733
- Hofmeister
CC, Czerlanis C, Forsythe S, Stiff PJ. Retrospective utility of
bronchoscopy after hematopoietic stem cell transplant. Bone Marrow
Transplant. 2006;38(10):693-698. https://doi.org/10.1038/sj.bmt.1705505 PMid:16980989
- Kasow
KA, King E, Rochester R, Woodard P, Handgretinger R and Hale G.
Diagnostic yield of bronchoalveolar lavage is low in allogeneic
hematopoietic stem cell recipients receiving immunosuppressive therapy
or with acute graft-versus-host disease: the St. Jude experience,
1990-2002. Biol Blood Marrow Transplant. 2007;13(7):831-837. https://doi.org/10.1016/j.bbmt.2007.03.008 PMid:17580261
- Dunagan
DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of
pulmonary infiltrates following bone marrow transplantation. Chest.
1997;111(1):135-141. Accessed May 25, 2014. https://doi.org/10.1378/chest.111.1.135 PMid:8996007
- Shorr
AF, Susla GM, O'Grady NP. Pulmonary infiltrates in the non-HIV-infected
immunocompromised patient: etiologies, diagnostic strategies, and
outcomes. Chest. 2004;125(1):260-271. https://doi.org/10.1378/chest.125.1.260 PMid:14718449
- Shannon
VR, Andersson BS, Lei X, Champlin RE, Kontoyiannis DP. Utility of early
versus late fiberoptic bronchoscopy in the evaluation of new pulmonary
infiltrates following hematopoietic stem cell transplantation. Bone
Marrow Transplant. 2010;45(4):647-655. https://doi.org/10.1038/bmt.2009.203 PMid:19684637
- Rañó
A, Agustí C, Jimenez P, Angrill J, Benito N, Danes C, Gonzalez J,
Rovira M, Pumarola T, Moreno A and Torres A. Pulmonary infiltrates in
non-HIV immunocompromised patients: a diagnostic approach using
non-invasive and bronchoscopic procedures. Thorax. 2001;56(5):379-387. https://doi.org/10.1136/thorax.56.5.379 PMid:11312407 PMCid:PMC1746047
- Peikert
T, Rana S, Edell ES. Safety, diagnostic yield, and therapeutic
implications of flexible bronchoscopy in patients with febrile
neutropenia and pulmonary infiltrates. Mayo Clin Proc.
2005;80(11):1414-1420. https://doi.org/10.4065/80.11.1414 PMid:16295020
- Azoulay
E, Mokart D, Rabbat A, Pene F, Kouatchet A, Bruneel F, Vincent F,
Hamidfar R, Moreau D, Mohammedi I, Epinette G, Beduneau G, Castelain V,
de Lassence A, Grusson D, Lemiale V, Renard B, Chevret S and Schlemmer
B. Diagnostic bronchoscopy in hematology and oncology patients with
acute respiratory failure: prospective multicenter data. Crit Care Med.
2008;36(1):100-107. https://doi.org/10.1097/01.CCM.0000295590.33145.C4 PMid:18090351
- Maschmeyer
G, Beinert T, Buchheidt D, Cornely OA, Einsela H, Heinz W, Heussel CP,
Kahl C, Kiehl M, Lorenz H, Hof H and Mattiuzzii G. Diagnosis and
antimicrobial therapy of lung infiltrates in febrile neutropenic
patients: Guidelines of the infectious diseases working party of the
German Society of Haematology and Oncology. Eur J Cancer.
2009;45(14):2462-2472. https://doi.org/10.1016/j.ejca.2009.05.001 PMid:19467584
- Jain
P, Sandur S, Meli Y, Arroliga AC, Stoller JK, Mehta AC. Role of
flexible bronchoscopy in immunocompromised patients with lung
infiltrates. Chest. 2004;125(2):712-722. https://doi.org/10.1378/chest.125.2.712 PMid:14769756
- Gilbert
CR, Lerner A, Baram M, Awsare BK. Utility of flexible bronchoscopy in
the evaluation of pulmonary infiltrates in the hematopoietic stem cell
transplant population -- a single center fourteen year experience. Arch
Bronconeumol. 2013;49(5):189-195. https://doi.org/10.1016/j.arbres.2012.11.012 PMid:23455477
- Yoo
H, Suh GY, Jeong B-H, Lim SY, Chung MP, Kwon OJ and Jeon K. Etiologies,
diagnostic strategies, and outcomes of diffuse pulmonary infiltrates
causing acute respiratory failure in cancer patients: a retrospective
observational study. Crit Care. 2013;17(4):R150. https://doi.org/10.1186/cc12829 PMid:23880212 PMCid:PMC4055964
- Chellapandian
D, Lehrnbecher T, Phillips B, Fisher BT, Zaoutis TE, Steinbach WJ,
Beyene J and Sung L. Bronchoalveolar lavage and lung biopsy in patients
with cancer and hematopoietic stem-cell transplantation recipients: a
systematic review and meta-analysis. J Clin Oncol. 2015;33(5):501-509. https://doi.org/10.1200/JCO.2014.58.0480 PMid:25559816
- Díaz
Couselo FA, Morero JL, Sánchez F, Dictar M, Zylberman M. [Pulmonary
infiltrates in cancer patients]. Medicina (B Aires).
2008;68(5):367-372. Accessed March 29, 2016.
- von
Eiff M, Zühlsdorf M, Roos N, Thomas M, Büchner T, van de Loo J.
Pulmonary infiltrates in patients with haematologic malignancies:
clinical usefulness of non-invasive bronchoscopic procedures. Eur J
Haematol. 1995;54(3):157-162. Accessed March 28, 2016. https://doi.org/10.1111/j.1600-0609.1995.tb00207.x PMid:7720835
- Hummel
M, Rudert S, Hof H, Hehlmann R, Buchheidt D. Diagnostic yield of
bronchoscopy with bronchoalveolar lavage in febrile patients with
hematologic malignancies and pulmonary infiltrates. Ann Hematol.
2008;87(4):291-297. https://doi.org/10.1007/s00277-007-0391-6 PMid:17932672
- Marchesi
F, Cattaneo C, Criscuolo M, Delia M, Dargenio M, Del Principe MI,
Spadea A, Fracchiolla NS, Melillo L, Perruccio K, Alati C, Russo D,
Garzia M, Brociner M, Cefalo M, Armiento D, Cesaro S, Decembrino N,
Mengarelli A, Tumbarello M, Busca A, Pagano L; Sorveglianza
Epidemiologica Infezioni nelle Emopatie (SEIFEM) Group. A
bronchoalveolar lavage-driven antimicrobial treatment improves survival
in hematologic malignancy patients with detected lung infiltrates: A
prospective multicenter study of the SEIFEM group. Am J Hematol.
2019;94(10):1104-1112. https://doi.org/10.1002/ajh.25585 PMid:31321791
- Fry
AM, Chittaganpitch M, Baggett HC, Peret TC, Dare RK, Sawatwong P,
Thamthitiwat S, Areerat P, Samasuttipun W, Fisher J, MAloney SA, Erdman
DD and Olsen SJ. The burden of hospitalized lower respiratory tract
infection due to respiratory syncytial virus in rural Thailand. Cowling
BJ, ed. PLoS One. 2010;5(11):e15098. doi:10.1371/journal.pone.0015098. https://doi.org/10.1371/journal.pone.0015098 PMid:21152047 PMCid:PMC2994907
- Marcone
DN, Videla C, Ricarte C, Carballal G, Vidaurreta S, Echavarría M.
Rhinovirus detection by real-time RT-PCR in children with acute
respiratory infection in Buenos Aires, Argentina. Rev Argent Microbiol.
44(4):259-265. Accessed March 27, 2018.
- Maertens
J, Maertens V, Theunissen K, Meersseman W, Meersseman P, Meers S,
Verhoef G, Van Eldere and Lagrou K. Bronchoalveolar lavage fluid
galactomannan for the diagnosis of invasive pulmonary aspergillosis in
patients with hematologic diseases. Clin Infect Dis.
2009;49(11):1688-1693. https://doi.org/10.1086/647935 PMid:19886801
- Oren
I, Hardak E, Zuckerman T, Geffen Y, Hoffman R, Yiglo M and Avivi I.
Does molecular analysis increase the efficacy of bronchoalveolar lavage
in the diagnosis and management of respiratory infections in
hemato-oncological patients? Int J Infect Dis. 2016;50:48-53. https://doi.org/10.1016/j.ijid.2016.07.011 PMid:27484225
- De
Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T,
Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson
TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler
CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A,
Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR,
Zaoutis T, Bennett JE; European Organization for Research and Treatment
of Cancer/Invasive Fungal Infections Cooperative Group; National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) Consensus Group. Revised definitions of invasive fungal
disease from the European Organization for Research and Treatment of
Cancer/Invasive Fungal Infections Cooperative Group and the National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) C. Clin Infect Dis. 2008;46(12):1813-1821. https://doi.org/10.1086/588660 PMid:18462102 PMCid:PMC2671227
- Crawford
SW, Bowden RA, Hackman RC, Gleaves CA, Meyers JD, Clark JG. Rapid
detection of cytomegalovirus pulmonary infection by bronchoalveolar
lavage and centrifugation culture. Ann Intern Med. 1988;108(2):180-185.
Accessed January 31, 2018. https://doi.org/10.7326/0003-4819-108-2-180 PMid:2829672
- Woods
GL, Thompson AB, Rennard SL, Linder J. Detection of cytomegalovirus in
bronchoalveolar lavage specimens. Spin amplification and staining with
a monoclonal antibody to the early nuclear antigen for diagnosis of
cytomegalovirus pneumonia. Chest. 1990;98(3):568-575. Accessed January
31, 2018. https://doi.org/10.1378/chest.98.3.568 PMid:2168309
- Jagasia
MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J,
Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF,
McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D,
Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler
CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME. National Institutes
of Health Consensus Development Project on Criteria for Clinical Trials
in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging
Working Group report. Biol Blood Marrow Transplant.
2015;21(3):389-401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001 PMid:25529383 PMCid:PMC4329079
- Yousem
SA. The histological spectrum of pulmonary graft-versus-host disease in
bone marrow transplant recipients. Hum Pathol. 1995;26(6):668-675.
Accessed January 31, 2018. https://doi.org/10.1016/0046-8177(95)90174-4
- Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711-5719. https://doi.org/10.1182/blood-2008-10-143560 PMid:19299333 PMCid:PMC2700312
- Ljungman
P, Hakki M, Boeckh M. Cytomegalovirus in Hematopoietic Stem Cell
Transplant Recipients. Infect Dis Clin North Am. 2010;24(2):319-337. https://doi.org/10.1016/j.idc.2010.01.008 PMid:20466273
- Marchesi
F, Pimpinelli F, Ensoli F, Mengarelli A. Cytomegalovirus infection in
hematologic malignancy settings other than the allogeneic transplant.
Hematol Oncol. June 2017. https://doi.org/10.1002/hon.2453 PMid:28660653
- Nseir
S, Di Pompeo C, Diarra M, Brisson H, Tissier S, Boulo M and Dorocher A.
Relationship between immunosuppression and intensive care unit-acquired
multidrug-resistant bacteria: a case-control study. Crit Care Med.
2007;35(5):1318-1323. https://doi.org/10.1097/01.CCM.0000261885.50604.20 PMid:17414081
- Luong
ML, Filion C, Labbé AC, Roy J, Pépin J, Cadrin-Tourigny J, Carignan S,
Sheppard DC, Laverdière M. Clinical utility and prognostic value of
bronchoalveolar lavage galactomannan in patients with hematologic
malignancies. Diagn Microbiol Infect Dis. 2010;68(2):132-139. https://doi.org/10.1016/j.diagmicrobio.2010.03.017 PMid:20846585
- Preliminary
Results for Surveillance of Invasive Mold Diseases (IMD) in Argentina
(AR), 2010-2013. M. C. Dignani, A. A. Cleveland, G. Davel, T. Chiller,
N. Refojo, S. Córdoba, A. Valledor, A. Laborde, M. L. Pereyra, I.
Roccia-Rossi, R. Jordán, F. Herrera, N. Tiraboschi, G. Guerrini, H.
Peretti, A. Zárate,J. Afeltra, A. Vila, L. Tula, C. Freuler, S.
López-Papucci, Register for Invasive Mycoses (REMIIN Group);53rd ICAAC
2013. Sep 10-13, Denver, CO. USA Abst# 1437
- Brownback
K, Simpson S. Association of bronchoalveolar lavage yield with chest
computed tomography findings and symptoms in immunocompromised
patients. Ann Thorac Med. 2013;8(3):153. https://doi.org/10.4103/1817-1737.114302 PMid:23922610 PMCid:PMC3731857
- Gruson
D, Hilbert G, Valentino R, Vargas F, Chene G, Bebear C, Allery A,
Pigneux A, Gbikpi-Benissan G, Cardinaud JP. Utility of fiberoptic
bronchoscopy in neutropenic patients admitted to the intensive care
unit with pulmonary infiltrates. Crit Care Med. 2000;28(7):2224-2230.
Accessed March 27, 2018. https://doi.org/10.1097/00003246-200007000-00007 PMid:10921544
- Cefalo
M., Puxeddu E., Sarmati L., Paterno G., Fontana C., Nasso D., Pane G.,
De Bellis E., Palmieri R., Buzzati E., Meconi F., Laureana R., Casciani
P., Zizzari A.G., Rogliani P., de Fabritiis P., Maurillo L., Buccisano
F., Cantonetti M., Arcese W., Venditti A., Del Principe M.I.Diagnostic
performance and safety of bronchoalveolar lavage in thrombocytopenic
haematological patients for invasive fungal infections diagnosis: a
monocentric, retrospective experience. Mediterr J Hematol Infect Dis
2019, 11(1): e2019065, DOI: https://doi.org/10.4084/mjhid.2019.065 PMid:31700590 PMCid:PMC6827601
- Wingard
JR, Hiemenz JW, Jantz MA. How I manage pulmonary nodular lesions and
nodular infiltrates in patients with hematologic malignancies or
undergoing hematopoietic cell transplantation. Blood. 2012;
120(9):1791-1800. https://doi.org/10.1182/blood-2012-02-378976 PMid:22692506
- Boch
T, Buchheidt D, Spiess B, Miethke T, Hofmann W-K, Reinwald M. Direct
comparison of galactomannan performance in concurrent serum and
bronchoalveolar lavage samples in immunocompromised patients at risk
for invasive pulmonary aspergillosis. Mycoses. 2016;59(2):80-85. https://doi.org/10.1111/myc.12434 PMid:26627577
- Moragues
MD, Amutio E, García-Ruiz JC, Pontón J. [Usefulness of galactomannan
detection in the diagnosis and follow-up of hematological patients with
invasive aspergillosis]. Rev Iberoam Micol. 2003;20(3):103-110. http://www.ncbi.nlm.nih.gov/pubmed/15456366. Accessed March 21, 2016.
- Acosta
J, Catalan M, del Palacio-Peréz-Medel A, Lora D, Montejo JC, Cuetara
MS, Moragues MD, Ponton J and del Palacio A. A prospective comparison
of galactomannan in bronchoalveolar lavage fluid for the diagnosis of
pulmonary invasive aspergillosis in medical patients under intensive
care: comparison with the diagnostic performance of galactomannan and
of (1→ 3)-β-d-glucan. Clin Microbiol Infect. 2011;17(7):1053-1060. https://doi.org/10.1111/j.1469-0691.2010.03357.x PMid:20825441
- Wahla
AS, Chatterjee A, Khan II, Conforti JF, Haponik E. Survey of academic
pulmonologists, oncologists, and infectious disease physicians on the
role of bronchoscopy in managing hematopoietic stem cell
transplantation patients with pulmonary infiltrates. J Bronchology
Interv Pulmonol. 2014;21(1):32-39. https://doi.org/10.1097/LBR.0000000000000042 PMid:24419184
[TOP]





