Yusuke Ito1, Kensuke Takaoka1, Kazuhiro Toyama1, Kazuki Taoka1, Yoshitaka Wakabayashi2, Aya Shinozaki-Ushiku3, Aiko Okazaki2, Kinuyo Chikamatsu4, Satoshi Mitarai4, Tetsuo Ushiku3 and Mineo Kurokawa1,5.
1 Department of Hematology and Oncology, Graduate School of Medicine, The University of Tokyo.
2 Department of Infectious Diseases, The University of Tokyo Hospital.
3 Department of Pathology, Graduate School of Medicine, The University of Tokyo.
4 Department of Mycobacterium Reference and Research, Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association.
5 Department of Cell Therapy and Transplantation Medicine, The University of Tokyo Hospital.
Correspondience to: Mineo Kurokawa, Professor.
Address: 7-3-1 Hongo, Bunkyo-City Tokyo, 113-8655 Japan. Tel:
+81-3-5800-9045, Fax: +81-3-5800-9045. E-mail:
kurokawa-tky@umin.ac.jp
Published: July 1, 2020
Received: February 14, 2020
Accepted: June 2, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020035 DOI
10.4084/MJHID.2020.035
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract This is the first case of concurrent Mycobacterium genavense
lymphadenitis and Epstein-Barr virus (EBV)-positive lymphoproliferative
disorder (LPD) in the same lymph node with no immunocompromised
history. M. genavense
infection is a rare opportunistic infection mainly for human
immunodeficiency virus (HIV)-infected patients. Although no
immunodeficiency was detected in our patient, our case indicates that
the immunodeficiency in the background of EBV latency type III and the
immunosuppression by malignant lymphoma itself might induce the M. genavense
lymphadenitis. This case highly alerts clinicians to the
immunosuppressive state of EBV-positive LPD with latency type III even
if any immunodeficient serological factors are not detected.
|
Introduction
Mycobacterium genavense
is a non-tuberculous mycobacterium (NTM), first isolated from 18
HIV-infected patients with CD4-positive T cell counts below 100 /μL in
1992. [1] The infection against non-HIV patients is extraordinarily unusual, and only 46 cases have been reported.[2]
Most cases are immunocompromised hosts, and the common underlying
complications are solid organ transplantation, sarcoidosis, and
hematopoietic stem cell transplantation. As for the relation with
malignant lymphoma, three cases have been reported, and all developed
the infection under the immunocompromised conditions due to
chemotherapy or immunosuppressive agents. Herein, we report the first
non-HIV case of concurrent M. genavense
lymphadenitis and Epstein-Barr virus (EBV)-positive lymphoproliferative
disorder (LPD) with no apparent immunocompromised history.
Case Report
The
patient was a 53-year-old male with no significant past medical
history. Since December 2017, the fever up to 40℃ emerged
intermittently, followed by weight loss and right inguinal
lymphadenopathy. In February 2018, a CT scan showed multiple subphrenic
lymphadenopathies. A blood culture detected the bloodstream infection
of methicillin-resistant Staphylococcus aureus
(MRSA), and a gastrointestinal endoscopy revealed the widespread
esophageal candidiasis. In March, he was complicated by herpes zoster
infection. The right inguinal lymph node biopsy showed mycobacterium
infection with malignant lymphoma, and he was transferred to our
hospital.
On admission, laboratory data showed a white blood cell
count of 14,400 /μL (band cell 3.0%, segmented cell 81.0%, monocyte
8.5%, lymphocyte 7.5%), hemoglobin level of 9.0 g/dL, platelet count of
18.3 x 104 /μL, CD4-positive T cell
count of 678 /μL (50.3% of T cells), aspartate transaminase (AST) of 16
U/L, alanine aminotransferase (ALT) of 15 U/L, blood urea nitrogen
(BUN) of 5.3 mg/dL, creatine of 0.60 mg/dL, C-reactive protein (CRP) of
26.52 mg/dL, immunoglobulin G of 1764 mg/dL, and soluble IL-2R of
16,523 U/mL. HIV antibody, HTLV-1 antibody, mycobacterium avium
complex (MAC) antibody, candida antigen, aspergillus antigen and
Interferon-Gamma release assay were negative. Polymerase chain reaction
(PCR) assays for the detection of clonally rearranged T cell receptors
in the peripheral blood showed no clonality,[3] and
lymphocyte blastoid transformation test by phytohemagglutinin (PHA) was
29,300 count per minute (cpm) (normal range: 20,500-56,800 cpm), which
suggested no apparent T cell dysfunction.
PET-CT demonstrated multiple enlargements of subphrenic lymph nodes (SUVmax 11.1 in the right inguinal lymph node) (Figure 1a-b).
The histopathological examination of the right inguinal lymph node
biopsy showed the destruction of normal structure and the mixture of
the proliferation of abnormal large lymphoma cells and epithelioid cell
granuloma. With small T cells and histiocytes as a background, Hodgkin
cells, Reed-Sternberg cells and Lacunar cells invaded. These malignant
cells were positive for CD30 and PD-L1, partially positive for CD15,
and negative for CD3, CD4, CD8, and CD20 in immunohistochemistry.
EBER-ISH was positive, and LMP-1 and EBNA-2 were also partially
positive, which suggested EBV infection with latency type III (Figure 2a-e).
This case showed more atypical and various cell appearance than Hodgkin
lymphoma (HL). EBV-associated HL typically shows EBV infection with
latency type II. Based on these pathological findings, EBV-positive LPD
with Hodgkin lymphoma-like features was diagnosed.
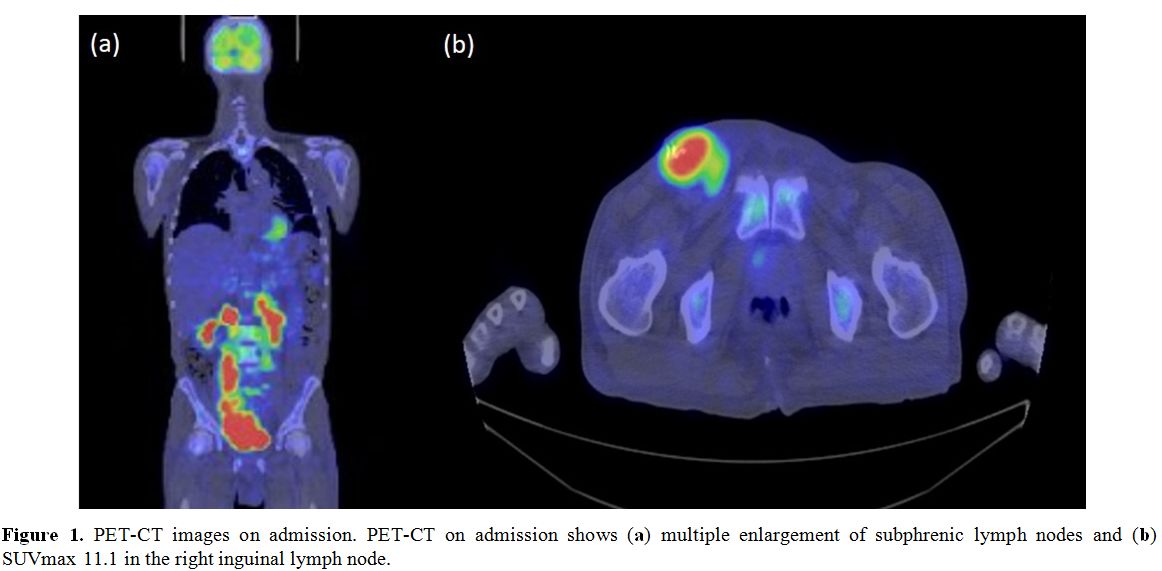 |
Figure 1. PET-CT images on admission. PET-CT on admission shows (a) multiple enlargement of subphrenic lymph nodes and (b) SUVmax 11.1 in the right inguinal lymph node. |
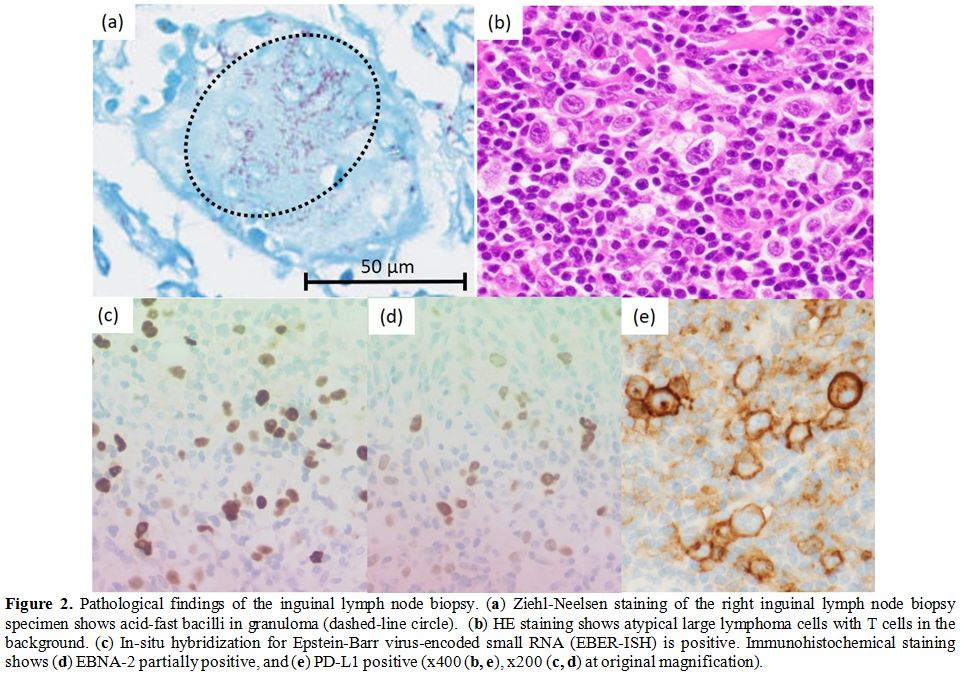 |
Figure 2. Pathological findings of the inguinal lymph node biopsy. (a)
Ziehl-Neelsen staining of the right inguinal lymph node biopsy specimen
shows acid-fast bacilli in granuloma (dashed-line circle). (b) HE staining shows atypical large lymphoma cells with T cells in the background. (c) In-situ hybridization for Epstein-Barr virus-encoded small RNA (EBER-ISH) is positive. Immunohistochemical staining shows (d) EBNA-2 partially positive, and (e) PD-L1 positive (x400 (b, e), x200 (c, d) at original magnification). |
PCR tests of the right inguinal lymph node were negative for Mycobacterium tuberculosis
and MAC, and culture tests of bacteria, fungi, and mycobacterium
species were also negative. However, Ziehl-Neelsen staining of the
biopsy specimen showed acid-fast bacilli in granulomas (Figure 2a). In PCR, we revealed 100% sequence identity of both 16s ribosomal RNA and heat shock protein 65 (hsp65) of M. genavense,[4] targeting 710 base pair (bp) sequences out of 1500 bp and 361 bp sequences out of 1623 bp respectively. The detection of M. genavense infection by culture is troublesome due to its fastidious growth requirements;[2] therefore, negative culture result cannot exclude M. genavense infection. Consequently, EBV-positive LPD and M. genavense lymphadenitis were concomitantly diagnosed. We treated him with rifampicin, ethambutol and clarithromycin against M. genavense,[5]
and adriamycin, vinblastine and dacarbazine for EBV-positive LPD. We
excluded bleomycin due to emphysema. Although fever and lymphadenopathy
promptly subsided with these double therapies, PET-CT after six cycles
showed multiple lymphadenopathies. The right inguinal lymph node
re-biopsy demonstrated the relapse of EBV-positive LPD with no signs of
mycobacterium infection. We started salvage chemotherapy and continued
triplet antibiotics. The optimal treatment duration against M. genavense remains unclear, and we continued the triplet therapy for more than one year.[2]
We stopped the triplet antibiotics after 17 months' duration, and
subsequently, the patient has had NTM free follow-up for 14 months.
Discussion
Our case is the first case with concomitant M. genavense lymphadenitis and malignant lymphoma in the same lymph node. Mycobacterium genavense
is a rare pathogen named after Geneva, which was first reported in a
series of 18 patients with acquired immune deficiency syndrome (AIDS).[1] M. genavense
infection used to be an opportunistic infectious disease for
HIV-infected patients with CD4-positive T cell counts less than 100/μL.[1] However, 46 non-HIV cases have been reported.[2]
Most of them were immunocompromised hosts, and the common underlying
conditions were solid organ transplantation (40%), sarcoidosis (14%),
autoimmune diseases (13%) and hematopoietic stem cell transplantation
(7%). 60% were on at least two immunosuppressants, and the median
CD4-positive T cell counts were 105/μL. The main symptoms were weight
loss, fever, lymphadenopathy and hepatosplenomegaly, which were similar
to those of malignant lymphoma.
Three cases reported the relation between M. genavense infection and lymphoma (Table 1A). An 80-year-old female patient with chronic lymphocytic leukemia,[6] a 51-year-old female patient with peripheral T cell lymphoma,[7] and a 63-year-old male patient with non-Hodgkin lymphoma (NHL)[8] caused M. genavense infection. All cases were under chemotherapy or immunosuppressive therapy when M. genavense infection was detected; thus, the situation is different from our patient with concurrent M. genavense infection and EBV positive LPD with no immunosuppressive therapy.
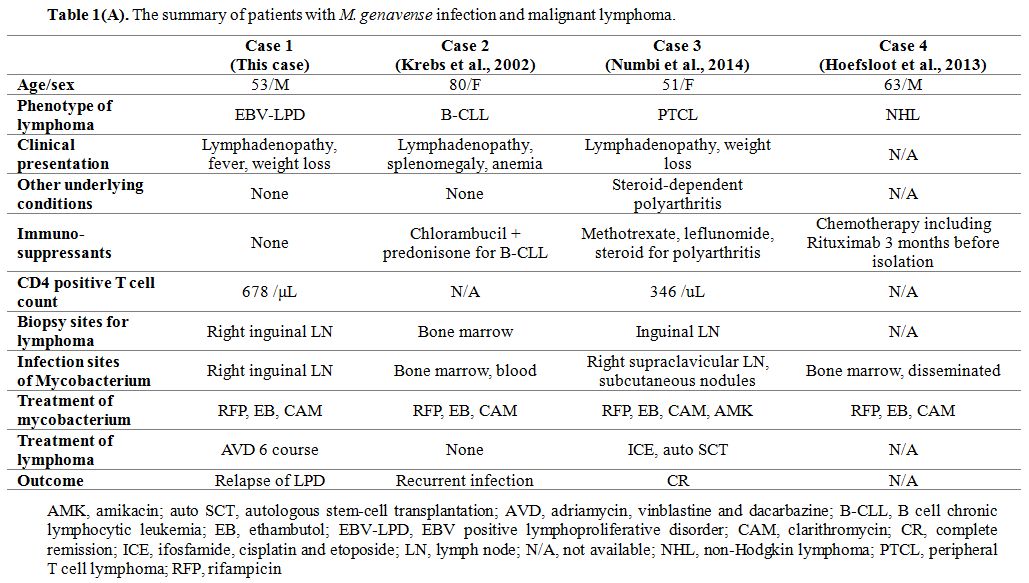 |
Table 1(A). The summary of patients with M. genavense infection and malignant lymphoma. |
Meanwhile, a simultaneous diagnosis of NTM infection and malignant lymphoma has been reported in four cases (Table 1B). Two of them were patients with AIDS, a 27-year-old male patient with MAC infection and HL,[9] and a 31-year-old male patient with NTM infection and NHL.[10]
Since NTM infection and malignant lymphoma are both included in
AIDS-defining diseases, the possibility of simultaneous onset may be
relatively high in AIDS patients. The other two cases were a
13-year-old male with M. avium infection and HL,[11] and a 5-year-old male with MAC infection and HL.[12]
These cases were compatible with the evidence that NTM lymphadenitis
has mainly occurred in children, and MAC accounts for 80-90%.[13] Consequently, our patient is the first adult non-HIV case with concomitant NTM lymphadenitis and lymphoma.
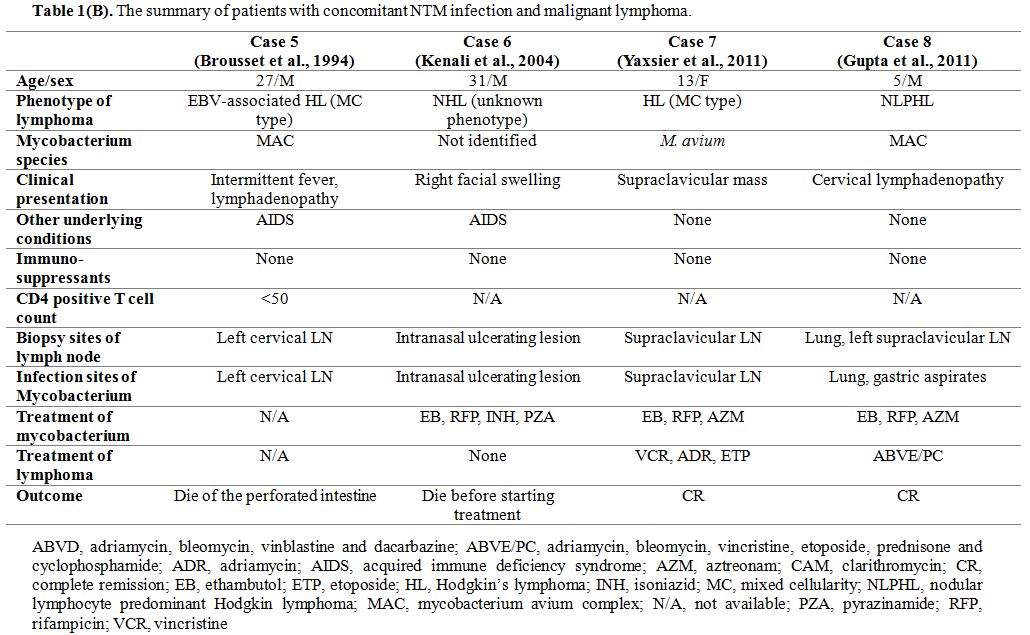 |
Table 1(B). The summary of patients with concomitant NTM infection and malignant lymphoma. |
This
patient presents with an EBV latency type III. It is typically observed
in immunodeficiency-associated LPD and a part of EBV-positive diffuse
large B cell lymphoma (DLBCL), not otherwise specified (NOS), which
indicates the highly immunodeficient background.[14] Furthermore, this patient suffered from M. genavense
lymphadenitis, MRSA bacteremia, widespread esophageal candidiasis, and
herpes zoster infection. These bacterial, fungal, and viral infections
further suggest an immunocompromised condition. However, this case did
not have primary immune disorders, HIV infection, or another iatrogenic
immunodeficiency, or pathological features of DLBCL. White blood count,
CD4-positive T cell count, and immunoglobulin levels were normal. T
cell receptors in the peripheral blood were polyclonal, and the
lymphocyte blastoid transformation test by phytohemagglutinin (PHA) was
normal, which suggested no apparent T cell dysfunction. Mycobacterial,
fungal, and viral infections can be caused by monocytopenia and
mycobacterial infection (MonoMAC) syndrome.[15]
However, the differential blood count, including the monocyte count of
this patient was normal, which exclude the possibility of MonoMAC
syndrome. Furthermore, we analyzed the sequence of GATA binding protein
2 (GATA2) using DNA extracted
from peripheral blood and found a single-nucleotide polymorphism c.490
G>A (p.A164T) and a silent mutation c.15 C>G. In addition, our
case did not have the age like suffering from severe immunosenescence,
which is critical for the pathogenesis of EBV-positive DLBCL, NOS.[14] Based on these results, no immunodeficiency could be detected in our patient.
Patients with HL are often complicated with tuberculosis.[16] HL cells are known to highly express PD-L1 and cause intratumoral T cell exhaustion, leading to T cell dysfunction.[17]
Generally, high PD-L1 expression on malignant lymphoma cells is due to
either the amplification of the PD-L1 locus on chromosome 9p24.1, which
is a recurrent abnormality seen in HL, or EBV infection.[18] EBV infection upregulates PD-L1 expression via EBNA2, the characteristic of EBV latency type III.[18]
In our case, EBNA2 induced PD-L1 expression on the lymphoma cells and
might activate PD-1/PD-L1 signaling on the surrounding T cells. Immune
checkpoint players such as PD-1, cytotoxic T lymphocyte antigen 4
(CTLA-4), and T cell immunoglobulin and mucin domain-containing
molecule 3 (TIM-3) have been well known for the role of not only cancer
immune escape but also immunosuppression during chronic infection.[19,20] For example, during chronic Mycobacterium tuberculosis infection, T cells express multiple inhibitory receptors, including PD-1 and TIM-3, which cause T cell exhaustion.[21] It promotes impairment of T cell function and impairs host resistance to M. tuberculosis.[21]
These reports suggest that T cell exhaustion may induce the
exacerbation of infections against mycobacterium species. Therefore,
the immunosuppressive effect through the PD-1/PD-L1 axis might promote
the simultaneous M. genavense
infection in our case. Consequently, our case indicates that the
immunodeficiency in the background of EBV latency type III and the
immunosuppression by malignant lymphoma itself might induce the M. genavense
lymphadenitis and other bacterial, fungal, and viral infections. Our
case highly alerts clinicians of the immunosuppressive state of
EBV-positive LPD with latency type III even if any immunodeficient
serological factors are not detected.
Conclusions
This is the first case of simultaneously diagnosed M. genavense
lymphadenitis and EBV-positive LPD with no immunocompromised history.
As patients with EBV-positive LPD with latency type III may be highly
susceptible to mycobacterium species and other opportunistic
infections, there should be increased awareness of their marked
immunocompromised condition regardless of the existence of any
immunodeficient serological findings.
References
- Böttger EC, Teske A, Kirschner P, Bost S, Chang HR,
Beer V, Hirschel B. Disseminated "Mycobacterium genavense" infection in
patients with AIDS. Lancet 1992;340:76-80 https://doi.org/10.1016/0140-6736(92)90397-L
- Mahmood
M, Ajmal S, Abu Saleh OM, Bryson A, Marcelin JR, Wilson JW.
Mycobacterium genavense infections in non-HIV immunocompromised hosts:
a systematic review. Infect Dis. 2018;50:329-39 https://doi.org/10.1080/23744235.2017.1404630 PMid:29157060
- van
Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL,
Delabesse E, Davi F, Schuuring E, García-Sanz R, van Krieken JH, Droese
J, González D, Bastard C, White HE, Spaargaren M, González M, Parreira
A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and
standardization of PCR primers and protocols for detection of clonal
immunoglobulin and T-cell receptor gene recombinations in suspect
lymphoproliferations: report of the BIOMED-2 Concerted Action
BMH4-CT98-3936. Leukemia 2003;17:2257-2317 https://doi.org/10.1038/sj.leu.2403202 PMid:14671650
- Pai
S, Esen N, Pan X, Musser JM. Routine rapid Mycobacterium species
assignment based on species-specific allelic variation in the
65-kilodalton heat shock protein gene (hsp65). Arch Pathol Lab Med.
1997;121:859-64
- Ombelet S, Van
Wijngaerden E, Lagrou K, Tousseyn T, Gheysens O, Droogne W, Doubel P,
Kuypers D, Claes KJ. Mycobacterium genavense infection in a solid organ
recipient: a diagnostic and therapeutic challenge. Transpl Infect Dis.
2016;18:125-31 https://doi.org/10.1111/tid.12493 PMid:26688125
- Krebs
T, Zimmerli S, Bodmer T, Lämmle B. Mycobacterium genavense infection in
a patient with long-standing chronic lymphocytic leukaemia. J Intern
Med.2000;248:343-8 https://doi.org/10.1046/j.1365-2796.2000.00730.x PMid:11086646
- Numbi
N, Demeure F, Van Bleyenbergh P, De Visscher N. Disseminated
Mycobacterium genavense infection in a patient with immunosuppressive
therapy and lymphoproliferative malignancy. Acta Clin Belg.
2014;69:142-5 https://doi.org/10.1179/0001551213Z.00000000016 PMid:24724760
- Hoefsloot
W, van Ingen J, Peters EJ, Magis-Escurra C, Dekhuijzen PN, Boeree MJ,
van Soolingen D. Mycobacterium genavense in the Netherlands: an
opportunistic pathogen in HIV and non-HIV immunocompromised patients.
An observational study in 14 cases. Clin Microbiol Infect.
2013;19:432-7 https://doi.org/10.1111/j.1469-0691.2012.03817.x PMid:22439918
- Brousset
P, Marchou B, Chittal SM, Delsol G. Concomitant Mycobacterium avium
complex infection and Epstein-Barr virus associated Hodgkin's disease
in a lymph node from a patient with AIDS. Histopathology 1994;24:586-8 https://doi.org/10.1111/j.1365-2559.1994.tb00583.x PMid:8063291
- Kenali
MS, Fadzilah I, Maizaton AA, Sani A. Concurrent mycobacterial infection
and non-Hodgkin's lymphoma at the same site in an AIDS patient. Med J
Malaysia. 2004;59:108-11
- de Armas Y,
Capó V, González I, Mederos L, Díaz R, de Waard JH, Rodríguez A, García
Y, Cabanas R. Concomitant Mycobacterium avium Infection and Hodgkin's
Disease in a Lymph Node from an HIV-negative Child. Pathol Oncol Res.
2011;17:139-140 https://doi.org/10.1007/s12253-010-9275-5 PMid:20467849
- Gupta
S, Cogbill CH, Gheorghe G, Rao AR, Kumar S, Havens PL, Camitta BM,
Warwick AB. Mycobacterium avium intracellulare Infection Coexistent
With Nodular Lymphocyte Predominant Hodgkin Lymphoma Involving the
Lung. J Pediatr Hematol Oncol. 2011;33:e127-31 https://doi.org/10.1097/MPH.0b013e3181faf89a PMid:21399527
- Garcia-Marcos
PW, Plaza-Fornieles M, Menasalvas-Ruiz A, Ruiz-Pruneda R, Paredes-Reyes
P, Miguelez SA. Risk factors of non-tuberculous mycobacterial
lymphadenitis in children: a case-control study. Eur J Pediatr.
2017;176:607-13 https://doi.org/10.1007/s00431-017-2882-3 PMid:28265761
- Castillo
JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM.
EBV-positive diffuse large B-cell lymphoma, not otherwise specified:
2018 update on diagnosis, risk-stratification and management. Am J
Hematol. 2018;93:953-62 https://doi.org/10.1002/ajh.25112 PMid:29984868
- Hsu
AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, Frucht DM, Vinh
DC, Auth RD, Freeman AF, Olivier KN, Uzel G, Zerbe CS, Spalding C,
Pittaluga S, Raffeld M, Kuhns DB, Ding L, Paulson ML, Marciano BE,
Gea-Banacloche JC, Orange JS, Cuellar-Rodriguez J, Hickstein DD,
Holland SM. Mutations in GATA2 are associated with the autosomal
dominant and sporadic monocytopenia and mycobacterial infection
(MonoMAC) syndrome. Blood. 2011;118:2653-5 https://doi.org/10.1182/blood-2011-05-356352 PMid:21670465 PMCid:PMC3172785
- Harris
J, Alexanian R, Hersh EM, Leary W. Hodgkin's Disease Complicated by
Infection with Mycobacterium kansasii. Can Med Assoc J. 1969;101:231-4
- Ansell
SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster
SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH,
Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1
Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N
Engl J Med. 2014;372:311-9 https://doi.org/10.1056/NEJMoa1411087 PMid:25482239 PMCid:PMC4348009
- Anastasiadou
E, Stroopinsky D, Alimperti S, Jiao AL, Pyzer AR, Cippitelli C, Pepe G,
Severa M, Rosenblatt J, Etna MP, Rieger S, Kempkes B, Coccia EM, Sui
SJH, Chen CS, Uccini S, Avigan D, Faggioni A, Trivedi P, Slack FJ.
Epstein−Barr virus-encoded EBNA2 alters immune checkpoint PD-L1
expression by downregulating miR-34a in B-cell lymphomas. Leukemia
2019;33:132-47 https://doi.org/10.1038/s41375-018-0178-x PMid:29946193 PMCid:PMC6327052
- Lutzky
VP, Ratnatunga CN, Smith DJ, Kupz A, Doolan DL, Reid DW, Thomson RM,
Bell SC, Miles JJ. Anomalies in T Cell Function Are Associated With
Individuals at Risk of Mycobacterium abscessus Complex Infection. Front
Immunol 2018;9:1319 https://doi.org/10.3389/fimmu.2018.01319 PMid:29942313 PMCid:PMC6004551
- Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97-111 https://doi.org/10.1111/imr.12520 PMid:28258697 PMCid:PMC5512889
- Jayaraman
P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, Anderson AC,
Kuchroo VK, Behar SM. TIM3 Mediates T Cell Exhaustion during
Mycobacterium tuberculosis Infection. PLoS Pathog. 2016;12:e1005490 https://doi.org/10.1371/journal.ppat.1005490 PMid:26967901 PMCid:PMC4788425
[TOP]



