Giacomo Marchi*1, Fabiana Busti*1, Acaynne Lira Zidanes1, Alice Vianello1 and Domenico Girelli1.
1 Department
of Medicine, Section of Internal Medicine, University of Verona,
EuroBloodNet Referral Center for Iron Metabolism Disorders, Azienda
Ospedaliera Universitaria Integrata Verona, 37138, Verona, Italy.
* Giacomo Marchi and Fabiana Busti equally contributed to the work.
Correspondence to: Fabiana Busti, Policlinico G. B. Rossi, P.le L.A.
Scuro 10, 37134 Verona (Italy). Tel. 0458128417; fax 0458027496.
E-mail:
fabiana.busti@univr.it
Published: July 1, 2020
Received: May 30, 2020
Accepted: May 5, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020043 DOI
10.4084/MJHID.2020.043
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Older
people are at risk for cobalamin (vitamin B12) deficiency because of a
number of common disorders (e.g., autoimmune gastritis) and drugs
(e.g., antacids) that may alter its absorption and utilization. The
prevalence of cobalamin deficiency increases with age, resulting,
particularly elevated, in frail and institutionalized subjects. At
variance with common sense, the diagnosis is far from simple. It
requires a high degree of suspicion, due to heterogeneity and
non-specificity of the signs and symptoms, ranging from macrocytosis
(with or without anemia) to neuropsychiatric manifestations, that
characterize several other aging-related disorders, like hematological
malignancies, diabetes, hypothyroidism or vasculopathy. Furthermore,
the detection of low levels of serum vitamin B12 appears poorly
sensitive and specific. Other biomarkers, like serum homocysteine or
methylmalonic acid, have improved the diagnostic possibilities but are
expensive, not widely available, and may be influenced by some
confounders (e.g., folate deficiency, or chronic renal failure). Early
recognition and treatment are crucial since a proportion of patients
develop severe complications, such as bone marrow failure and
irreversible neurological impairment. High-dose oral treatment has
proven to be as effective as the parenteral route, even in subjects
with malabsorption, ensuring the complete resolution in the majority of
cases. In this review, we trace the essential role of cobalamin in
humans, the possible causes and impact of deficiency, the diagnostic
challenges and the therapeutic options, between old and emerging
concepts, with a particular focus on the elderly.
|
Role of Cobalamin in Metabolic Processes
Vitamin
B12, also known as cobalamin (Cbl) is a complex water-soluble molecule,
containing a cobalt atom in the center of a tetrapyrrolic ring,
synthesized only by bacteria. Humans obtain Cbl from foods of animal
origin, such as meat, eggs, and dairy products. Body stores, primarily
located in the liver, usually contain about 2-5 mg of Cbl, with a daily
turnover of less than 0.1%. Deficiency manifests when stores drop below
300 µg, a process that may take several years.[1]
Cbl
is crucial for several metabolic functions, including cell
proliferation and survival, energy production, and nervous system
integrity, as it represents a pivotal cofactor for two[2] ubiquitously expressed enzymes, the cytosolic methionine-synthase, and the mitochondrial methylmalonyl coenzyme A mutase (Figure 1).
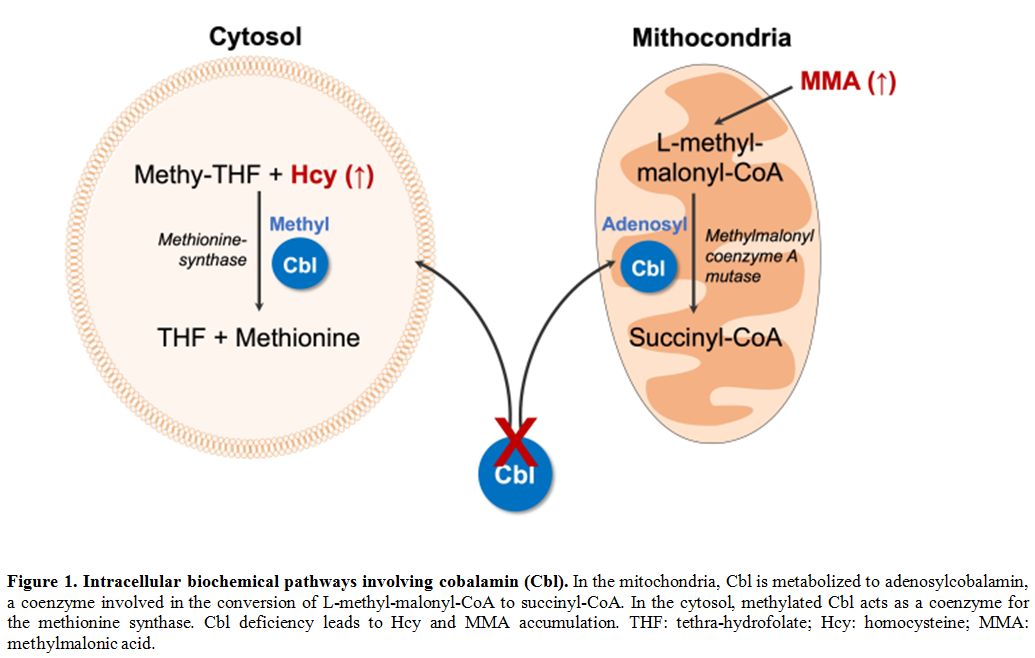 |
Figure 1. Intracellular biochemical pathways involving cobalamin (Cbl).
In the mitochondria, Cbl is metabolized to adenosylcobalamin, a
coenzyme involved in the conversion of L-methyl-malonyl-CoA to
succinyl-CoA. In the cytosol, methylated Cbl acts as a coenzyme for the
methionine synthase. Cbl deficiency leads to Hcy and MMA accumulation.
THF: tethra-hydrofolate; Hcy: homocysteine; MMA: methylmalonic acid. |
The
methionine-synthase catalyzes the conversion of methyl-tetrahydrofolate
(methyl-THF) and homocysteine (Hcy) into THF and methionine, a basilar
step toward DNA synthesis. The enzyme dysfunction is responsible for
the nucleus-cytoplasm maturation asynchrony affecting cells with an
elevated regenerative rate, predominantly the hematopoietic precursors
(leading to megaloblastic anemia), but also epithelial and mucous cells
(causing glossitis). Moreover, it causes a reduction of the
methionine-derived metabolite S-adenosylmethionine (SAM), required for
neurotransmitters and phospholipids synthesis, eventually compromising
cell membrane structure and fluidity, myelin formation, and
neurotransmission.[3]
The methylmalonyl coenzyme
A mutase (MUT) catalyzes the isomerization of L-methyl-malonyl-CoA in
succinyl-CoA, a key molecule in the tricarboxylic acid cycle, essential
for ATP generation, ketone bodies metabolism, myelinization, and heme
biosynthesis.
Prevalence of Cbl Deficiency (CblD) in the Elderly
Although it is known that Cbl levels tend to decline with advancing age,[4]
there are little data about the true prevalence of CblD in the elderly.
This is partly explained by the vast differences among subjects
included in epidemiological studies, varying for age, ethnicity, food
consumption (e.g., fortified or not), and comorbidities.
Further
uncertainties derive from the absence of "gold standard" tests and
cut-offs. Many studies, in fact, considered serum Cbl levels alone
(with different standard intervals), others utilized Cbl reduction in
combination with additional serum biomarkers, like homocysteine (Hcy)
and/or methylmalonic acid (MMA).
Currently, the estimated prevalence of CblD ranges from 4-5% in community-living elderly[5,6] to about 30-40% in institutionalized subjects with multiple comorbidities.[7] Among the latter, CblD was responsible for anemia in 4% of cases.[8] Since the presence of anemia or macrocytosis does not accurately predict CblD,[9] some Authors have advocated generalized biochemical screening for CblD in the aged population.[9,10]
Diagnosis of CblD
The diagnosis of CblD cannot be made through a single reliable laboratory test.[11]
Instead, it should be based on a thorough history, clinical
manifestations, and the combined use of multiple biochemical and
hematological indicators. In some cases, a trial with Cbl replacement
can be very useful, with the appearance of reticulocytosis and/or
improvement of neurological symptoms virtually confirming CblD.[1] Of note, peripheral blood smear is of great value as a first step in suggesting CblD and guiding differential diagnosis,[2] as it shows typical alterations of erythrocytes and neutrophils (see below).
The measurement of circulating Cbl
is often the first-line test to be performed. The reference intervals
vary among laboratories, but, in general, levels below 150 pmol/l (200
pg/ml) are consistent with deficiency, while levels above 300 pmol/l
(400 pg/ml) are considered normal. However, this test has reduced
sensitivity and specificity.[1] Diagnosis may be
missed in the presence of falsely normal circulating Cbl levels, as it
has been observed in ordinary conditions, such as chronic liver
diseases, myeloproliferative neoplasms, or in the presence of
anti-intrinsic factor antibodies.[1,12] Moreover, the assay measures the two endogenous forms of Cbl, holohaptocorrin, and the only biologically active holotranscobalamin
(HoloTC). A reduction in total Cbl levels may actually reflect a mere
impairment of holohaptocorrin synthesis (e.g., during cancer,
pregnancy, liver disease, and autoimmune disorders), with little (if
any) clinical significance.
Over the past 30 years, it has become evident that serum Hcy and MMA levels represent more sensitive and early indicators of CblD.[1,12]
Despite this, their use in clinical practice is hampered by scarce
availability, the lack of validated methods and thresholds, and
relatively higher costs. In addition, their levels tend to rise per se with aging.
In
the absence of impaired renal function, an elevation of MMA (>350
nmol/l) is the most specific biomarker. The Hcy increase (>15 µmol/l)
is sensitive but less specific, also rising in the case of folate
deficiency, B6 vitamin deficiency, hypothyroidism, and decreased GFR.
MMA and Hcy play a role in subjects with borderline Cbl values (i.e.,
150-300 pmol/l), or whenever there is a discrepancy between a clinical
picture suggesting CblD and apparently normal Cbl levels.[13,14]
Both can be helpful in confirming a true CblD after replacement
therapy, as they usually normalize within a week. MMA elevation has
also been associated with poorer functional outcomes in subjects with
reduced Cbl levels.[15]
Finally, the HoloTC assay has the best accuracy[16]
theoretically, but its clinical usefulness is precluded by the scarce
availability and the lack of reference values and standardization among
laboratories.
Recently, a combined index of the Cbl status (named
4cB12), based on Cbl, Hcy, MMA, and HoloTc levels, has been suggested
to improve the recognition of CblD, particularly in the early
subclinical stages.[17]
A possible algorithm for the diagnosis of CblD in the elderly is depicted in Figure 2.
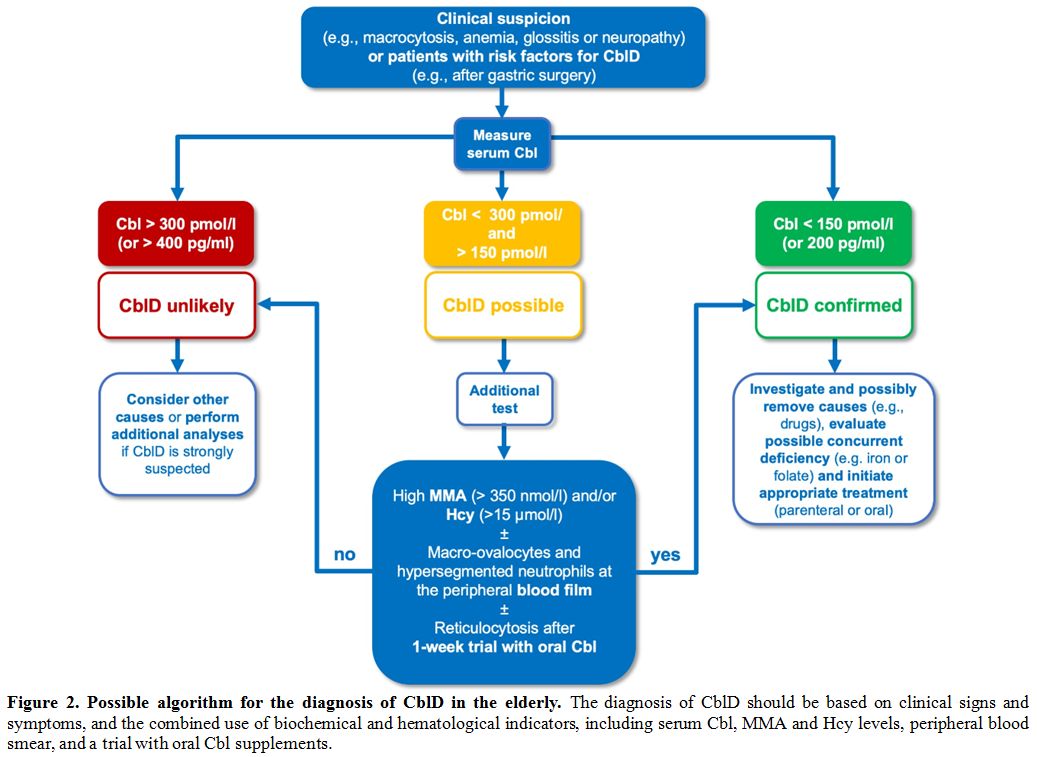 |
Figure 2. Possible algorithm for the diagnosis of CblD in the elderly.
The diagnosis of CblD should be based on clinical signs and symptoms,
and the combined use of biochemical and hematological indicators,
including serum Cbl, MMA and Hcy levels, peripheral blood smear, and a
trial with oral Cbl supplements. |
Causes of CblD
Cbl in food is protein-bound, and its acquisition depends on the function of the salivary glands, gastric, and ileal mucosa.[2]
Briefly, food Cbl is released in the stomach, in the presence of an
adequate acid pH, through the digestive action of pepsin. Cbl then
binds the salivary proteins haptocorrins (HC) and is conveyed in the
small intestine, where pancreatic proteases dissociate Cbl from HC.
Subsequently, Cbl forms a complex with the intrinsic factor (IF), a
protein secreted by gastric parietal cells. Such complex is absorbed
through a specific receptor (cubilin) expressed by enterocytes in the
terminal ileum and then released to plasma, where it is bound to a
family of transport proteins known as transcobalamins (TC). Finally,
Cbl enters the cells through an endocytosis process mediated by TC
receptors and is metabolized into adenosylcobalamin or methylcobalamin.[2]
Of
note, a relatively small fraction of ingested Cbl can be absorbed along
the entire intestine by passive diffusion, and that explains why
high-dose oral therapy may be effective even in people with
malabsorption.
Multiple conditions can interfere with the
complicated multi-step journey of Cbl from food to cells. The main
etiologies (summarized in Table 1)
comprise: 1) pernicious anemia (PA), 2) maldigestion (eventually
leading to the so-called "food-bound Cbl malabsorption," FBCM), 3)
ileum disorders, 4) insufficient intake, and 5) increased consumption.
PA and FBCM represent the primary causes in all age groups and are
particularly frequent in the elderly, while an insufficient dietary
intake is quite uncommon.[6] In addition to acquired
causes, sporadic congenital disorders (e.g., transcobalamin deficiency)
can lead to CblD, but they typically manifest in newborns and are not
relevant in the elderly. On the other hand, CblD in older people is
frequently multi-factorial.[12]
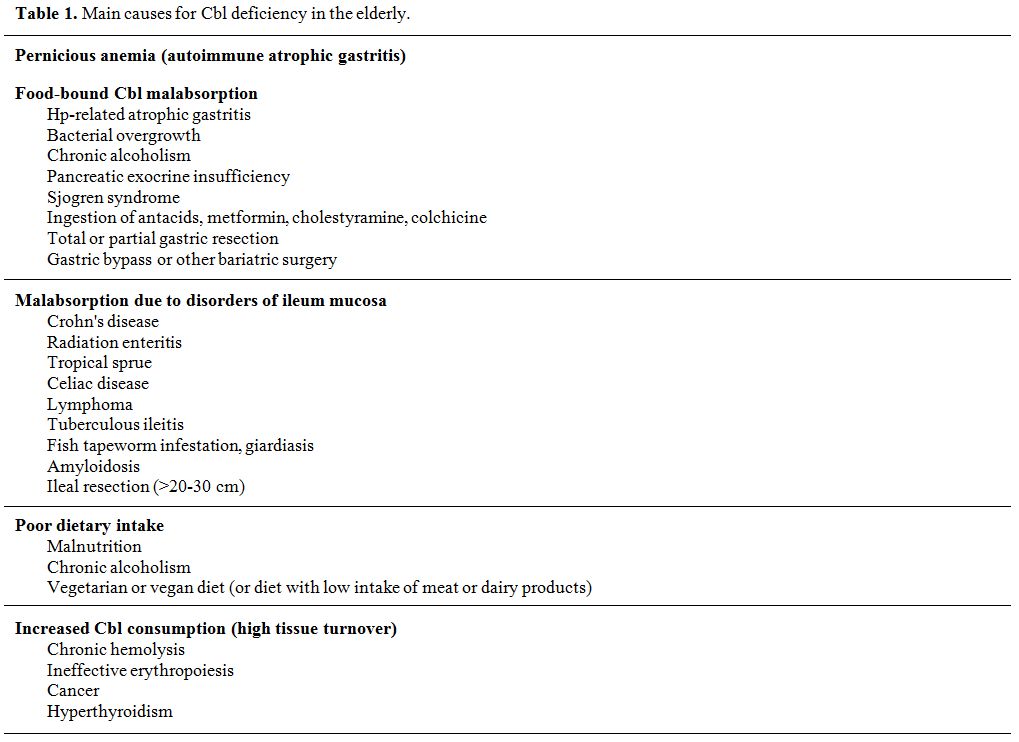 |
Table 1. Main causes for Cbl deficiency in the elderly. |
Pernicious Anemia
PA (considered fatal or "pernicious" before Cbl was purified in the liver, in 1948) is a common cause of CblD worldwide.[6]
It is particularly relevant in people older than 60 years, in whom the
estimated prevalence ranges from 2 to 12%, and increases with aging.[18]
The
disorder is a consequence of severe immune-mediated damage of the
gastric mucosa, which causes atrophy (atrophic autoimmune gastritis,
AAG), especially in the fundus and the body, with a spared antrum.
Histological confirmation of gastric atrophy needs for PA diagnosis. In
addition, diagnosis is confirmed by the presence of two types of
auto-antibodies, targeting the acid-producing H+/K+ ATPase of parietal
cells (Parietal Cells Antibody or PCA), or the IF/IF binding site in
the small bowel (Intrinsic Factors).
Antibody or IF), respectively.[18]
PCA causes hypo- or achlorhydria through the destruction of the
parietal cells, also impairing the production of IF. Their presence may
precede clinically overt atrophic gastritis by several years. PCA has
high sensitivity (80-90%) in the early stage of the disease, but much
lower specificity (50%), since they are also present in other
autoimmune diseases (e.g., thyroiditis, type 1 diabetes, Addison's
disease, and vitiligo), as well as in healthy elderly without AAG.[18]
IFA, which hamper the Cbl-IF complex formation or its binding to
enterocytes, are considered more specific markers but have lower
sensitivity (around 60%).[18]
Fasting hypergastrinemia (present in 75% of subjects) and low Pepsinogen I levels may be useful in the diagnosis of PA,[19]
while the Shilling test that specifically investigated IF-mediated
malabsorption has been abandoned due to its complexity and the need of
using of isotope-labeled Cbl.
The diagnostic workup should also
include an evaluation of iron status. Indeed, achlorhydria causes iron
malabsorption and may lead to iron deficiency (ID) that typically
precede megaloblastic anemia. PCA and endoscopic atrophic gastritis are
encountered in about 20-30% of patients with unexplained or refractory
ID.[20]
Moreover, screening for autoimmunity is indicated,[21]
as a proportion of patients (especially those with genetic
susceptibility associated with specific HLA-DR pattern) may develop
other organ-specific immune-mediated disorders. Recent studies
demonstrated that thyroid disorders (particularly Hashimoto's
thyroiditis) affect up to 40% of patients with AAG and may be
asymptomatic in the majority, leading to diagnostic and treatment
delays.[22] Thyroiditis with AAG (formerly known as
"thyrogastric syndrome") is currently considered part of the
polyglandular autoimmune syndromes, which include several endocrine and
nonendocrine manifestations.[23]
Finally,
patients with PA harbor almost 7-fold higher risk of developing gastric
neoplasms (adenocarcinomas, lymphomas, and carcinoids)[24]
as an end-stage evolution of gastric atrophy, achlorhydria and
compensatory hypergastrinemia, which causes cellular metaplasia. For
this reason, many experts recommend that adults with PA undergo
endoscopic surveillance at baseline and every 3 to 5 years for life,
although this practice is not universally accepted.[25]
Food-bound cobalamin malabsorption
FCBM
syndrome is characterized by the inability to release Cbl from food or
intestinal binding-proteins, generally as a consequence of
achlorhydria, in the presence of normal ileal mucosa.[6]
Despite the name, more appropriately, this entity refers to conditions
characterized by inadequate digestion, i.e., caused by non-immune
atrophic gastritis (e.g., Helicobacter pylori-related gastritis), intestinal bacterial overgrowth,[26]
chronic alcoholism, pancreatic exocrine insufficiency, Sjogren's
syndrome. Multiple drugs can also determine FCBM, such as long-term
ingestion of proton pump inhibitors (PPI), antacids, H2-receptor
antagonists (H2-RA), and metformin. FBCM typically produces a slow,
progressive depletion of Cbl. Clinical manifestations tend to be
subtle, although progression to more severe forms can still occur in a
minority of patients.
H. pylori-related gastritis. The most relevant form of FBCM is caused by chronic H. pylori (HP) infection, a common disorder in aged people.[27]
HP is strongly associated with atrophic gastritis. The mechanisms by
which HP provokes gastritis are still unclear, but the production of
antibodies cross-reacting with parietal cells H+/K+ ATPase may be
involved. Interestingly, HP eradication has been reported to improve
not only anemia and mean corpuscular volume (MCV), but also Cbl levels.[28]
The same was not observed in subjects in whom eradication therapy was
unsuccessful. In successful cases, the positive Cbl balance may be
related not only to HP eradication per se but also to the eradication of other small intestine bacteria potentially interfering with Cbl uptake.[26]
Treating HP is also important to reduce the risk of gastric cancer,
which is increased in patients with long-standing infection.[29,30]
Drugs. In the elderly, long-term polypharmacy for comorbidities may favor CblD.[31]
PPI and H2-RA suppress both gastric acid secretion and IF production. A
study including >200,000 subjects showed that CblD was more common
in people assuming PPI or H2-RA for >2-years, especially in those
treated with the highest dose.[32] Similarly, a recent systematic review and meta-analysis[33] were consistent with a higher risk of CblD in people chronically using PPI.
Metformin
interferes with the Cbl absorption via a dose-dependent reduction of
intestinal free calcium ions required for uptake of the Cbl¬-IF complex
by ileal enterocyte receptors.[34]
Although
PPI, H2-RA, and metformin appear to reduce Cbl bioavailability, the
clinical significance of such an effect is still controversial. In
clinical practice, it is crucial to keep in mind this as an additional
cofactor in subjects with other predisposing factors (e.g., in those
with high Cbl need due to chronic hemolysis), as well as in those with
anemia and neurologic/cognitive impairments. Anyway, a regular
reassessment of actual benefits and risks associated with these drugs
is recommended, especially in the elderly.
Gastric surgery.
Total or partial gastrectomy are relatively common causes of CblD.
Achlorhydria and the absence of pepsin lead to impaired Cbl
dissociation from food, and the reduced IF production impairs Cbl
absorption. In patients with total gastrectomy, CblD occurs relatively
early (after about 15 months), while in partial distal resections
presentation is delayed by several years, mainly in patients with low
pre-operative Cbl stores.[35] Cbl supplementation is always required after gastric surgery.
Malabsorption Due to Small Intestine Disorders
Several
disorders of the small intestine have the potential to interfere with
Cbl absorption, especially if the ileum is involved. These conditions
include inflammatory bowel disease (IBD), radiation enteritis, tropical
sprue, celiac disease, lymphoma, tuberculous ileitis, Diphillobotrium latum infestation (deriving from the ingestion of raw freshwater fish[36]), amyloidosis, and ileal resection (especially greater than 20-30 cm).[38] Cbl levels should be periodically monitored (e.g., every six months) in these conditions.
Dietary Poor Intake
A
typical Western diet provides around 5-30 µg daily of Cbl, 15-20% of
which is absorbed. This amount is higher than the Recommended Daily
Allowance (RDA), varying among different regions from 1 µg (Europe) to 2.4-2.8 µg (USA). Therefore, CblD is rarely attributable to pure nutritional deficiency, even in the elderly.[6]
However, a clinically relevant CblD can develop during very restrictive
diets that exclude animal-source foods, such as in vegans, or less
frequently, in vegetarians.[39,40] In these subjects,
routine Cbl supplements should be recommended. Physicians should be
aware that some individuals may be reluctant to take supplements due to
misconceptions and aversion to artificially manipulated food products.[40]
Increased Cbl Consumption
Elevated
Cbl consumption may characterize conditions with increased cell
turnover, such as chronic hemolytic disorders with erythropoiesis
expansion, neoplasms, and hyperthyroidism.
Clinical Manifestations
The
clinical manifestations of CblD are insidious and heterogeneous, with
some subjects being more prone to hematological disorders and others
developing preferentially severe neurological impairments in the
absence of anemia and/or macrocytosis. Many cases of CblD are
overlooked for years and sometimes even misdiagnosed, possibly leading
to irreversible sequelae. Non-specific symptoms include asthenia,
diarrhea, inappetence, lethargy, poor memory. The typical CblD features
are megaloblastic anemia (MA) and neurological disorders.
Megaloblastic Anemia
The
first description of MA dates back to the mid-1800s in a patient with
concomitant myeloneuropathy and glossitis. MA defines a condition
characterized by the presence of macro-ovalocytes in the peripheral
blood, associated with megaloblasts (i.e., abnormal erythroblasts with
elements larger than average and asynchronous nucleus-cytoplasm
maturation) at variable proportion in the bone marrow, which confer the
typical "blue" appearance.[2] The anemia is macrocytic
(MCV 100-150 fl or more), while isolated macrocytosis and anisocytosis
(increased red cell distribution width, RDW) may precede anemia by
several months. MA is slowly progressive and, as such, generally
well-tolerated, so that very low Hb values (5-6 g/dl) are often
detected at diagnosis. Of note, increased MCV may be poorly informative
in the elderly, in whom ID (with opposite effect on MCV) is highly
prevalent.[41] Table 2 summarizes the main causes of macrocytic anemia in the elderly.
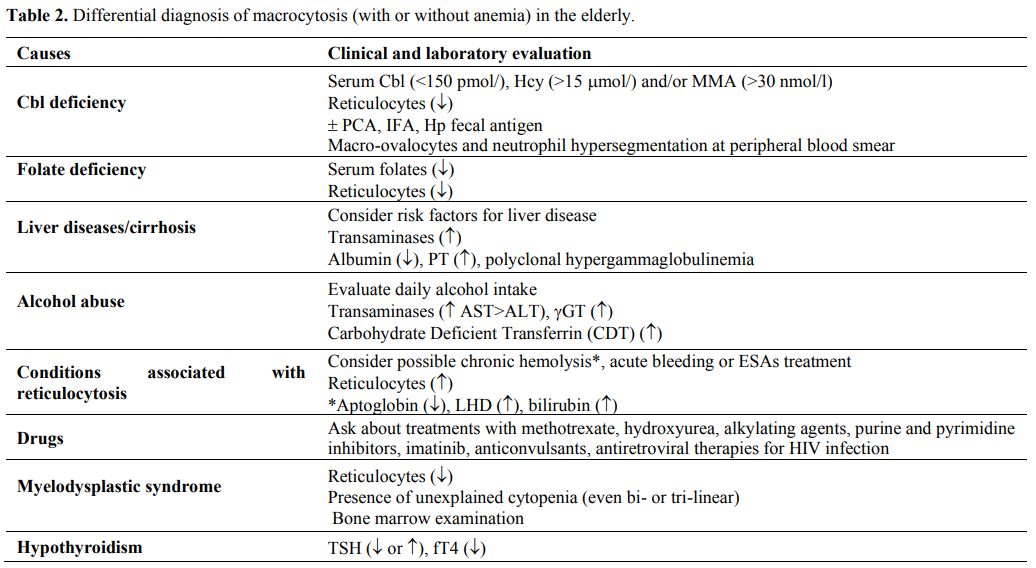 |
Table 2. Differential diagnosis of macrocytosis (with or without anemia) in the elderly. |
In
addition to ineffective erythropoiesis, the erythrocytes released into
the circulation have increased rigidity, which may be responsible for
peripheral hemolysis, leading to haptoglobin consumption and elevation
of both serum bilirubin and lactate dehydrogenase (LDH). The peripheral
blood smear may show anisocytosis, poikilocytosis, stomatocytes,
dacriocytes, red cell fragments, and target cells (Figure 3A). Thrombocytopenia and leukopenia may also occur. Neutrophils typically present hypersegmentation of their nuclei[2] (Figure 3B).
Detection of at least 3 neutrophils with at least 5 lobes, or one
containing at least 6, is considered specific for CblD. Hypersegmented
neutrophils are an early sign of megaloblastosis, but they have scarce
sensitivity and may persist for days after Cbl levels correction. In
severe cases, the initial differential diagnosis can include
myelodysplastic syndromes, hemolytic anemias, or even acute leukemia.
Severe CblD could lead to a picture that mimics thrombotic
microangiopathy (TMA),[42] also known as pseudo-TMA.
Both conditions are characterized by red cell fragmentation coupled
with thrombocytopenia. An evaluation of reticulocytes count (reduced in
severe CblD, elevated in TMA) is generally useful for differentiating
the disorders[43] and is critical to avoid unnecessary/complex treatment for TMA.[44]
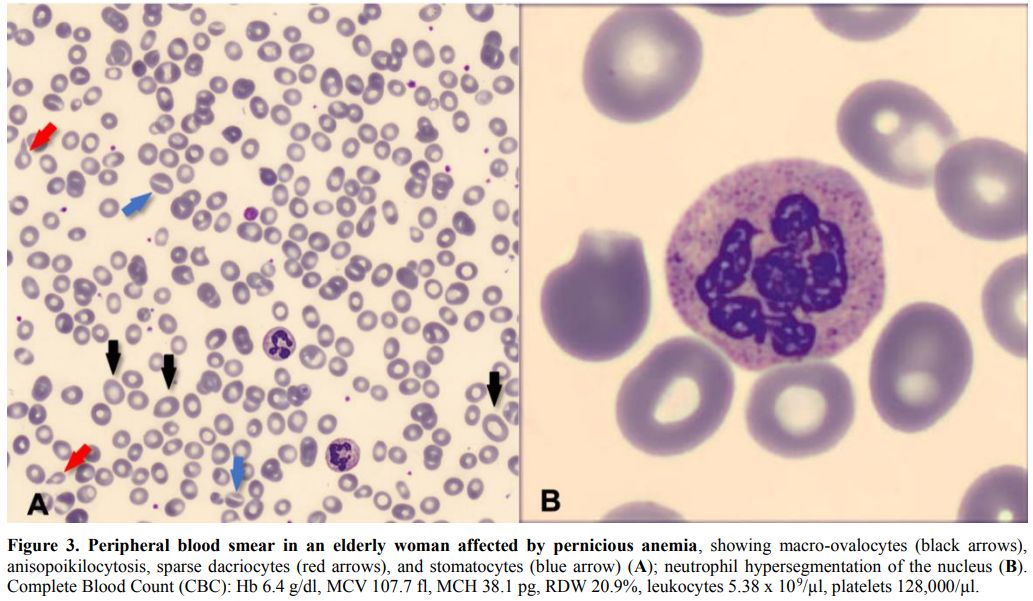 |
Figure 3. Peripheral blood smear in an elderly woman affected by pernicious anemia,
showing macro-ovalocytes (black arrows), anisopoikilocytosis, sparse
dacriocytes (red arrows), and stomatocytes (blue arrow) (A); neutrophil
hypersegmentation of the nucleus (B). Complete Blood Count (CBC): Hb
6.4 g/dl, MCV 107.7 fl, MCH 38.1 pg, RDW 20.9%, leukocytes 5.38 x
109/µl, platelets 128,000/µl. |
Neuropsychiatric Impairment
CblD has been associated with a broad spectrum of neurologic, cognitive, and psychiatric manifestations.[45] Of note, it could contribute to reducing responsivity to antidepressants.[46]Recognizing
CblD as the etiology of neuropsychiatric signs in the elderly requires
a high degree of suspicion, since they may develop before or even in
the absence of anemia or macrocytosis in around 20% of patients.[36,47]
Neurological impairment is usually heralded by proprioception and
vibration loss due to peripheral sensory neuropathy. Other common
neurological findings include paresthesia, gait ataxia, abnormal
reflexes, bowel/bladder incontinence, optic atrophy, altered smell and
taste, lethargy, and extrapyramidal signs. Autonomic dysfunction can
also occur, leading to orthostatic hypotension and syncope.[45] CblD in the elderly can be associated with poor coordination, walking difficulties, falls, and loss of function.Subacute
combined degeneration (SCD) of the spinal cord due to demyelination is
a rare complication of CblD, which, if untreated, may cause
irreversible spastic ataxia. SCD can be detected by MRI in T2-weighted
images, showing symmetrical hyperintensity of posterior and lateral
columns in the cervical and thoracic spinal cord, although imaging
sensitivity appears quite low.[48]In advanced stages, cognitive decline, psychosis with hallucinations,[45] and depression[49] may be observed. Severe CblD in the elderly may predispose to delirium,[45,50,51] although this association has been confuted by a recent report.[52]Of
note, recent trials do not support Cbl supplementation in the elderly
with normal to low Cbl levels for preventing cognitive deterioration.[53,54]
Other Manifestations
CblD
may lead to epithelial changes, including glossitis, angular
stomatitis, skin hyperpigmentation, dermatitis, nail, and hair
abnormalities.[55]Low
Cbl levels, with or without hyperhomocysteinemia, has been associated
with high markers of bone turnover and increased fracture risk.[56]
However, the clinical relevance of such association is debated, and, at
present, supplementation cannot be recommended for preventing fracture
in the elderly.[57,58]Finally, hyperhomocysteinemia resulting from CblD has been associated with endothelial dysfunction,[59] and accelerated atherosclerosis.[60,61]
However, studies evaluating Hcy-lowering treatment by B-vitamins
supplementation have failed to demonstrate an improvement in
cardiovascular outcomes.[62,63]
Treatment
In
many patients, the causes of CblD cannot be removed, and lifelong Cbl
replacement therapy is required. CblD occurs over months or years, and
usually, there is no need for urgent action. However, in some
circumstances, it may be warranted to correct CblD rapidly, as in the
case of neurologic symptoms, due to the risk of irreversible sequelae,
or in severe/symptomatic anemia. Special considerations should be made
for the elderly, who often take many medications and may be poorly
compliant with oral therapy.[64,65]Cbl
can be administered orally and parenterally (intramuscularly, IM).
Subcutaneous, transdermal, sublingual, and nasal formulations are also
available, but their role in clinical practice appears marginal,
because of their variable effectiveness and higher costs.[65] Two formulations are currently available, cyanocobalamin and hydroxocobalamin.Initial
parenteral administration is appropriated in the subjects with (e.g.,
PA, or gastric resections) and in those with symptoms, requiring a
prompt correction.[45,64] The
typical schedule consists of 1 injection (1,000 mg, of which about 10%
is retained) three times a week for 1 to 2 weeks, followed by weekly
injections for a month. Maintenance therapy is based on monthly
administration for cyanocobalamin, once every other month, for
hydroxocobalamin.Oral
Cbl (50-150 μg/day) represents a cheaper and easier route of
administration, more comfortable for the patients and effective in the
majority of mild-moderate cases.[66] It is also more
suitable in patients under anticoagulant therapy, in whom IM injections
may be contraindicated. Recently, its role has been re-evaluated even
in subjects with malabsorption or FBCM, in which high-dose oral Cbl
(1,000 μg daily) has proven as non-inferior to the parenteral
route.[67,68] Indeed, small amounts of Cbl (0.5-4%) can be passively
absorbed by the entire bowel, via an IF-independent pathway.[69]
Therefore, high oral doses of 1,000 μg deliver at least 5 μg of Cbl,
which are largely sufficient to satisfy daily requirements. However, in
clinical practice, the role of high-dose oral Cbl in PA or
malabsorption is still debated, and injectable Cbl remains frontline
therapy. Food alters oral Cbl absorption; thus, it should preferably be
assumed on an empty stomach.Monitoring
the hematological and clinical response to Cbl replacement therapy is
essential, as it is useful to confirm the diagnosis. Typically, the
reticulocyte crisis occurs in 1 week, anemia and macrocytosis improve
within 3-4 weeks, and normalization of Hb and MCV is generally achieved
within sixth-eighth weeks. The neurological response is less
predictable and can take from 1 week to 3 months.[64] Neurological
irreversible damages have been described in about 6% of cases, and are
more frequent in patients with ≥6-months treatment delay.[36]Monitoring
serum Cbl levels is scantly informative since they rapidly rise with
supplementation regardless of the actual repletion of Cbl body stores.
Serum MMA and Hcy levels tend to decrease or even normalize by the
first week (unless renal failure coexists), and this may further
support the diagnosis in uncertain cases.[64]Particular
attention has to be paid to other possible causes of anemia, such as
folate and iron deficiency. In patients with both Cbl and folate
deficiency, Cbl should be given first in order to avoid the risk of
precipitating SCD of the spinal cord.[36]Moreover,
some drugs may interfere with Cbl metabolism and absorption. This is
particularly true for PPI, which are often inappropriately prescribed
in the elderly,[37] and whose cessation should be considered whenever
clear indications for their use are not present.Cbl
supplements are generally well-tolerated even when prescribed at high
doses. Adverse effects may include hot flushes, acneiform eruptions,[70]
and, quite rarely, severe allergic reactions (i.e., anaphylaxis),
especially in subjects with cobalt sensitivity.[36,55] Transient
hypokalemia can be observed when severe anemias respond to Cbl as a
consequence of potassium uptake by growing hematopoietic cells, but its
clinical relevance has never been proven.[1]Concerns
have been raised about the safety of generalized Cbl supplementation,
especially regarding a possible increased risk of lung cancer.[71,72,73]
However, a meta-analysis of randomized controlled trials (RCTs) denied
any effects of Cbl supplementation on cancer incidence or mortality,
rather showing a lower risk of melanoma.[74]
Monitoring circulating Cbl levels in lifelong treated high-risk
patients (e.g., male smokers) could be a reasonable approach to avoid
overtreatment.Finally,
the use of multivitamin supplements is becoming very popular among
older people. Taking these supplements, which often contain low-dose
Cbl (3-5 μg/day) in association with vitamin D, iron, or proteins, may
be theoretically useful for short periods, for instance, to compensate
poor nutrition after a disabling disease. However, there is currently
no evidence of their efficacy in preventing CblD, and probably they
have little (if any) effects in treating CblD in the elderly.
Conclusions
CblD
is relatively common in the elderly, but often underrecognized because
of non-specificity and heterogeneity of clinical manifestations, as
well as the lack of reliable laboratory tests. Increasing clinicians'
awareness is essential to avoid misdiagnosis. Further research is
needed to identify better biomarkers of CblD, to define the relevance
of subclinical CblD in the elderly, as well as the usefulness of
screening programs and the long-term safety of Cbl supplements,
including novel nasal or sublingual formulations.
References
- Stabler, SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013; 368(2): 149-60. https://doi.org/10.1056/NEJMcp1113996 PMid:23301732
- Green R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. 2017; 129(19): 2603-11. https://doi.org/10.1182/blood-2016-10-569186 PMid:28360040
- Lu SC. S-Adenosylmethionine. Int J Biochem Cell Biol. 2000; 32(4): 391-95. https://doi.org/10.1016/S1357-2725(99)00139-9
- Clarke
R, Grimley Evans J, Schneede J, Nexo E, Bates C, Fletcher A, Prentice
A, Johnston C, Ueland PM, Refsum H, Sherliker P, Birks J, Whitlock G,
Breeze E, Scott JM. Vitamin B12 and folate deficiency in later life.
Age Ageing. 2004; 33(1): 34-41. https://doi.org/10.1093/ageing/afg109 PMid:14695861
- Lindenbaum
J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of
cobalamin deficiency in the Framingham elderly population. Am J Clin
Nutr. 1994; 60(1): 2-11. https://doi.org/10.1093/ajcn/60.1.2 PMid:8017332
- Andres
E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB, Perrin AE,
Noblet-Dick M, Maloisel F, Schlienger JL, Blicklé JF. Vitamin B12
(cobalamin) deficiency in elderly patients. CMAJ. 2004; 171(3): 251-59.
https://doi.org/10.1503/cmaj.1031155 PMid:15289425 PMCid:PMC490077
- Wong
CW, Ip CY, Leung CP, Leung CS, Cheng JN, Siu CY. Vitamin B12 deficiency
in the institutionalized elderly: A regional study. Exp Gerontol. 2015;
69: 221-25. https://doi.org/10.1016/j.exger.2015.06.016 PMid:26122132
- Andres
E, Federici L, Kaltenbach G. Hematological manifestations related to
cobalamin deficiency in elderly patients. Eur J Intern Med. 2008;
19(2): 149-50. https://doi.org/10.1016/j.ejim.2007.05.006 PMid:18249317
- Loikas
S, Koskinen P, Irjala K, Löppönen M, Isoaho R, Kivelä SL, Pelliniemi
TT. Vitamin B12 deficiency in the aged: a population-based study. Age
Ageing. 2007; 36(2): 177-83. https://doi.org/10.1093/ageing/afl150 PMid:17189285
- Wong CW. Vitamin B12 deficiency in the elderly: is it worth screening? Hong Kong Med J. 2015; 21(2): 155-64. https://doi.org/10.12809/hkmj144383 PMid:25756278
- Devalia
V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment
of cobalamin and folate disorders. Br J Haematol. 2014; 166(4):
496-513. https://doi.org/10.1111/bjh.12959 PMid:24942828
- Wolffenbuttel
BHR, Wouters HJCM, Heiner-Fokkema MR, van der Klauw MM. The Many Faces
of Cobalamin (Vitamin B12) Deficiency. Mayo Clin Proc Innov Qual
Outcomes. 2019; 3(2): 200-14. https://doi.org/10.1016/j.mayocpiqo.2019.03.002 PMid:31193945 PMCid:PMC6543499
- Vashi
P, Edwin P, Popiel B, Lammersfeld C, Gupta D. Methylmalonic Acid and
Homocysteine as Indicators of Vitamin B-12 Deficiency in Cancer. PLoS
One. 2016; 11(1): e0147843. https://doi.org/10.1371/journal.pone.0147843 PMid:26807790 PMCid:PMC4725715
- Lee
SM, Oh J, Chun MR, Lee SY. Methylmalonic Acid and Homocysteine as
Indicators of Vitamin B12 Deficiency in Patients with Gastric Cancer
after Gastrectomy. Nutrients. 2019; 11(2). https://doi.org/10.3390/nu11020450 PMid:30795564 PMCid:PMC6412945
- Wolffenbuttel
BHR, Wouters HJCM, de Jong WHA, Huls G, van der Klauw MM. Association
of vitamin B12, methylmalonic acid, and functional parameters. Neth J
Med. 2020; 78(1): 10-24.
- Jarquin Campos
A, Risch L, Nydegger U, Wiesner J, Vazquez Van Dyck M, Renz H, Stanga
Z, Risch M. Diagnostic Accuracy of Holotranscobalamin, Vitamin B12,
Methylmalonic Acid, and Homocysteine in Detecting B12 Deficiency in a
Large, Mixed Patient Population. Dis Markers. 2020; 2020: 7468506. https://doi.org/10.1155/2020/7468506 PMid:32089757 PMCid:PMC7017578
- Fedosov
SN. Biochemical markers of vitamin B12 deficiency combined in one
diagnostic parameter: the age-dependence and association with cognitive
function and blood hemoglobin. Clin Chim Acta. 2013; 422: 47-53. https://doi.org/10.1016/j.cca.2013.04.002 PMid:23583557
- Bizzaro N, Antico A. Diagnosis and classification of pernicious anemia. Autoimmun Rev. 2014; 13(4-5): 565-68. https://doi.org/10.1016/j.autrev.2014.01.042 PMid:24424200
- Annibale B, Lahner E, Fave GD. Diagnosis and management of pernicious anemia. Curr Gastroenterol Rep. 2011; 13(6): 518-24. https://doi.org/10.1007/s11894-011-0225-5 PMid:21947876
- Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014; 123(3): 326-33. https://doi.org/10.1182/blood-2013-10-512624 PMid:24215034
- Andres E, Serraj K. Optimal management of pernicious anemia. J Blood Med. 2012; 3: 97-103. https://doi.org/10.2147/JBM.S25620 PMid:23028239 PMCid:PMC3441227
- Lahner
E, Conti L, Cicone F, Capriello S, Cazzato M, Centanni M, Annibale B,
Virili C.Thyro-entero-gastric autoimmunity: Pathophysiology and
implications for patient management. Best Pract Res Clin Endocrinol
Metab. 2019; 101373. https://doi.org/10.1016/j.beem.2019.101373 PMid:31864909
- Toh
BH, Chan J, Kyaw T, Alderuccio F. Cutting edge issues in autoimmune
gastritis. Clin Rev Allergy Immunol. 2012; 42(3): 269-78. https://doi.org/10.1007/s12016-010-8218-y PMid:21174235
- Murphy
G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A, Lin SW, Abnet
CC, Freedman ND. Cancer Risk After Pernicious Anemia in the US Elderly
Population. Clin Gastroenterol Hepatol. 2015; 13(13): 2282-89 e1-4. https://doi.org/10.1016/j.cgh.2015.05.040 PMid:26079040 PMCid:PMC4655146
- Lahner
E, Esposito G, AnnibaleD. Pernicious Anemia: Time to Justify Endoscopic
Monitoring? Clin Gastroenterol Hepatol. 2016; 14(2): 322. https://doi.org/10.1016/j.cgh.2015.07.038 PMid:26240006
- Sachdev
AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and
clinical significance. Ther Adv Chronic Dis. 2013; 4(5): 223-31. https://doi.org/10.1177/2040622313496126 PMid:23997926 PMCid:PMC3752184
- Zendehdel
A, Roham M. Role of Helicobacter pylori infection in the manifestation
of old age-related diseases. Mol Genet Genomic Med. 2020; e1157. https://doi.org/10.1002/mgg3.1157 PMid:32067423 PMCid:PMC7196471
- Kaptan
K, Beyan C, Ural AU, Cetin T, Avcu F, Gülşen M, Finci R, Yalçín A.
Helicobacter pylori--is it a novel causative agent in Vitamin B12
deficiency? Arch Intern Med. 2000; 160(9): 1349-53. https://doi.org/10.1001/archinte.160.9.1349 PMid:10809040
- Bae
SE, Choi KD, Choe J, Kim SO, Na HK, Choi JY, Ahn JY, Jung KW, Lee J,
Kim DH, Chang HS, Song HJ, Lee GH, Jung HY. The effect of eradication
of Helicobacter pylori on gastric cancer prevention in healthy
asymptomatic populations. Helicobacter. 2018; 23(2): e12464. https://doi.org/10.1111/hel.12464 PMid:29345408
- Choi
IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH.
Helicobacter pylori Therapy for the Prevention of Metachronous Gastric
Cancer. N Engl J Med: 2018; 378(12): 1085-95. https://doi.org/10.1056/NEJMoa1708423 PMid:29562147
- Hesdorffer CS, Longo DL. Drug-Induced Megaloblastic Anemia. N Engl J Med. 2015; 373(17): 1649-58. https://doi.org/10.1056/NEJMra1508861 PMid:26488695
- Lam
JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and
histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA.
2013; 310(22): 2435-42. https://doi.org/10.1001/jama.2013.280490 PMid:24327038
- Jung
SB, Nagaraja V, Kapur A, Eslick GD. Association between vitamin B12
deficiency and long-term use of acid-lowering agents: a systematic
review and meta-analysis. Intern Med J. 2015; 45(4): 409-16. https://doi.org/10.1111/imj.12697 PMid:25583062
- Miller
JW. Proton Pump Inhibitors, H2-Receptor Antagonists, Metformin, and
Vitamin B-12 Deficiency: Clinical Implications. Adv Nutr. 2018; 9(4):
511S-518S. https://doi.org/10.1093/advances/nmy023 PMid:30032223 PMCid:PMC6054240
- Hu
Y, Kim HI, Hyung WJ, Song KJ, Lee JH, Kim YM, Noh SH. Vitamin B(12)
deficiency after gastrectomy for gastric cancer: an analysis of
clinical patterns and risk factors. Ann Surg. 2013; 258(6): 970-75. https://doi.org/10.1097/SLA.0000000000000214 PMid:24096753
- Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014; 349: g5226. https://doi.org/10.1136/bmj.g5226 PMid:25189324
- Schepisi
R, Fusco S, Sganga F, Falcone B, Vetrano DL, Abbatecola A, Corica F,
Maggio M, Ruggiero C, Fabbietti P, Corsonello A, Onder G, Lattanzio F.
Inappropriate Use of Proton Pump Inhibitors in Elderly Patients
Discharged From Acute Care Hospitals. J Nutr Health Aging. 2016; 20(6):
665-70. https://doi.org/10.1007/s12603-015-0642-5 PMid:27273358
- Battat
R, Kopylov U, Szilagyi A, Saxena A, Rosenblatt DS, Warner M, Bessissow
T, Seidman E, Bitton A. Vitamin B12 deficiency in inflammatory bowel
disease: prevalence, risk factors, evaluation, and management. Inflamm
Bowel Dis. 2014; 20(6): 1120-28. https://doi.org/10.1097/MIB.0000000000000024 PMid:24739632
- Pawlak
R, Lester SE, Babatunde T. The prevalence of cobalamin deficiency among
vegetarians assessed by serum vitamin B12: a review of literature. Eur
J Clin Nutr. 2014; 68(5): 541-48. https://doi.org/10.1038/ejcn.2014.46 PMid:24667752
- Rizzo
G, Laganà AS, Rapisarda AM, La Ferrera GM, Buscema M, Rossetti P, Nigro
A, Muscia V, Valenti G, Sapia F, Sarpietro G, Zigarelli M, Vitale SG.
Vitamin B12 among Vegetarians: Status, Assessment and Supplementation.
Nutrients. 2016; 8(12). https://doi.org/10.3390/nu8120767 PMid:27916823 PMCid:PMC5188422
- Girelli, D., G. Marchi, and C. Camaschella, Anemia in the Elderly. HemaSphere. 2018; 2(3): e40. https://doi.org/10.1097/HS9.0000000000000040 PMid:31723768 PMCid:PMC6745992
- Sabry
W, Elemary M, Burnouf T, Seghatchian J, Goubran H. Vitamin B12
deficiency and metabolism-mediated thrombotic microangiopathy (MM-TMA).
Transfus Apher Sci. 2019; 102717. https://doi.org/10.1016/j.transci.2019.102717 PMid:31902683
- Tran
PN, Tran MH. Cobalamin deficiency presenting with thrombotic
microangiopathy (TMA) features: A systematic review. Transfus Apher
Sci. 2018; 57(1): 102-06. https://doi.org/10.1016/j.transci.2018.01.003 PMid:29454538
- Hall
JA, Mason J, Choi J, Holguin M. B12 deficiency leading to marked
poikilocytosis versus true schistocytosis, a pernicious problem.
Transfus Apher Sci. 2017; 56(4): 576-77. https://doi.org/10.1016/j.transci.2017.06.003 PMid:28711333
- Lachner
C, Steinle NI, Regenold WT. The neuropsychiatry of vitamin B12
deficiency in elderly patients. J Neuropsychiatry Clin Neurosci. 2012;
24(1): 5-15. https://doi.org/10.1176/appi.neuropsych.11020052 PMid:22450609
- Kate
N, Grover S, Agarwal M. Does B12 deficiency lead to lack of treatment
response to conventional antidepressants? Psychiatry (Edgmont). 2010;
7(11): 42-44.
- Lavoie MR, Cohen NC,
Gregory TA, Weber PV. Subacute combined degeneration: a case of
pernicious anaemia without haematological manifestations. BMJ Case Rep.
2020; 13(3). https://doi.org/10.1136/bcr-2020-234276 PMid:32209580
- Jain
KK, Malhotra HS, Garg RK, Gupta PK, Roy B, Gupta RK. Prevalence of MR
imaging abnormalities in vitamin B12 deficiency patients presenting
with clinical features of subacute combined degeneration of the spinal
cord. J Neurol Sci. 2014; 342(1-2): 162-66. https://doi.org/10.1016/j.jns.2014.05.020 PMid:24857760
- Penninx
BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin
B(12) deficiency and depression in physically disabled older women:
epidemiologic evidence from the Women's Health and Aging Study. Am J
Psychiatry. 2000; 157(5): 715-21. https://doi.org/10.1176/appi.ajp.157.5.715 PMid:10784463
- Sanford AM, Flaherty JH. Do nutrients play a role in delirium? Curr Opin Clin Nutr Metab Care. 2014; 17(1): 45-50. https://doi.org/10.1097/MCO.0000000000000022 PMid:24296414
- Sevuk
U, Baysal E, Ay N, Altas Y, Altindag R, Yaylak B, Alp V, Demirtas E.
Relationship between cobalamin deficiency and delirium in elderly
patients undergoing cardiac surgery. Neuropsychiatr Dis Treat. 2015;
11: 2033-39. https://doi.org/10.2147/NDT.S87888 PMid:26300642 PMCid:PMC4535547
- Vahdat
Shariatpanahi M, Velayati A, Jamalian SA, Babevaynejad M, Vahdat
Shariatpanahi Z. The relationship between serum cobalamin, folic acid,
and homocysteine and the risk of post-cardiac surgery delirium.
Neuropsychiatr Dis Treat. 2019; 15: 1413-19. https://doi.org/10.2147/NDT.S201620 PMid:31190843 PMCid:PMC6536132
- Dangour
AD, Allen E, Clarke R, Elbourne D, Fletcher AE, Letley L, Richards M,
Whyte K, Uauy R, Mills K. Effects of vitamin B-12 supplementation on
neurologic and cognitive function in older people: a randomized
controlled trial. Am J Clin Nutr. 2015; 102(3): 639-47. https://doi.org/10.3945/ajcn.115.110775 PMid:26135351 PMCid:PMC4548176
- Rutjes
AW, Denton DA, Di Nisio M, Chong LY, Abraham RP, Al-Assaf AS, Anderson
JL, Malik MA, Vernooij RW, Martínez G, Tabet N, McCleery J. Vitamin and
mineral supplementation for maintaining cognitive function in
cognitively healthy people in mid and late life. Cochrane Database Syst
Rev. 2018; 12: CD011906. https://doi.org/10.1002/14651858.CD011906.pub2 PMCid:PMC6353240
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015; 16(1): 27-33. https://doi.org/10.1007/s40257-014-0107-3 PMid:25559140
- Dhonukshe-Rutten
RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA.
Homocysteine and vitamin B12 status relate to bone turnover markers,
broadband ultrasound attenuation, and fractures in healthy elderly
people. J Bone Miner Res. 2005; 20(6): 921-29. https://doi.og/10.1359/JBMR.050202 PMid:15883631
- Enneman
AW, Swart KM, van Wijngaarden JP, van Dijk SC, Ham AC, Brouwer-Brolsma
EM, van der Zwaluw NL, Dhonukshe-Rutten RA, van der Cammen TJ, de Groot
LC, van Meurs J, Lips P, Uitterlinden AG, Zillikens MC, van Schoor NM,
van der Velde N. Effect of Vitamin B12 and Folic Acid Supplementation
on Bone Mineral Density and Quantitative Ultrasound Parameters in Older
People with an Elevated Plasma Homocysteine Level: B-PROOF, a
Randomized Controlled Trial. Calcif Tissue Int. 2015; 96(5): 401-09. https://doi.org/10.1007/s00223-015-9968-6 PMid:25712255 PMCid:PMC4415946
- van
Wijngaarden JP, Swart KM, Enneman AW, Dhonukshe-Rutten RA, van Dijk SC,
Ham AC, Brouwer-Brolsma EM, van der Zwaluw NL, Sohl E, van Meurs JB,
Zillikens MC, van Schoor NM, van der Velde N, Brug J, Uitterlinden AG,
Lips P, de Groot LC. Effect of daily vitamin B-12 and folic acid
supplementation on fracture incidence in elderly individuals with an
elevated plasma homocysteine concentration: B-PROOF, a randomized
controlled trial. Am J Clin Nutr. 2014; 100(6): 1578-86. https://doi.org/10.3945/ajcn.114.090043 PMid:25411293
- Balint
B, Jepchumba VK, Guéant JL, Guéant-Rodriguez RM. Mechanisms of
homocysteine-induced damage to the endothelial, medial and adventitial
layers of the arterial wall. Biochimie. 2020 [Epub ahead of print]. https://doi.org/10.1016/j.biochi.2020.02.012 PMid:32105811
- Wald
DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence
on causality from a meta-analysis. BMJ. 2002; 325(7374): 1202. https://doi.org/10.1136/bmj.325.7374.1202 PMid:12446535 PMCid:PMC135491
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015; 14: 6. https://doi.org/10.1186/1475-2891-14-6 PMid:25577237 PMCid:PMC4326479
- Toole
JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides
EG, Wang CH, Stampfer M. Lowering homocysteine in patients with
ischemic stroke to prevent recurrent stroke, myocardial infarction, and
death: the Vitamin Intervention for Stroke Prevention (VISP) randomized
controlled trial. JAMA. 2004; 291(5): 565-75. https://doi.org/10.1001/jama.291.5.565 PMid:14762035
- Marti-Carvajal
AJ, Solà I, Lathyris D, Dayer M. Homocysteine-lowering interventions
for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;
8: CD006612. https://doi.org/10.1002/14651858.CD006612.pub5 PMCid:PMC6483699
- Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008; 112(6): 2214-21. https://doi.org/10.1182/blood-2008-03-040253 PMid:18606874 PMCid:PMC2532799
- Andres
E, Zulfiqar AA, Vogel T. State of the art review: oral and nasal
vitamin B12 therapy in the elderly. QJM. 2020; 113(1): 5-15. https://doi.org/10.1093/qjmed/hcz046 PMid:30796433
- Andres
E, Zulfiqar AA, Serraj K, Vogel T, Kaltenbach G. Systematic Review and
Pragmatic Clinical Approach to Oral and Nasal Vitamin B12 (Cobalamin)
Treatment in Patients with Vitamin B12 Deficiency Related to
Gastrointestinal Disorders. J Clin Med. 2018; 7(10). https://doi.org/10.3390/jcm7100304 PMid:30261596 PMCid:PMC6210286
- Wang
H, Li L, Qin LL, Song Y, Vidal-Alaball J, Liu TH. Oral vitamin B12
versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane
Database Syst Rev. 2018; 3: CD004655. https://doi.org/10.1002/14651858.CD004655.pub3 PMCid:PMC6494183
- Butler
CC, Vidal-Alaball J, Cannings-John R, McCaddon A, Hood K, Papaioannou
A, Mcdowell I, Goringe A. Oral vitamin B12 versus intramuscular vitamin
B12 for vitamin B12 deficiency: a systematic review of randomized
controlled trials. Fam Pract. 2006; 23(3): 279-85. https://doi.org/10.1093/fampra/cml008 PMid:16585128
- Berlin
H, Berlin R, G Brante G. Oral treatment of pernicious anemia with high
doses of vitamin B12 without intrinsic factor. Acta Med Scand. 1968;
184(4): 247-58. https://doi.org/10.1111/j.0954-6820.1968.tb02452.x PMid:5751528
- Veraldi
S, Benardon S, Diani M, Barbareschi M. Acneiform eruptions caused by
vitamin B12: A report of five cases and review of the literature. J
Cosmet Dermatol. 2018; 17(1): 112-15. https://doi.org/10.1111/jocd.12360 PMid:28594082
- Ebbing
M, Bønaa KH, Nygård O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen
K, Njølstad I, Refsum H, Nilsen DW, Tverdal A, Meyer K, Vollset SE.
Cancer incidence and mortality after treatment with folic acid and
vitamin B12. JAMA. 2009; 302(19): 2119-26. https://doi.org/10.1001/jama.2009.1622 PMid:19920236
- Fanidi
A, Carreras-Torres R, Larose TL, Yuan JM, Stevens VL, Weinstein SJ,
Albanes D, Prentice R, Pettinger M, Cai Q, Blot WJ, Arslan AA,
Zeleniuch-Jacquotte A, McCullough ML, Le Marchand L, Wilkens LR, Haiman
CA, Zhang X, Stampfer MJ, Smith-Warner SA, Giovannucci E, Giles GG,
Hodge AM, Severi G, Johansson M, Grankvist K, Langhammer A, Brumpton
BM, Wang R, Gao YT, Ericson U, Bojesen SE, Arnold SM, Koh WP, Shu XO,
Xiang YB, Li H, Zheng W, Lan Q, Visvanathan K, Hoffman-Bolton J, Ueland
PM, Midttun Ø, Caporaso NE, Purdue M, Freedman ND, Buring JE, Lee IM,
Sesso HD, Michael Gaziano J, Manjer J, Relton CL, Hung RJ, Amos CI,
Johansson M, Brennan P; LC3 consortium and the TRICL consortium. Is
high vitamin B12 status a cause of lung cancer? Int J Cancer. 2019;
145(6): 1499-1503. https://doi.org/10.1002/ijc.32033 PMid:30499135 PMCid:PMC6642017
- Brasky
TM, White E, Chen CL. Long-Term, Supplemental, One-Carbon
Metabolism-Related Vitamin B Use in Relation to Lung Cancer Risk in the
Vitamins and Lifestyle (VITAL) Cohort. J Clin Oncol. 2017; 35(30):
3440-48. https://doi.org/10.1200/JCO.2017.72.7735 PMid:28829668 PMCid:PMC5648175
- Zhan
SL, Chen TS, Ma CY, Meng YB, Zhang YF, Chen YW, Zhou YH. Effect of
vitamin B supplementation on cancer incidence, death due to cancer, and
total mortality: A PRISMA-compliant cumulative meta-analysis of
randomized controlled trials. Medicine (Baltimore). 2016; 95(31):
e3485. https://doi.org/10.1097/MD.0000000000003485 PMid:27495015 PMCid:PMC4979769
TOP]

