Ginevra Lolli1, Beatrice Casadei1, Lisa Argnani1, Laura Nanni1, Michele Cavo1 and Pier Luigi Zinzani1
1 Institute of Hematology “L. e A. Seràgnoli”, University of Bologna, Bologna, Italy.
Correspondence to: Pier Luigi Zinzani, Institute of Hematology “Lorenzo
e Ariosto Seràgnoli”, University of Bologna, Via Massarenti, 9- 40138
Bologna, Italy. Tel +39 051 214 3680. Fax +39 051 636 4037. Email:
pierluigi.zinzani@unibo.it
Published: July 1, 2020
Received: April 29, 2020
Accepted: June 8, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020040 DOI
10.4084/MJHID.2020.040
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Sézary syndrome (SS) is a rare lymphoproliferative neoplasm, almost
incurable outside the setting of allogeneic transplantable patients.
The prognosis for relapsed/refractory patients remains poor, as the
available drugs confer short-lasting remission. In this setting, the
anti-chemokine receptor type 4 (CCR4) monoclonal antibody mogamulizumab
demonstrated efficacy in an international, open-label, randomized
controlled phase 3 trial (MAVORIC) versus vorinostat.
Case description:
A heavily pretreated 57-year-old SS woman (stage IVA) was randomized in
the mogamulizumab arm of MAVORIC at our Institution. She quickly
achieved a response, but after 30 cycles, she was discontinued from
therapy due to cutaneous toxicity. Nevertheless, she is still in
complete response (CR).
Conclusions:
mogamulizumab is an anti-CCR4 monoclonal antibody that can induce
long-lasting response also in very heavily pretreated patients not
responding to any previous treatment. The extraordinary characteristic
of our patient is that she is still in CR after 2.5 years since
treatment discontinuation.
|
Introduction
Sezàry
syndrome (SS) is a rare, aggressive leukemic variant of cutaneous
T-cell lymphomas with a 5-year overall survival (OS) of 26%.[1,2]
Outside the setting of allogeneic transplantation, SS is considered
incurable and requires chronic therapy because a relapse occurs shortly
after treatment discontinuation.[3] The prognosis for
relapsed/refractory patients is poor, with a low response rate and
short remission duration. There are some therapeutic options
(alemtuzumab, vorinostat, brentuximab vedotin) but no standard of care.[4]
In this setting, patients can benefit from a new therapeutic approach:
mogamulizumab, a humanized, glycoengineered IgG1κ monoclonal antibody
directed against the chemokine receptor type 4 (CCR4). This drug
demonstrated an overall response rate (ORR) of 28% in cutaneous T-cell
lymphomas in an international, open-label, randomized, controlled phase
3 trial (MAVORIC, NCT01728805) versus vorinostat, with a peak of 37% in
SS.
Case Report
A 57-year-old woman was diagnosed with SS in stage IVA (T4NXM0B2) in 2011.[5]
This patient was treated in first-line with CHOP (cyclophosphamide,
doxorubicin, vincristine, prednisone every two weeks) for three cycles,
but this therapy was interrupted for intolerance and absence of
response. She subsequently underwent several treatments from May 2011
to April 2014: extracorporeal phototherapy plus bexarotene in 2011,
total skin external body irradiation in 2013, extracorporeal
phototherapy plus bexarotene again in 2014 and finally gemcitabine
(1000mg/m2) plus oxaliplatin (100 mg/m2)
for 11 cycles in 2015, with progressive disease after each of them.
Because of this reason, in July 2015, this patient was referred to our
Institution, where she was enrolled in the MAVORIC trial, and she was
randomized in the mogamulizumab arm. She was erythrodermic and
symptomatic for intense pruritus and skin exfoliation (Figures 1A and 1B).
The patient received mogamulizumab 1mg/kg once a week for the first
cycle, in a 28-days cycle, and then a dosage of 1mg/kg every two weeks.
The treatment per protocol was intended until progression.
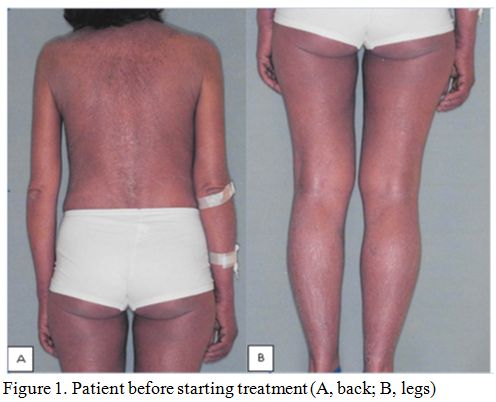 |
Figure 1. Patient before starting treatment (A, back; B, legs). |
Our patient obtained
an impressively rapid improvement of symptoms already from the third
cycle, while a partial response (PR) was achieved after the fifth
cycle. A complete response (CR) was documented after the 10th cycle (Figures 2A and 2B).[5] Therapy was well tolerated and went on without complications until the 27th
cycle when the patient developed a plaque skin lesion in the zygomatic
area (without pruritus). In the suspect of disease relapse, a
punch-cutaneous biopsy was performed in September 2017 and then again
in October 2017 for the persistence of this lesion. Results of both
biopsies were consistent with a drug-related lesion, with no signs of
active disease. Our patient received 30 cycles of mogamulizumab
overall, then we decided to discontinue her from the treatment protocol
in October 2017, due to the persistence of this lesion compatible with
persistent grade 2 drug toxicity, histologically documented.
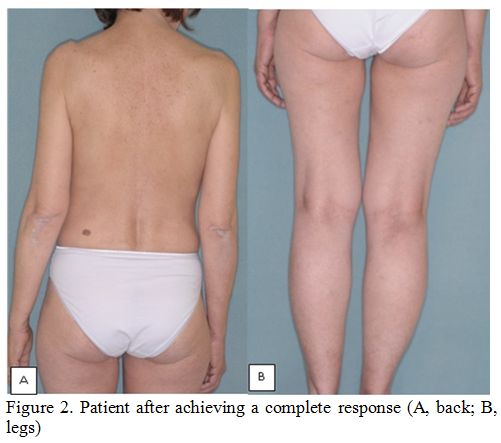 |
Figure 2. Patient after achieving a complete response (A, back; B, legs). |
After mogamulizumab
discontinuation, this patient was admitted to the follow-up phase. The
cutaneous zygomatic lesion quickly disappeared, and no further lesions
appeared after that. At the latest available follow-up, 2.5 years after
therapy discontinuation, she is still in CR without having undergone
further therapy after mogamulizumab.
The patient gave written, informed consent to publish her data and images.
Discussion
SS
is a challenging disease to face, but some new therapeutic options are
now available. Among them, we underline the role of the anti-CCR4
monoclonal antibody mogamulizumab. CCR4 is a receptor detectable in a
large group of patients with SS, and it plays a central role in the
T-lymphocytes homing and migration to the skin.[4,6,7]
CCR4 is also expressed on T regulatory cells (T-Regs), a subset of
lymphocytes involved in immunotolerance. The activity of mogamulizumab
against T-Regs is long-lasting, and it can lead to a loss of
immunotolerance. This aspect represents another important way of action
of the drug, and, in particular, the restoration of immunosurveillance
could explain why it is able to induce a long-lasting response, not
limited by the persistence of the drug.[8] In this
difficult-to-treat disease, the first-in-class anti-CCR4 antibody
mogamulizumab demonstrated an ORR of 37% in the MAVORIC trial, with a
median progression-free survival of 7.7 months, and a median duration
of remission of 17.3 months.[6] The brilliant results
of this trial led to mogamulizumab approval by FDA for mycosis
fungoides and SS relapsing after one or more lines of therapy in 2018.
Our
patient demonstrates that, in line with the data coming from MAVORIC,
mogamulizumab can induce good responses. These responses include few
CRs, also if very rare (only 5 out of 186 patients in MAVORIC), and the
time to achieve a response is quite short also in heavily pretreated
patients. In our case, a clinical response was achieved after the third
cycle, and a PR was documented after the fifth one, in line with the
median time to mogamulizumab response of 3.3 months.[6]
Another observation that our case suggests is that that this drug can
induce skin lesions to distinguish from those of the relapsing disease.
Unfortunately, we have no more tissue slides to perform the research of
T-regs depletion in our patient, nor the CCR4 mutational status.
Analysis of T-regs depletion and mutational status could be a very
stimulating starting point to perform new studies and to deepen
knowledge about the properties of mogamulizumab in patients who are
going to receive it in the future. Skin toxicity was durable and led to
treatment discontinuation in our patient, but this case report also
showed that this adverse event was reversible and did not invalidate
the response. To our knowledge, there is not an update about MAVORIC
after its publication in 2018; thus, we do not know if our patient is
the only one who achieved this extraordinary long-lasting response, or
if also other patients did. All the other patients enrolled in the
MAVORIC study at our Institution progressed and required subsequent
therapy.
Conclusion
Mogamulizumab
is an anti-CCR4 monoclonal antibody that can induce long lasting
response also in very heavily pretreated patients not responding to any
previous treatment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Cristofoletti C, Narducci MG, Russo G. Sézary Syndrome, recent biomarkers and new drugs. Chin Clin Oncol 2019;8:2. https://doi.org/10.21037/cco.2018.11.02 PMid:30525758
- Willemze
R, Hodak E, Zinzani PL, et al ESMO Guidelines Committee. Primary
cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol 2018;29:iv30-iv40. https://doi.org/10.1093/annonc/mdy133 PMid:29878045
- Campbell
J, Clark RA, Watanabe R, et al. Sézary syndrome and mycosis fungoides
arise from distinct T-cell subsets: a biologic rationale for their
distinct clinical behaviors. Blood 2010;116:767-71. https://doi.org/10.1182/blood-2009-11-251926 PMid:20484084 PMCid:PMC2918332
- Ollila
TA, Sahin I, Olszewski AJ. Mogamulizumab: a new tool for management of
cutaneous T-cell lymphoma. Onco Targets Ther 2019;12:1085-94. https://doi.org/10.2147/OTT.S165615 PMid:30799938 PMCid:PMC6369856
- Narducci
MG, Scala E, Bresin A, et al. Skin homing of Sezary cells involves
SDF-1-CXCR4 signaling and down-regulation of CD26/ dipeptidylpeptidase
IV. Blood 2006;107:1108-15. https://doi.org/10.1182/blood-2005-04-1492 PMid:16204308
- Kim
YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in
previously treated cutaneous T-cell lymphoma (MAVORIC): an
international, open-label, randomised, controlled phase 3 trial. Lancet
Oncol 2018;19:1192-204. https://doi.org/10.1016/S1470-2045(18)30379-6
- Olsen
EA, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and
classification of mycosis fungoides and Sézary syndrome: a proposal of
the International Society for Cutaneous Lymphomas (ISCL) and the
Cutaneous Lymphoma Task Force of the European Organization of Research
and Treatment of Cancer (EORTC). Blood 2007;110:1713-22. https://doi.org/10.1182/blood-2007-03-055749 PMid:17540844
- Kurose
K, Ohue Y, Wada H et al. Phase Ia study of FoxP3þ CD4 Treg depletion by
infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer
patients. Clin Cancer Res 2015;21:4327-36. https://doi.org/10.1158/1078-0432.CCR-15-0357 PMid:26429981
[TOP]

