Vincenzo De Sanctis1, Ashraf T. Soliman2, Shahina Daar3, Duran Canatan4, Salvatore Di Maio5 and Christos Kattamis6.
1 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
2 Department of Pediatrics, University of Alexandria, Alexandria, Egypt.
3 Department of Haematology, College of Medicine and Health Sciences, Sultan Qaboos University, Sultanate of Oman.
4 Antalya Genetic Diseases Center, Antalya, Turkey.
5 Emeritus Director in Pediatrics, Children’s Hospital “Santobono-Pausilipon,” Naples, Italy.
6
First Department of Paediatrics, National Kapodistrian University of
Athens, “Aghia Sophia” Children’s Hospital, Athens, Greece.
Correspondence to: Vincenzo De Sanctis MD, Pediatric
and Adolescent Outpatient Clinic, Quisisana Hospital, 44121 Ferrara,
Italy. Tel: +39 0532 770243. E-mail:
vdesanctis@libero.it
Published: May 1, 2020
Received: March 23, 2020
Accepted: April 17, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020032 DOI
10.4084/MJHID.2020.032
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Women
with sickle cell disease (SCD) are of particular concern regarding the
significantly increased risk of pregnancy-related morbidity, mortality,
and adverse outcomes. They have limited knowledge of pregnancy and
childbirth risks, as well as of the benefits and risks of
contraceptives. Thus, there is an urgent need for appropriate
information about reproductive family planning to reduce unintended
pregnancy. Any decision regarding the use of contraceptives has to be
based on the efficacy and risk/benefit ratio of the method used. Both
the World Health Organization (WHO) and the Centers for Disease Control
(CDC) have developed, published, and updated evidence-based guidelines
for medical providers for the use of contraceptives in patients with
specific medical chronic conditions. This article provides an overview
of the present knowledge on the use of contraceptives in women with
SCD. We believe that the collaboration between health care
professionals (hematologists, obstetricians, endocrinologists, and
primary care providers) can play a major role in identifying the safer
contraceptive method to abolish the risks of unintended pregnancy and
preserve the health status of patients with SCD.
|
Introduction
Sickle
cell disease (SCD) represents one of the most common monogenic blood
disorders worldwide, with an incidence of over 300,000 newborns
affected annually, two-thirds of whom are in Africa.[1-3]
Owing to population migration, SCD is now an increasing health problem
worldwide with increasing numbers of affected individuals in Europe.[4]
The
term SCD refers to a set of disorders characterized by the inheritance
of the structural HbS variant. When the HbS or sickle cell variant is
inherited in a homozygous state (HbSS) it is defined as sickle cell
anaemia; however, it is usually clinically silent when inherited in a
heterozygous form (HbAS). SCD complex also includes compound
heterozygotes disorders in which the HbS variant is coinherited with
another hemoglobinopathy, e.g. ß-thalassemia heterozygotes (HbS/ß0-thal, HbS/ß+-thal and HbS/(δβ)0-thal) known as microdrepanocytic disease, or with other ß-globin structural variants.[5]
Patients with HbSS, HbSC, HbSD or HbS/ß0
disease lack normal β-globin chains, and so they have no HbA. In HbSS,
the HbS levels are usually above 80%. The HbF levels are usually
increased up to 20%; high HbF levels are negatively related to the
severity of clinical symptoms. The presence of HbA and increased HbF in
patients with HbS/β+ thal ameliorate the clinical symptoms of patients and in particular the obstructive and hemolytic crises.[1,2,5]
HbSS and HbS/ß0-thal
disease have identical hematological phenotypes and the most severe
clinical and hematological phenotypes, but in both cases, the clinical
severity varies markedly. HbS/ß+-thal
presents with a broader clinical spectrum depending on the severity of
the mutation of the ß -thalassemia gene and the levels of HbA.
SCD
is associated with chronic activation of coagulation and with an
increased risk for venous thrombosis. The phenotypic variation in SCD
is thought to be related to a complex interaction between hemolysis,
vaso-occlusion, endothelial dysfunction, and hyperviscosity.[1,2]
Patients with SCD suffer from a variety of clinical events due to small
and large vessels occlusion, including vaso-occlusive painful episodes,
strokes, and acute chest syndrome.[6] Such episodes
may be associated with derangements of plasma and cellular haemostatic
mechanisms that may impart a thrombophilic tendency.[6]
Reported changes include an increase in thrombin generation, platelet
activation and decreased levels of circulating anticoagulants such as
protein C and S.[7]
In addition, venous
thromboembolism (VTE), which includes both deep vein thrombosis and
pulmonary embolism, is increasingly recognized as a critical
complication of SCD resulting from the hypercoagulable state that is
elicited by the disease. It has been described in children and adults
with SCD,[8,9] HbS/ß+-thalassemia[10] and sickle cell trait.[8,11,12]
By the age of 30 years, up to 25% of patients with SCD experience ≥ 1 episode of VTE.[13,14]
Splenectomy,
which is a known risk factor for VTE in other hemoglobinopathies such
as β-thalassemia intermedia and major has also been associated with VTE
and in sickle cell variant syndrome of microdrepanocytic disease.[10,14]
Information on contraception in women with SCD is limited.[15-18]
The course of SCD can be worsened by pregnancy which is associated with
high rates of maternal morbidity despite advances in management.
Pregnancy is associated with an increased incidence of painful
episodes, infection, pulmonary complications, VTE, antepartum bleeding,
and increased risk of pre-eclampsia.[19-21] Because
of this, pregnancy should be timed during a period of relative disease
stability, although this may prove impossible as the disease is
unpredictable in its course. Therefore, appropriate consultation both
for pregnancy planning and effective contraception is of paramount
importance.[22]
In normal females, the synthetic
steroids used in contraception induce metabolic changes on lipoprotein,
insulin response to glucose and coagulation factors, all of which have
been associated with cardiovascular and venous thrombosis. VTE is a
rare event in normal women of reproductive age, and its incidence
increases with age.[23] In recent reviews and
meta-analysis studies, an increased risk has been demonstrated in users
of combined hormonal contraceptives (COCs) containing ethinylestradiol
(EE) and different progestins.[24,25] Although the
risk is increased approximately 4-fold as compared with non-users, the
absolute risk is low (about 7/10,000 women-years) and lower than the
risk of pregnancy.[26]
The main objective of
this article is to provide a brief overview of the present knowledge of
the available options for hormonal contraception in adolescent and
young adult women with SCD and consider the current risk-benefit
analysis of available contraceptive methods.
A. Clinical Implications and Hormonal Contraception in General Practice
Patients
with SCD seen in haematology practice are generally clinically
heterogeneous with a spectrum of clinical findings and symptoms.
For
an appropriate decision, women with SCD should be provided with
up-to-date research-based evidence regarding suitable methods for
hormonal contraception and be referred (if needed) to a contraceptive
specialist for further advice. Adolescents (males and females) with SCD
should be allowed to privately discuss their family planning needs and
receive care in the context of the relevant law.[27]
Before
prescribing any contraceptive method, a careful history of past and
present medical conditions, drugs use, and family history, followed by
physical examination and laboratory assessment is required to exclude
conditions or risk factors that might be a contraindication to
contraceptive use.
Specifically, information regarding migraine,
risk factors for cardiovascular disease (smoking, hypertension,
obesity, glucose intolerance, dyslipidemia, thrombophilia, previous
VTE), blood pressure measurement and body mass index (BMI) are
essential. A pregnancy test ensures the initiation of contraception
before pregnancy.
Patients also require reliable information on
the correct use of the pill, together with detailed information on how
to avoid sexually transmitted diseases (STDs) by combining pill use
with a condom.[28] Discussion about emergency
contraception should occur at each visit when providing anticipatory
guidance strategies regarding safe sex practices.
B. Overview of Hormonal Contraceptive Choices
Globally,
it has been recognised that adolescents and young women with SCD are at
high risk of unintended pregnancy. In a study, only 33% of a group of
women with SCD used any form of contraception compared to 66% in the
control group.[18]
When choosing a hormonal
contraceptive method, it is important to recognize the distinct
advantages and disadvantages of each method and consider the following
factors: efficacy, ease of dosing/duration of action, impact on
menstrual bleeding, time to return to fertility, side effects, cost,
non-contraceptive benefits, and medical contraindications. The
products, utilized to inhibit conception, exert their contraceptive
actions at the levels of ovarian-produced hormones or block the sperm
from fertilizing the egg.
Non-hormonal barrier and behavioural
methods include male and female condoms, diaphragms, caps, shields,
intrauterine devices (IUDs), spermicides, withdrawal, fertility
awareness and natural family planning.
a. Short-acting Reversible Contraception (SARC)
methods include two main groups: the combined hormonal contraceptives
(COCs) with estrogen and progestin components and the progestin-only
pills (POPs).
Contraceptive action is provided by: (a) ovulation
suppression by inhibiting follicle-stimulating hormone (FSH) and
luteinizing hormone (LH); (b) cervical mucosal changes that inhibit
sperm penetration; and (c) endometrial changes that reduce the chances
of successful implantation.[29,30]
1. Combined hormonal contraceptives (COCs) include the following methods:
• Oral
• Transdermal patches
• Vaginal rings
Combined oral contraceptives (COCs) remain the most frequently prescribed form of contraception.
The
majority of COCs contains ethinylestradiol (EE) as the estrogen
component. There are a considerable number of different combinations of
COCs concerning both compounds and doses. COCs vary in dose and type of
estrogen, dose and type of progestin, regime (monophasic, biphasic,
triphasic or quadriphasic) and route of administration (oral, patch,
vaginal ring or subcutaneous implant). The prescription pattern differs
between different parts of the world.
The estrogen content of
the COCs ranges from 15 to 50 µg per active tablet. Although EE and
estradiol are the only estrogens used in COC, many progestins are
currently available. Their content varies considerably dependent upon
the potency differences in the compound used.
Two of the newer
progestogens, (desogestrel and gestodene) have been associated with a
small increase in the risk of venous thromboembolism. In the late
1980s, three new “third-generation” progestogens were introduced
(norgestimate, desogestrel and gestodene) which were designed to have
less androgenic side-effects (such as adverse effects on the lipid
profile, acne, hirsutism, and androgenic weight gain). A low-dose pill
has been developed containing the progestogen drospirenone, which has
mineralocorticoid activities.[31]
COCs are
typically taken in a regimen of 21 “active” hormone pills followed by a
hormone-free interval of seven days, during which withdrawal bleeding
occurs. The monophasic agents consist of fixed amounts of the
estrogen/progestin ingredients in all 21 active tablets. The biphasic
and triphasic formulations have 2 or 3 different tablets, respectively,
containing varying amounts of hormones, which more closely approximates
the usual levels experienced during a woman’s menstrual cycle.
Lengthening
the hormone-free interval by missing pills at the beginning or end of a
cycle may increase the risk of pregnancy by allowing follicular
development and ovulation in some patients.[32]
The
disadvantages of COCs use for adolescents include the need to take the
pill every day (preferably at the same time each day), and the lack of
protection against STDs.[32]
Adolescents may
choose to start hormonal contraception on the first day of the next
menstrual cycle or do a “Sunday start”. Starting on the first day of
the menstrual cycle allows an adolescent to be reasonably sure that
they are not pregnant. Initiating on a Sunday allows for a withdrawal
bleed to occur on a Monday, assuming a seven-day hormone-free interval.[28,29,32]
Adolescents
often have an irregular lifestyle, difficulties in assessing risk of
unintended pregnancy and consequently run a high risk of contraceptive
failure and unintended pregnancies. Winner et al.[33]
showed that among users of pills, patches, or rings, those who were
less than 21 years of age had a risk of unintended pregnancy that was
almost twice as high as the risk among older women. In the event of
missing a pill, only 25% would use additional contraceptive measures
such as condoms.[34]
Other widely used SARC
methods are the vaginal ring (delivers 15 µg of EE and 120 µg of
etonogestrel daily) and the patch (delivers 20 µg of EE and 150 µg of
norelgestromin daily). Medical eligibility and side effect profiles of
both compounds are considered to be the same as for the COCs.[28]
The
vaginal ring is a flexible silicone ring measuring 5,4 cm at the outer
diameter with 4 mm thickness. The ring is inserted in the vagina and
left in place for 3 weeks to release on average 0.120 mg/d of
etonogestrel and 0.015 mg/d of ethinyl estradiol hormones daily for
birth control. After 3 weeks, it is removed for 1 ring-free week.[28]
Patients should be counselled that if the ring is occasionally removed,
it must be replaced within 3 hours to maintain optimal efficacy.
The
patch is worn for three consecutive weeks (each patch for 7 days),
followed by a patch-free week to allow for withdrawal bleeding. The
thin plastic patch is worn on the skin (upper extremities, back, lower
abdomen, or buttocks but not on the breasts) where it provides a
constant flow of these hormones into the bloodstream.[28]
According to data from the manufacturer, there is an increase of 60% in
the area under the curve for EE compared to a 35 µg COC preparation.
COCs use is associated with a 3.0 to 3.5-fold increase in the relative risk of VTE.[32]
However, if there are no additional risk factors, the absolute risk of
VTE associated with 20 µg of EE dose is lower, particularly when
compared to the risk during pregnancy and post-partum.[32] The risk of VTE is highest in the first few months after initiating COC and lessens over the first year of use.[32]
The
safety of different progestogens is conflicting; however, there is
evidence that COCs containing levonorgestrel or norethisterone may be
associated with lower rates of VTE, stroke, and myocardial infarction
than COCs containing the newer generation progestogens.[32]
If
the patient has additional risk factors for VTE, the absolute risk is
higher, and COCs should not be used. The conditions in which COCs
should not be used or are not usually recommended are the same for
adolescents and adults.
Estrogen-containing COCs are
contraindicated for those with a history of thromboembolism or
thrombophilia due to factor V Leiden mutation or to protein C, protein
S, or antithrombin III deficiencies; pulmonary artery hypertension;
systemic lupus erythematosus associated with antiphospholipid antibody
syndrome or renal disease (particularly that associated with
hypertension) or severe hepatic dysfunction.[32]
Breast
tension, headache and nausea in particular. are much less frequent in
women taking very low-dose formulations. Weight gain may be a
significant discomfort associated with COCs although a Cochrane
analysis did not reveal convincing evidence that use of COCs affects
body weight or composition, and if any effect exists, it is likely
mild.[35]
For combined hormonal contraceptives
(COCs, patches, vaginal rings) interactions with drugs, we suggest
consulting an up-to-date medicine formulary.
2. Progestogen-only pills.
Progestogen-only pills formulations (POPs) are a suitable alternative
for those who wish to use an oral contraceptive but have
contraindications to oestrogen use or prefer not to use COCs. POPs
thicken cervical mucosa to inhibit sperm penetration and may also
prevent ovulation (50% of cycles).[36]
POPS are
oestrogen-free oral contraceptives containing 0.35 mg of norethindrone
and are taken daily with no hormone-free days. There are no inactive
pills in the POP pack and no break required between packs. POPs can be
initiated on any day of the menstrual cycle; however, if starting six
or more days after the onset of menses, condoms should be used for the
first two days (48 hours) of hormone pills.[36]
Norethisterone
and levonorgestrel-only pills must be taken within three hours of the
regular dosing time each day. Desogestrel-only pills have a wider
window for error and must be taken within 12 hours of the regular
dosing time.[36]
Most women achieve decreased
menstrual bleeding, and 10% achieve complete amenorrhea. Breakthrough
bleeding is the most common side effect. POPs should be used with
caution in those with some liver diseases, e.g. decompensated cirrhosis
or positive for antiphospholipid antibodies.[37]
b. Long-Acting Reversible Contraception (LARC).
LARC are defined as methods that require administration less than once
per cycle or month. The methods listed below fall within this
definition:
Progestogen-only injectable:
• Progestogen-only injectables (Medroxy-progesterone acetate: DMPA, given intramuscularly or subcutaneously)
• Progestogen subdermal implants
Intrauterine contraception:
• Copper intrauterine device (IUCD)
• Levonorgestrel releasing intrauterine system (LNG-IUS)
Subdermal progestogen implants
1. Depot medroxyprogesterone acetate (DMPA).
The primary effect of DMPA is to reduce the chance of ovulation by
limiting follicle-stimulating hormone and luteinizing hormone
secretion.[32] Besides, DMPA injections can alter
cervical mucosa to prevent sperm penetration, as well as thin the
endometrial lining to make it unsuitable for implantation.[32]
DMPA
injections are administered at 12-week intervals (11–13 weeks) for
optimal effect. It may be given in a single dose of 150 mg
intramuscularly. Lower dose injections of DMPA containing 30% less
hormone, given by subcutaneous injections every 13 weeks, are available
in some countries. The upper outer quadrant of the buttock (i.e.
dorsogluteal site) is the preferred IM injection site; the first
injection should be given within the first five days of starting of a
menstrual cycle.
This method is convenient for women who do not
want to remember to take the pill daily, cannot use the patch, or a
contraceptive method at the time of intercourse.38 Other advantages
include lack of estrogen-related adverse effects.
There are 2 specific areas of concern for the use of DMPA in teenagers:
(a) Weight gain: In some patients, DMPA causes increased appetite and weight gain.[39,40] Diet and exercise should be a point of counselling at all visits for patients who are overweight or obese.
(b) Bone health:
Another notable side effect is the potential decrease of bone mineral
density (BMD), particularly after prolonged use. This is of significant
concern in teenagers because girls accrue approximately 30% to 40% of
their bone mass during adolescence. The BMD loss appears to be
reversible after stopping the DMPA.[41] Adolescent
DMPA-users should be counselled for adequate calcium and vitamin D
intake, weight-bearing activity, and avoidance of alcohol, caffeine,
and smoking which can also contribute to BMD loss.[42]
2. Progestogen-containing contraceptive implant.
Long-acting progestogen subdermal implants have been proven to be
highly effective and safe. The currently available
etonogestrel-releasing subdermal implant is a single rod that measures
4 cm in length and 2 mm in diameter and is composed of an inner
ethylene vinyl acetate core embedded with crystals of the progestin
active ingredient, etonogestrel.[43]
The
single-rod implant consists of a small plastic rod, about the size of a
matchstick, placed just under the skin of the upper arm that releases
small amounts of progestogen into the body. Implants contain no
oestrogen and so are therefore suitable for most women (including
breastfeeding) or cannot, or do not wish to use oestrogen. The implants
prevent pregnancy by inhibiting ovulation, as well as preventing sperm
penetration by altering cervical mucosa.[43] They are
the most effective form of reversible contraception and can protect for
a period of up to five years. Unlike estrogen-containing contraceptive
methods, use of the implant can safely be encouraged in patients with a
history of thromboembolic disease, hypertension, those who are
overweight or obese, smoke, or are aged 35 and older.[44]
Insertion
and removal complications are rare, reported in 0.3% to 1% of
insertions and 0.2% to 1.7% of removals. They include local irritation,
allergic reaction, infection, and hematoma.[45]
Irregular
bleeding is the most common side effect, especially in the first 6 to
12 months. For most women, periods become shorter and lighter, but some
will have longer, heavier periods with increased spotting.[46] Skin irritation may occur at the site of placement of the contraceptive rod.
3. Intrauterine contraceptive device (IUD).
There are currently two types of intrauterine devices (IUD):
levonorgestrel-releasing intrauterine system (LNG-IUD) and copper (Cu)
IUD. An IUD provides contraception by preventing fertilisation and
preventing implantation of the fertilised egg(s). When used
appropriately, IUDs are a generally safe and effective method of
contraception with a failure rate of less than 1%.[47,48]
The
LNG-IUD consists of a T-shaped polyethylene frame (T-body) with a
steroid reservoir around the vertical stem. The reservoir consists of a
white cylinder, made of a mixture of levonorgestrel and silicon.[47] The LNG-IUS’s (LNG-IUS 20, LNG-IUS 12, LNG-IUS 8) contain different amounts of levonorgestrel in their reservoir.
After
insertion of an LNG-IUD, unpredictable bleeding may occur for the first
3–6 months; however, most women will see overall reduced menstrual
bleeding thereafter.
The Cu-IUDs may either have a frame
(usually T-shaped) or be frameless and contain a varying amount of
copper. The Cu-IUD is associated with increased duration and volume of
menstrual bleeding.[48]
The success rate for
insertion in adolescents is 96%. Before providing or placing an IUD,
absolute and relative contraindications should be reviewed, and the
procedure should be carefully explained, including the possibility of
discomfort or pain during the gynaecological examination and device
insertion. The most common side effects are bleeding pattern
alterations, vulvovaginitis, abdominal/ pelvic pain, acne, ovarian
cysts, and headache.[49-51]
Available options
for hormonal contraception in adolescent and young women, including
limitations and side-effects are summarized in table 1.
All contraceptive methods require reliable information on correct use,
together with detailed information on how to avoid sexually transmitted
diseases (STDs). Additional use of condoms is advised for dual
protection.
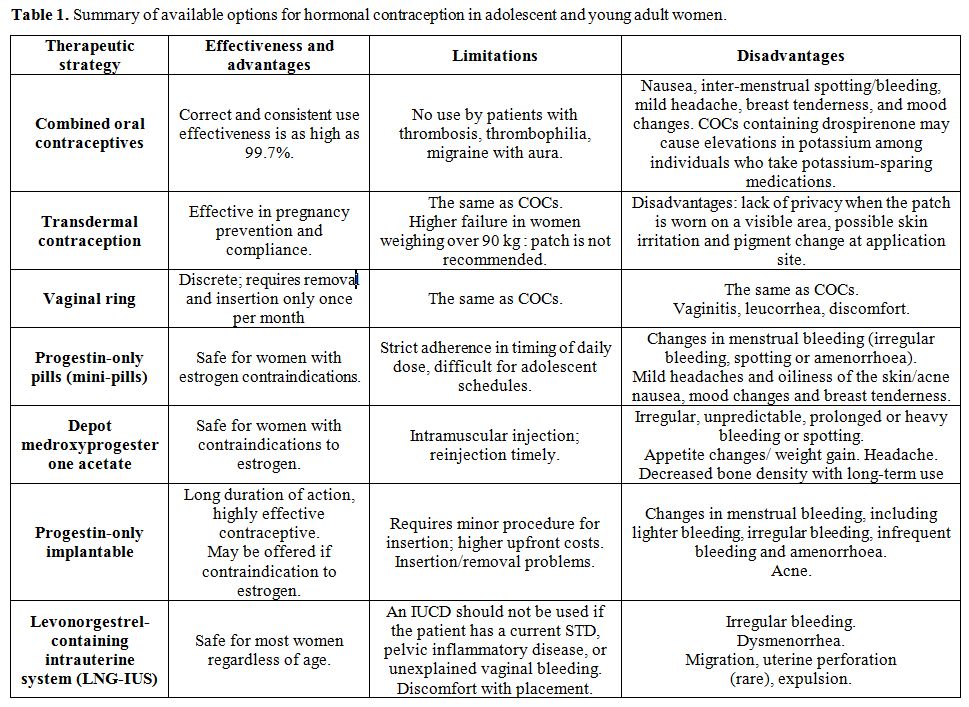 |
Table
1. Summary of available options for hormonal contraception in adolescent and young adult women. |
C. Overview of Contraceptive Practices Followed by Women with SCD and Treatment Safety
Available data on contraceptive methods chosen by women with SCD and on the safety of treatment are limited.
In 1984, Samuels-Reid, [52]
interviewed 52 patients with HbSS, HbSC, HbS/ß-thal and sickle cell
trait. 33% of the study group used a contraceptive method compared with
66% of healthy controls. The most common method in both groups was
the birth control pill (39% in the sickle cell group and 86% in the
control group). The sickle cell group used a greater variety of
contraceptive methods, with the cumulative majority choosing the
diaphragm (23%), intrauterine device (15.4%), and foam (23%).
Howard et al.[53]
investigated the use of contraceptives and complications in 102 women
with HbSS disease, 42 with HbSC, and 12 with HbS/ß-thal. COCs were
taken by 67 women (45%); 30 used POPs (20%), 28 intrauterine
contraceptive device (19%), and 36 injectable DMPA (17%). These
findings were similar to those from North America, where 39% used COCS
and 15.4% the intrauterine device.[54]
In this
cohort of 156 women using the combined contraceptive pill, four
complained of increased frequency of crises (3 of 102 with HbSS disease
and 1of 12 HbS/ß-thal), while two reported deep vein thrombosis. The
type of pill was not stated, but both were assumed to be low dose
preparations because of the prescribing policy of the clinic concerned.
Both had HbSS disease.[53]
The fact that over
50% of SCD pregnancies were still unplanned in a 2010 survey confirms
that there is a continuing unmet need for effective contraceptive
advice for this group of patients, suggesting that further intensive
efforts on this issue are needed to educate health care professionals,
as well as initiatives to include contraceptive advice in the routine
medical care of young women with SCD.[55]
Legardy and Curtis[56]
searched the MEDLINE database for articles published between 1966 and
September 2004 on the use of progestogen-only contraceptives in women
with SCD. Of the 70 articles identified, 8 met the criteria for this
review. These studies did not identify any adverse event, or clinically
or statistically significant adverse changes in haematological or
biochemical parameters associated with the use of progestogen-only
contraceptive methods. Six studies suggested that users experienced a
decrease in clinical symptoms and less frequent and severe painful
crises compared with nonusers.
A Cochrane review by Manchikanti et al.[57]
reported similar results. DMPA use in women with HbSS was a safe
contraceptive option. In addition, DMPA reduced painful sickle episodes
(OR 0.23; 95% CI 0.05 to 1.02). No trial involving estrogen products
was reported.
A systematic review that examined the safety of
hormonal and intrauterine contraceptive use among women with SCD was
performed in 2012. Eight articles met the inclusion criteria. The
evidence was of fair to poor quality and suggested that progestin-only
and combined hormonal contraception did not affect the frequency of
sickle crises or other adverse events and no effect on hematologic
parameters associated with sickle crises.[58] No
studies examined the risk of thromboembolism in combined hormonal
contraceptive users with SCD. There was insufficient evidence to
comment on the safety of intrauterine devices.
We searched PubMed
for all articles published thereafter, between 2014 and March 2020.
Four studies were identified that met the inclusion criteria
"contraceptive methods, combined oral contraceptives, short- and
long-acting reversible contraception, safety and effectiveness of
contraceptive methods, oral contraceptive use and incident stroke,
sickle cell disease". The main reported findings are summarized in table 2.
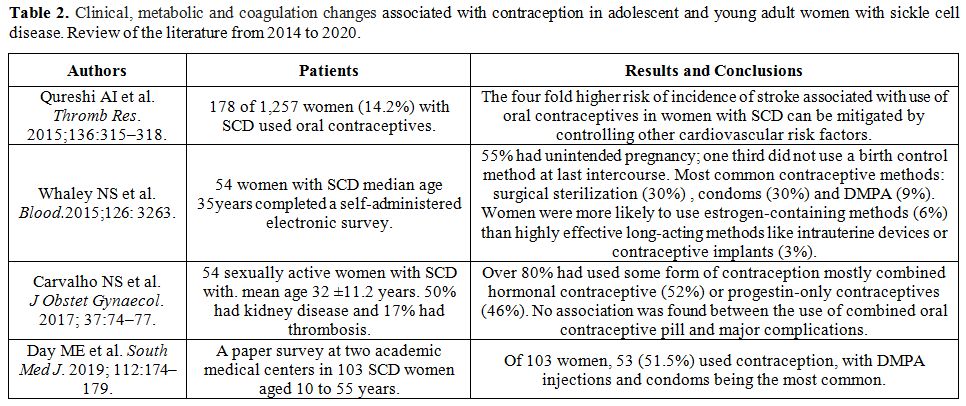 |
Table 2. Clinical,
metabolic and coagulation changes associated with contraception in
adolescent and young adult women with sickle cell disease. Review of
the literature from 2014 to 2020. |
D. Medical Eligibility Contraception (MEC) Recommendations
Recommendations
for the safety of contraception in women with certain characteristics
or medical conditions are provided in the form of MEC from WHO, CDC,
Faculty of Sexual and Reproductive Healthcare, and other international
organizations.[59-61] WHO and CDC also developed
Selective Practice Recommendations for Contraceptive Use recommending
which tests and examinations should be performed before providing
contraception.[62,63]
The international
recommendations are intended to assist health care providers in
counselling women, men, and couples about the choice of contraceptive
method. For each medical condition/characteristic, contraceptive
methods are classified in one of four categories to determine
contraceptive eligibility:
• Category 1 = Conditions for which there are no restrictions for the use of the contraceptive method.
• Category 2 = Conditions for which the advantages of using the method generally outweigh the theoretical or proven risks.
• Category 3
= Conditions for which theoretical or observed risks usually outweigh
the advantages of using the method. The implementation of a method
requires expert clinical judgement and/or referral to a specialist
contraceptive provider since the method is not usually recommended
unless other more appropriate methods are not available or not
acceptable.
• Category 4 = Conditions that represent an unacceptable health risk if the contraceptive method is used.
SCD
is considered a “prothrombotic” state because of abnormal RBC rheology,
hyperviscosity, endothelial dysfunction, and red cells adhesion;[64,65]
increased platelet activation; venous sludging and abnormal coagulation
associated with increased thrombotic complications in patients
receiving estrogens.[66]
Moreover, VTE, defined
as deep vein thrombosis (DVT) or pulmonary embolism (PE), is a frequent
and severe clinical complication in adults with SCD, and is likely, at
least in part, to be the result of this hypercoagulable state. Up to
12% of patients with SCD have a VTE by 40 years of age.[67]
Therefore, the use of certain contraceptives may exacerbate medical disorder and the risk of complications.
In
women with SCD, COCs are classified as level 2, meaning that “the
advantages of using the method generally outweigh the theoretical or
proven risks.” The benefits of estrogen-containing methods usually
outweigh the risks of unintended pregnancy. The progestin-only pill,
injection, implant and IUD all received a “1” rating (substantially can
be used without restriction) from the CDC.[60]
There
are few contraindications to progestin-only methods: current breast
cancer (Category 4), breast cancer remission within five years, severe
cirrhosis, hepatocellular adenoma, malignant liver tumour, and
unexplained vaginal bleeding (Category 3),[60] use of
medications to treat seizures or tuberculosis (i.e., phenytoin,
carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, or
rifampicin),[72] and evidence suggesting an increased risk of VTE with the use of injectable DMPA.[73]
The copper IUD has a “2” classification rating due to the possibility of heavier menses with this method.[59,60]
These
recommendations are meant to serve as a source of clinical guidance;
however, individual patient's decision needs to be considered in
special risk situations for SCD patients. Such decisions often require
interdisciplinary consultation, particularly when the patient suffers
from a specific medical condition that is outside the gynaecological
sphere, such as: previous stroke, pulmonary hypertension, renal
impairment, autosplenectomy, and hepatobiliary complications.
At
present, more than 50% of SCD patients survive beyond the fifth decade.
This improvement in survival in developed countries has resulted from
the close clinical and laboratory follow-up and symptomatic treatment.[74]
As patients with SCD get older, there are general or specific risks for
developing co-morbidities that were not or rarely seen in the younger
SCD population, e.g. silent infarcts, which do not manifest overtly but
can accumulate over time, renal failure, and iron overload (especially
on frequent transfusions).[75]
Sickle cell
hepatopathy is a spectrum of disease manifestations with varying levels
of severity due to acute or chronic changes of the hepatobiliary
system.[76,77]
In addition to the risk of VTE
associated with low-dose COCs use, estrogens and progestogens are
cleared through hepatic metabolism, and estrogens act directly on the
liver independently of administration route.[78] Long-term use of COCs may be related to the development of hepatocellular carcinomas and adenomas.[79]
Finally, estrogens can alter the biliary function and increase
cholesterol saturation, which requires special caution (Category 3)
when COCs are used in patients with gall bladder disease.[59-61] Hormonal contraceptive use has also been incriminated as a risk of hepatobiliary damage.[80]
Overall,
before selecting the appropriate contraceptive method for a woman with
SCD, the prescriber should carefully evaluate her medical history and
current disease status. The prescriber has to consider not only the
WHO-MEC, which are evolving, but also the international guidelines and
specialised books[81] to determine the possible
contraindications to the contraceptive methods desired by the woman or
couple, and decide the most appropriate, and avoiding risk factors
especially: obesity, smoking, immobilization, lower extremity injury
and surgery wherever possible (tables 3-6).[82]
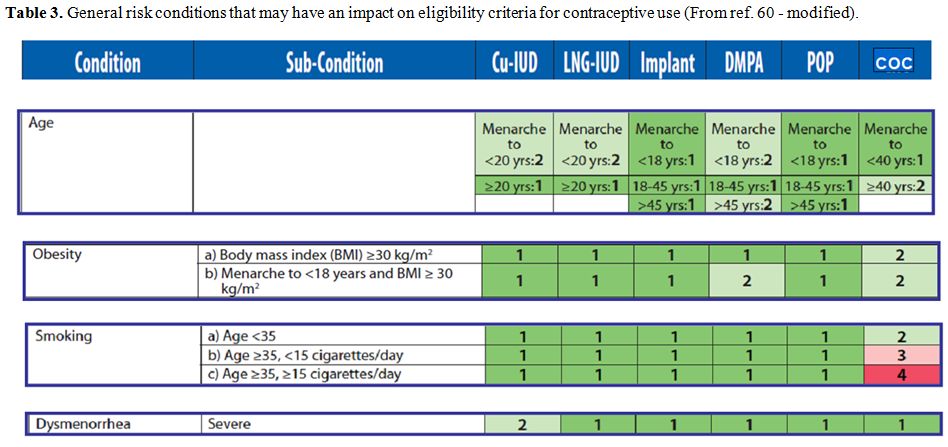 |
Table 3.
General risk conditions that may have an impact on eligibility criteria for contraceptive use (From ref. 60 - modified). |
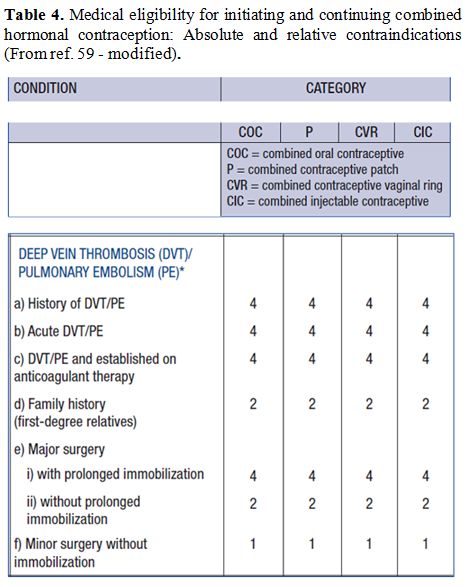 |
Table 4. Medical
eligibility for initiating and continuing combined hormonal
contraception: Absolute and relative contraindications (From ref. 59 -
modified). |
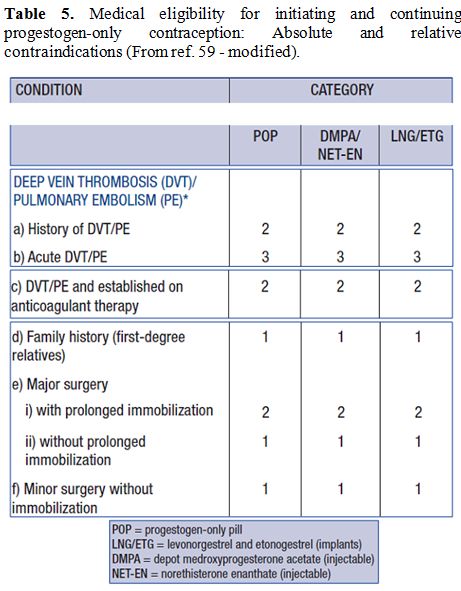 |
Table 5. Medical eligibility for
initiating and continuing progestogen-only contraception: Absolute and
relative contraindications (From ref. 59 - modified). |
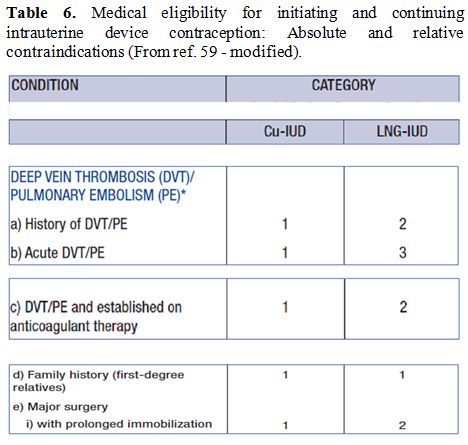 |
Table 6. Medical eligibility for
initiating and continuing intrauterine device contraception: Absolute
and relative contraindications (From ref. 59 - modified). |
E. Reproductive Medical Counselling
Over
the past 20 years, we have steadily progressed in the management of
patients with SCD. Long-term therapies, with chronic transfusions,
hydroxyurea (HU), and hematopoietic stem cell transplantation (HSCT)
have reduced SCD-related morbidity and mortality. Thus, many more
children and adolescents with SCD grow into adulthood and face serious
considerations regarding childbearing, which is one of the most
important factors for quality of life.
There has been very little research on reproductive attitudes, beliefs and health knowledge of patients with SCD.[83-87]
The decision to have a child is influenced by the risks of the genetic
transmission, the perceptions of the disease severity and the risks of
pregnancy to the mother and fetus. All are essential components in the
clinical management of SCA patients with significant medical,
psychological, social, ethical and legal implications.
Accordingly,
it is of utmost importance that health providers have a fundamental
understanding of the disease and be aware of relevant professional
management guidelines to encourage knowledgeable reproductive health
decisions.
Although there is a paucity of information on
provider knowledge and practice related to hemoglobinopathies
internationally, the Royal College of Obstetricians and Gynaecologists[88]
has established guidelines for the management of hemoglobinopathies in
pregnancy and released recent recommendations for the management of SCD
in pregnancy based on the available evidence.
Conclusions and Recommendations
Patients
with SCD seen in haematology practice are incredibly heterogeneous in
clinical and haematological phenotypes with multiple clinical issues
that must be faced. Acute and chronic vessel(s) occlusion causes
significant complications in various organs, including brain, kidneys,
bones, lungs, liver, spleen, and gastrointestinal tract.
Furthermore,
women with SCD are known to have high-risk pregnancies, mainly
affecting the foetus. The mothers also face serious maternal risks,
such as an increased risk of both medical complications (infections and
thromboembolic events) and pregnancy-related complications
(preeclampsia, eclampsia, preterm labour, placental abruption, and
fetal growth retardation).[19] The maternal and fetal mortality rates during pregnancy can attain 11.4% and 20%, respectively.[20,89]
Women with SCD also have higher rates of Caesarean deliveries.[90]
The
cornerstones of treatment for SCD patients involve the management of
painful vaso-occlusive, hemolytic and aplastic rises, hemolytic
anaemia, other disease complications, and prevention of infection.
Blood transfusions (especially exchange transfusions), the first
disease-modifying therapy used for SCD, reduces the percentage of
circulat¬ing RBCs with HbS. How¬ever, the need for repeated venous
access and the associated risks and complications, such as
alloimmunization and iron overload, limits its use.[85,86,91,92] Chelation therapy can be used to remove excess iron in patients with evidence of iron overload.[91] During pregnancy, chelation should be restricted for cases where the potential benefit outweighs the potential fetal risk.
HU
improves several clinical outcomes, such as decrease of vaso-occlusive
crisis (VOC) and acute chest syndrome (ACS), reduction of mortality,
and decrease for RBC transfusions and hospital¬izations. HU works
primarily by increasing the level of fetal haemoglobin (HbF), which
prevents sickling.[92-95] At present, it is
recommended that HU should be discontinued at least 3 months before
conception96 due to the risk of teratogenic side effects.[97-99]
Women with SCD have little knowledge about the risks associated with contraceptive use,[100] and thus, they need guidance for adequate reproductive family planning and unintended pregnancy.
Women
with SCD primarily received contraceptive counselling from
gynaecologist providers, and only 30% reported a different source.[100]
Contraception
should be discussed during transitional care and at regular review; the
woman should be fully informed on the advantages and disadvantages of
the available methods. The full range of choices should be offered to
women with SCD, though some methods may be more suitable.
When
assessing the safety of contraceptive methods in women with SCD, any
co-existing medical conditions that contraindicate the use of a
specific method must be considered carefully.
Women should be
informed that in the general population, the risk of venous
thromboembolism with the use of COCs is approximately doubled compared
to non-users. For SCD patients, there is a lack of evidence on whether
this risk is further increased and whether the risk of VTE is reduced
in subjects taking COC with a low dose of EE.
The World Health
Organization recommends that all contraceptive methods may be
prescribed for women with SCD, but the progestogen-only contraceptive
methods are preferred (due to no reported increased incidence of venous
or arterial thrombosis). The benefits of estrogen-containing methods
usually outweigh the risks of unintended pregnancy (Level 2).
However,
there is reluctance on the part of physicians to prescribe hormonal
contraception in women with SCD based on the assumption that additional
risks may be compounded to the underlying disease process; they are
often instinctively reluctant to propose the use of the intrauterine
contraceptive device because of the potential complications of
menorrhagia, exacerbating the chronic anaemia of SCD. That may provoke
potentially sickling episodes and infections.
Appropriate
treatment requires the active involvement of health care professionals
with experience in the management and treatment of SCD, usually a
haematologist working in conjunction with a multidisciplinary team,
although subspecialists may also have limited experi¬ence in the care
of SCD-related complications.
Therefore, there is a real need
for an integrated approach for selecting suitable contraception for
women with SCD by a team of haematologists, gynaecologists,
endocrinologists, and primary care providers to support sound
communication strategies and collaborative efforts, and share
responsibility, mutual understanding and acceptance of each provider’s
role, within the practice. We hope that this synergy can play a
significant role in identifying the safest contraceptive method that
preserves patient health status and abolish the risks of unintended
pregnancy.
References
- Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N Engl J Med. 2017;376:1561-1573. https://doi.org/10.1056/NEJMra1510865 PMid:28423290
- Stuart MJ, Nagel RL. Sickle cell disease. Lancet. 2004;364:1343-1360. https://doi.org/10.1016/S0140-6736(04)17192-4
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762-9. https://doi.org/10.1056/NEJM199709113371107 PMid:9287233
- Chakravorty
S, Williams TN. Sickle cell disease: a neglected chronic disease of
increasing global health importance. Arch Dis Child. 2015;100:48-53. https://doi.org/10.1136/archdischild-2013-303773 PMid:25239949 PMCid:PMC4285890
- Ashley-Koch
A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell
disease: a HuGE review. Am. J. Epidemiol. 2000;151, 839-845. https://doi.org/10.1093/oxfordjournals.aje.a010288 PMid:10791557
- Yee
DL, Edward RM, Mueller BU, Teruya J. Thromboelastrographic and
hemostatic characteristics in pediatric patients with sickle cell
disease. Arch Pathol Lab Med. 2005;129:760-765.
- Stuart
MJ, Setty BN. Hemostatic alterations in sickle cell disease:
Relationship to disease pathophysiology. Pediatr Pathol Mol Med.
2001;20:27-46. https://doi.org/10.1080/15513810109168816
- Naik
RP, Streiff MB, Lanzkron S. Sickle cell disease and venous
thromboembolism: what the anticoagulation expert needs to know. J
Thromb Thrombolysis. 2013;35:352-358. https://doi.org/10.1007/s11239-013-0895-y PMid:23435703 PMCid:PMC4335704
- Kumar
R, Stanek J, Creary S, Dunn A, O'Brien SH. Prevalence and risk factors
for venous thromboembolism in children with sickle cell disease: an
administrative database study.Blood Adv. 2018;2:285-291. https://doi.org/10.1182/bloodadvances.2017012336 PMid:29431623 PMCid:PMC5812330
- Yu
TT, Nelson J, Streiff MB, Lanzkron S, Naik RP. Risk factors for venous
thromboembolism in adults with hemoglobin SC or Sß(+) thalassemia
genotypes. Thromb Res. 2016;141:35-38. https://doi.org/10.1016/j.thromres.2016.03.003 PMid:26962984 PMCid:PMC4856579
- Little
I, Vinogradova Y, Orton E, Kai J, Qureshi N. Venous thromboembolism in
adults screened for sickle cell trait: a population-based cohort study
with nested case-control analysis. BMJ Open. 2017;7: e012665. doi:
10.1136/bmjopen-2016-012665 https://doi.org/10.1136/bmjopen-2016-012665 PMid:28360235 PMCid:PMC5372149
- Noubiap
JJ, Temgoua MN, Tankeu R, Tochie JN, Wonkam A, Bigna JJ. Sickle cell
disease, sickle trait and the risk for venous thromboembolism: a
systematic review and meta-analysis.Thromb J. 2018; 6: 27. Published
online 2018 Oct 4. https://doi.org/10.1186/s12959-018-0179-z PMid:30305805 PMCid:PMC6171302
- Naik
RP, Streiff MB, Haywood C Jr, Nelson JA, Lanzkron S. Venous
thromboembolism in adults with sickle cell disease: a serious and
under-recognized complication. Am J Med. 2013;126:443-449. https://doi.org/10.1016/j.amjmed.2012.12.016 PMid:23582935 PMCid:PMC3627211
- Crary
SE, George R. Buchanan GR. Vascular complications after splenectomy for
hematologic disorders. Blood. 2009;114: 2861-2868. https://doi.org/10.1182/blood-2009-04-210112 PMid:19636061 PMCid:PMC2756197
- Chavis WM, Norman GS. Sexuality and sickle cell disease. J Natl Med Assoc. 1993;85:113-116.
- Cobo
VDA, Chapadeiro CA, Ribeiro JB, Moraes-Souza H, Martins PR. Sexuality
and sickle cell anemia. Rev Bras Hematol Hemoter. 2013;35: 89-93. https://doi.org/10.5581/1516-8484.20130027 PMid:23741184 PMCid:PMC3672116
- Smith
M, Aguirre RT. Reproductive attitudes and behaviors in people with
sickle cell disease or sickle cell trait: a qualitative interpretive
meta-synthesis. Soc Work Health Care. 2012;51:757-779. https://doi.org/10.1080/00981389.2012.693580 PMid:23078010
- Howard RJ, Tuck SM. Contraception and sickle cell disease. IPPF Med Bull. 1994;28:3-4. https://doi.org/10.1007/978-1-4471-2473-3_1 PMid:25685685
- Jain
D, Atmapoojya P, Colah R, Lodha P. Sickle cell disease and pregnancy.
Mediterr J Hematol Infect Dis. 2019, 11(1): e2019040, DOI:
http://dx.doi.org/10.4084/MJHID.2019.040. https://doi.org/10.4084/mjhid.2019.040 PMid:31308916 PMCid:PMC6613624
- Boga
C, Ozdogu H. Pregnancy and sickle cell disease: A review of the current
literature. Crit Rev Oncol Hematol. 2016;98:364-374. https://doi.org/10.1016/j.critrevonc.2015.11.018 PMid:26672916
- Oteng-Ntim
E, Meeks D, Seed PT, Webster L, Howard J, Doyle P, Chappell LC. Adverse
maternal and perinatal outcomes in pregnant women with sickle cell
disease: systematic review and meta-analysis. Blood.
2015;125:3316-3325. https://doi.org/10.1182/blood-2014-11-607317 PMid:25800049
- Tuck SM, Studd JW, White JM. Pregnancy in sickle cell disease in the UK. Br J Obstet Gynaecol.1983; 90:112-117. https://doi.org/10.1111/j.1471-0528.1983.tb08893.x PMid:6824610
- Naess
IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR,
Hammerstrom J. Incidence and mortality of venous thrombosis: a
population-based study. J Thromb Haemost. 2007;5:692-629. https://doi.org/10.1111/j.1538-7836.2007.02450.x PMid:17367492
- Manzoli
L, De Vito C, Marzuillo C, Boccia A, Villari P. Oral contraceptives and
venous thromboembolism: a systematic review and meta-analysis. Drug
Saf. 2012;35:191-205. https://doi.org/10.2165/11598050-000000000-00000 PMid:22283630
- Plu-Bureau
G, Hugon-Rodin J, Maitrot-Mantelet L, Canonico M. Hormonal
contraceptives and arterial disease: an epidemiological update. Best
Pract Res Clin Endocrinol Metab. 2013;27:35-45. https://doi.org/10.1016/j.beem.2012.11.003 PMid:23384744
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3-14. https://doi.org/10.1007/s11239-015-1311-6 PMid:26780736 PMCid:PMC4715842
- Ott MA, Sucato GS; Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014; 134: e1257-e1281. https://doi.org/10.1542/peds.2014-2300 PMid:25266435
- McGregor
JA, Hammill HA. Contraception and sexually transmitted diseases:
interactions and opportunities. Am J Obstet Gynecol.
1993;168:2033-2041. https://doi.org/10.1016/S0002-9378(12)90946-1
- Burke AE, Blumenthal PD. Successful use of oral contraceptives. Semin Reprod Med. 2001;19:313-321. https://doi.org/10.1055/s-2001-18639 PMid:11727173
- Speroff L, Darney PD. A Clinical Guide for Contraception. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Kuhl H. Pharmacology of progestogens. J Reproduktionsmed Endokrinol. 2011;8:157-176.
- Faculty of Sexual and Reproductive Healthcare. FSRH guideline: combined hormonal contraception. 2019. Available from: https://www.fsrh.org/standards-and-guidance/documents/combined-hormonal-contraception/ (Accessed Jan, 2019).
- Winner
B , Peipert J, Zhao Q, Buckel C, Madden T , Allsworth J. Secura GM.
Effectiveness of long-acting reversible contraception. N Engl J Med.
2012;366: 1998-2007. https://doi.org/10.1056/NEJMoa1110855 PMid:22621627
- Goldstuck
ND, Hammar E, Butchart A. Use and misuse of oral contraceptives by
adolescents attending a free-standing clinic. Adv Contracept.1987;
3:335-339. https://doi.org/10.1007/BF01849291 PMid:3445802
- Gallo
MF, Lopez LM, Grimes DA, Carayon F, Schulz KF, Helmerhorst FM.
Combination contraceptives: effects on weight. Cochrane Database Syst
Rev. 2014;(1):CD003987. Published 2014 Jan 29. https://doi.org/10.1002/14651858.CD003987.pub5
- Faculty
of Sexual and Reproductive Healthcare. Clinical Effectiveness Unit
guidance. Progestogen only pills. 2015. Available from: https://www.fsrh.org/documents/ceuguidanceprogestogenonlypills/ (Accessed Jan, 2019).
- White
K, Potter JE, Hopkins K, Fernández L, Amastae J, Grossman D.
Contraindications to progestin-only oral contraceptive pills among
reproductive-aged women. Contraception. 2012;86:199-203. https://doi.org/10.1016/j.contraception.2012.01.008 PMid:22364816 PMCid:PMC3368072
- Darroch
JE, Singh S, Frost JJ. Differences in teenage pregnancy rates among
five developed countries: the roles of sexual activity and
contraceptive use Fam Plann Perspect. 2001;33:244-250. https://doi.org/10.2307/3030191 PMid:11804433
- Lopez
LM, Edelman A, Chen M, Otterness C, Trussell J, Helmerhorst FM.
Progestin-only contraceptives: effects on weight. Cochrane Database
Syst Rev. 2013;7(7):CD008815. Published 2013 Jul 2.
doi:10.1002/14651858.CD008815.pub3. https://doi.org/10.1002/14651858.CD008815.pub3
- Clark
MK, Dhillon JS, Sowers M, Nichols S. Weight, fat mass and central
distribution of fat increase when women use depot-medroxyprogesterone
acetate for contraception. Int J Obes. 2005;29:1252-1258. https://doi.org/10.1038/sj.ijo.0803023 PMid:15997247
- Scholes
D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral
density among adolescent women using and discontinuing depot
medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med.
2005;159:139-144. https://doi.org/10.1001/archpedi.159.2.139 PMid:15699307
- Golden
NH, Abrams SA; Committee on Nutrition. Optimizing bone health in
children and adolescents. Pediatrics. 2014;134:e1229-e1243. https://doi.org/10.1542/peds.2014-2173 PMid:25266429
- Croxatto HB.Mechanisms that explain the contraceptive action of progestin implants for women. Contraception.2002;65:21-27. https://doi.org/10.1016/S0010-7824(01)00294-3
- Meckstroth KR. Implant contraception. Semin Reprod Med. 2001;19:339-354. https://doi.org/10.1055/s-2001-18642 PMid:11727176
- Grentzer
J, McNicholas C, Peipert JF. Use of the etonogestrel releasing
contraceptive implant. Expert Rev of Obstet Gynecol. 2013;8:337-344. https://doi.org/10.1586/17474108.2013.811941
- Trussell
J Guthrie K. Choosing a contraceptive: efficacy, safety, and personal
considerations. In: Hatcher RA, Trussell J, Nelson AL eds.
Contraceptive Technology. 19th revised ed. New York, NY: Ardent Media, Inc; 2007:19-47.
- National Institutes for Health and Care Excellence (NICE). Long-acting reversible contraception. 2014. Available from: https://www.nice.org.uk/guidance/cg30 (Accessed Feb, 2019).
- Wan
LS, Stiber A, Lam L. The levonorgestrel two-rod implant for long-acting
contraception: 10 years of clinical experience. Obstet Gynecol.
2003;102:24-26. https://doi.org/10.1097/00006250-200307000-00007 PMid:12850601
- Steyn PS, Goldstuck ND. Contraceptive needs of the adolescent. Best Pract Res Clin Obstet Gynaecol. 2014;28:891-901. https://doi.org/10.1016/j.bpobgyn.2014.04.012 PMid:24947598
- Apter
D. Contraception options: Aspects unique to adolescent and young adult.
Best Pract Res Clin Obstet Gynaecol. 2018;48:115-127. https://doi.org/10.1016/j.bpobgyn.2017.09.010 PMid:29032945
- Lidegaard
O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of
non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ.
2012;344:e2990. Published 2012 May 10. doi:10.1136/bmj.e2990. https://doi.org/10.1136/bmj.e2990 PMid:22577198 PMCid:PMC3349780
- Samuels-Reid
JH, Scott RB, Brown WE. Contraceptive practices and reproductive
patterns in sickle cell disease. J Natl Med Assoc.1984;76:879-883.
- Howard
RJ, Lillis C, Tuck SM. Contraceptives, counselling, and pregnancy in
women with sickle cell disease. BMJ.1993;306:1735-1737. https://doi.org/10.1136/bmj.306.6894.1735 PMid:8343632 PMCid:PMC1678269
- Foster HW. Contraceptives in sickle cell disease. South Med J.1981;74:543-545. https://doi.org/10.1097/00007611-198105000-00009 PMid:7244708
- Eissa
AA, Tuck SM, Rantell K, Stott D. Trends in family planning and
counselling for women with sickle cell disease in the UK over two
decades. J Fam Plann Reprod Health Care. 2015;41:96-101. https://doi.org/10.1136/jfprhc-2013-100763 PMid:24860151
- Legardy
JK, Curtis KM. Progestogen-only contraceptive use among women with
sickle cell anemia: a systematic review. Contraception.
2006;73:195-204. https://doi.org/10.1016/j.contraception.2005.08.010 PMid:16413850
- Manchikanti
A, Grimes DA, Lopez LM, Schulz KF. Steroid hormones for contraception
in women with sickle cell disease. Cochrane Database Syst Rev.
2007;(2):CD006261. Published 2007 Apr 18. https://doi.org/10.1002/14651858.CD006261.pub2
- Haddad
LB, Curtis KM, Legardy-Williams JK, Cwiak C, Jamieson DJ. Contraception
for individuals with sickle cell disease: a systematic review of the
literature. Contraception. 2012;85:527-537. https://doi.org/10.1016/j.contraception.2011.10.008 PMid:22152587
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 5th Edition. Geneva, Switzerland. 2015.
- Centers
for Disease Control and Prevention. U.S. Medical Eligibility Criteria
for Contraceptive Use, 2016. Morbidity and Mortality Weekly Report.
2016;65:1-103.
- Faculty of Sexual &
Reproductive Healthcare. UK Medical Eligibility Criteria for
Contraceptive Use. UKMEC 2016 (Amended September 2019). Last Accessed
date: January 5, 2020. Available from: https://www.fsrh.org/standards-and-guidance/documents/ukmec-2016
- World Health Organization. Selected practice recommendations for contraceptive use. Third Edition. Geneva, Switzerland. 2016.
- Curtis
KM, Jatlaoui TC, Tepper NK, Zapata LB, Horton LG, Jamieson DJ, Whiteman
MK. U.S. Selected Practice Recommendations for Contraceptive Use, 2016.
MMWR Recomm Rep. 2016;65:1-66. https://doi.org/10.15585/mmwr.rr6504a1
- Smith-Whitley K. Reproductive issues in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2014;2014:418-424. https://doi.org/10.1182/asheducation-2014.1.418 PMid:25696888
- Wun T, Brunson A. Sickle cell disease: an inherited thrombophilia. Hematology Am Soc Hematol Educ Program. 2016;2016:640-647. https://doi.org/10.1182/asheducation-2016.1.640 PMid:27913540 PMCid:PMC6142455
- Freie HM. Sickle cell diseases and hormonal contraception. Acta Obstet Gynecol Scand. 1983;62:211-217. https://doi.org/10.3109/00016348309155794 PMid:6624392
- Brunson
A, Lei A, Rosenberg AS, White RH, Keegan T, Wun T. Increased incidence
of VTE in sickle cell disease patients: risk factors, recurrence and
impact on mortality. Br J Haematol. 2017;178:319-326. https://doi.org/10.1111/bjh.14655 PMid:28369826
- Barsoum
MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is
progestin an independent risk factor for incident venous
thromboembolism? A population-based case-control study. Thromb Res.
2010;126:373-378. https://doi.org/10.1016/j.thromres.2010.08.010 PMid:20833412 PMCid:PMC2975753
- Lidegaard
Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E.Risk of venous
thromboembolism from use of oral contraceptives containing different
progestogens and oestrogen doses: Danish cohort study. 2001-9. BMJ.
2011;343:d6423. https://doi.org/10.1136/bmj.d6423 PMid:22027398 PMCid:PMC3202015
- Mantha
S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the
risk of venous thromboembolic events in women taking progestin-only
contraception: a meta-analysis. BMJ. 2012; 345:e4944. https://doi.org/10.1136/bmj.e4944 PMid:22872710 PMCid:PMC3413580
- van
Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous
thrombosis associated with injectable depot-medroxyprogesterone acetate
contraceptives or a levonorgestrel intrauterine device. Arterioscler
Thrombo Vasc Biol. 2010;30:2297-2300. https://doi.org/10.1161/ATVBAHA.110.211482 PMid:20798377
- White
K, Potter JE, Hopkins K, Fernández L, Amastae J, Grossman D.
Contraindications to progestin-only oral contraceptive pills among
reproductive-aged women. Contraception. 2012;86:199-203. https://doi.org/10.1016/j.contraception.2012.01.008 PMid:22364816 PMCid:PMC3368072
- Le
Moigne E, Tromeur C, Delluc A, Gouillou M, Alavi Z, Lacut K, Mottier D,
Le Gal G. Risk of recurrent venous thromboembolism on progestin-only
contraception: a cohort study. Haematologica. 2016;101:e12-e14. https://doi.org/10.3324/haematol.2015.134882 PMid:26452982 PMCid:PMC4697900
- Brunson
A, Lei A, Rosenberg AS, White RH, Keegan T, Wun T. Increased incidence
of VTE in sickle cell disease patients: risk factors, recurrence and
impact on mortality. Br J Haematol. 2017;178:319-326. https://doi.org/10.1111/bjh.14655 PMid:28369826
- Ugochi
O, Ogu UO, Billet HH. Comorbidities in sickle cell disease: Adult
providers needed! Indian J Med Res. 2018; 147: 527-529. https://doi.org/10.4103/ijmr.IJMR_1019_18 PMid:30168482 PMCid:PMC6118144
- Lee
MG, Thirumalai CH, Terry SI, Serjeant GR. Endoscopic and gastric acid
studies in homozygous sickle cell disease and upper abdominal pain.
Gut.1989; 30: 569-572. https://doi.org/10.1136/gut.30.5.569 PMid:2731748 PMCid:PMC1434224
- Gage TP, Gagnier JM. Ischemic colitis complicating sickle cell crisis. Gastroenterology.1983; 84:171-174. https://doi.org/10.1016/S0016-5085(83)80183-8
- Kapp N. WHO provider brief on hormonal contraception and liver disease. Contraception. 2009;80:325-326, https://doi.org/10.1016/j.contraception.2009.01.020 PMid:19751854
- Ledda
C, Loreto C, Zammit C, Marconi A, Fago L, Matera S, Costanzo V, Fuccio
Sanzà G, Palmucci S, Ferrante M, Costa C, Fenga C, Biondi A, Pomara C,
Rapisarda V. Non-infective occupational risk factors for hepatocellular
carcinoma: a review. Mol Med Rep. 2017;15:511-533, https://doi.org/10.3892/mmr.2016.6046 PMid:28000892 PMCid:PMC5364850
- Björnsson
ES. Drug-induced cholestasis. In: Carey EJ, Lindor KD, editors.
Cholestatic liver disease, clinical gastroenterology. pag. 13. https://doi.org/10.1007/978-1-4939-1013-7_2
- Guillebeaud J. Contraception today. 8th edition CRC Press; 2016. https://doi.org/10.1201/b19127 PMCid:PMC4858975
- Römer T. Medical eligibility for contraception in women at increased risk. Dtsch Arztebl Int. 2019; 116: 764-774. https://doi.org/10.3238/arztebl.2019.0764 PMid:31776000 PMCid:PMC6916704
- McLaughlin
BN, Martin RW, Morrison JC. Clinical management of sickle cell
hemoglobinopathies during pregnancy. Clin Perinatol. 1985;12:585-597. https://doi.org/10.1016/S0095-5108(18)30857-1
- Koshy M, Burd L. Management of pregnancy in sickle cell syndromes. Hematol Oncol Clin North Am.1991;5:585-596. https://doi.org/10.1016/S0889-8588(18)30433-7
- Koshy
M, Burd L, Wallace D, Moawad A, Baron J. Prophylactic red-cell
transfusions in pregnant patients with sickle cell disease. A
randomized cooperative study. N Engl J Med. 1988;319:1447-1452. https://doi.org/10.1056/NEJM198812013192204 PMid:3054555
- Mikobi
TM, Lukusa PT, Muamba JMM, Rhama T. Homozygous deletion
alpha-thalassemia and hereditary persistence of fetal hemoglobin, two
genetic factors
predictive the reduction of morbidity and mortality during pregnancy in
sickle cell patients. a report from the democratic republic of congo.
Mediterr J Hematol Infect Dis 2019, 11(1): e2019039, https://doi.org/10.4084/mjhid.2019.039 PMid:31308915 PMCid:PMC6613621
- Rogers DT, Molokie R. Sickle cell disease in pregnancy. Obstet Gynecol Clin North Am. 2010;37:223-237. https://doi.org/10.1016/j.ogc.2010.02.015 PMid:20685550
- Royal
College of Obstetricians and Gynaecologists. Management of Sickle Cell
Disease in Pregnancy. RCOG Green-top Guideline. 2011;61:1-20.
- Howard RJ. Management of sickling conditions in pregnancy. Br J Hosp Med. 1996;56:7-10.
- Villers
MS, Jamison MG, De Castro LM, James AH. Morbidity associated with
sickle cell disease in pregnancy. Am J Obstet Gynecol.
2008;199(2):125.e1-125.e1255. doi:10.1016/j.ajog.2008.04.016. https://doi.org/10.1016/j.ajog.2008.04.016 PMid:18533123
- Chou
ST. Transfusion therapy for sickle cell disease: a balancing act.
Hematology Am Soc Hematol Educ Program. 2013;2013:439-446. https://doi.org/10.1182/asheducation-2013.1.439 PMid:24319217
- Klings
ES, Kato GJ, Gladwin MT. Management of patients with sickle cell
disease. JAMA. 2015; 313(1):91.doi:10.1001/jama.2014.15898. https://doi.org/10.1001/jama.2014.15898 PMid:25562274 PMCid:PMC4896487
- Halsey C, Roberts IAG. The role of hydroxyurea in sickle cell disease. Br J Haematol. 2003;120:177-186. https://doi.org/10.1046/j.1365-2141.2003.03849.x PMid:12542474
- Ballas
SK, McCarthy WF, Guo N, DeCastro L, Bellevue R, Barton BA, Waclawiw AM,
Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Exposure to
hydroxyurea and pregnancy outcomes in patients with sickle cell anemia.
J Natl Med Assoc. 2009;101:1046-1051. https://doi.org/10.1016/S0027-9684(15)31072-5
- Scott JP. Hydroxurea and sickle cell disease: Its been a long, long time coming. Pediatr Blood Cancer. 2010;54: 185-186. https://doi.org/10.1002/pbc.22340 PMid:19908298
- Andemariam B, Browning SL. Current management of sickle cell disease in pregnancy. Clin Lab Med. 2013; 33:293-310. https://doi.org/10.1016/j.cll.2013.03.023 PMid:23702119
- Chaube S, Murphy ML. The effects of hydroxyurea and related compounds on the rat foetus. Cancer Res. 1996; 20:1448-1457.
- Gwer
SO, Onyango KO. Prevalence and incidence of congenital anomalies
amongst babies born to women with sickle cell disease and exposed to
hydroxyurea during pregnancy: a systematic review protocol. JBI
Database System Rev Implement Rep. 2018;16:1135-1140. https://doi.org/10.11124/JBISRIR-2017-003548 PMid:29762306
- Diav-Citrin
O, Hunnisett L, Sher GD, Koren G. Hydroxyurea use during pregnancy: a
case report in sickle cell disease and review of the literature. Am J
Hematol. 1999; 60:148-150. https://doi.org/10.1002/(SICI)1096-8652(199902)60:2<148::AID-AJH12>3.0.CO;2-I
- Whaley NS, Lanzkron S, Burke A. Contraceptive in Women with Sickle Cell Disease: A Survey Study. Blood.2015;126:3263. https://doi.org/10.1182/blood.V126.23.3263.3263
[TOP]





