Vincenzo de Sanctis1, Duran Canatan2, Joan Lluis Vives Corrons3, Mehran Karimi4, Shahina Daar5, Christos Kattamis6 and Ashraf T. Soliman7, (Steering Committee); Yasser Wali8, Salam Alkindi5, Valeh Huseynov9, Afag Nasibova9, Tarık Onur Tiryaki10, Melike Sezgin Evim11, Adalet Meral Gunes11, Zeynep Karakas12, Soteroula Christou13, Saveria Campisi14, Tahereh Zarei4, Doaa Khater15, Yesim Oymak16, Valeriya Kaleva17, Denka Stoyanova18, Atanas Banchev18, Maria Concetta Galati19, Mohamed A Yassin20, Shruti Kakar21, Myrto Skafida22, Yurdanur Kilinc23 (Participants); Saif Alyaarubi24, Narmin Verdiyevas9, Iva Stoeva25, Giuseppe Raiola26, Demetris Mariannis27, Leopoldo Ruggiero28, Salvatore Di Maio29 (Collaborators).
1 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy,
2 Antalya Genetic Diseases Diagnostic Center, Antalya, Turkey,
3
Red Blood Cell, and Haematopoietic Disorders Unit, Institute for
Leukaemia Research Josep Carreras (IJC) and University of Barcelona,
Catalonia, Spain. ENERCA Coordinator,
4 Hematology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran,
5 Department of Haematology, College of Medicine and Health Sciences, Sultan Qaboos University, Sultanate of Oman,
6 First Department of Paediatrics, National Kapodistrian University of Athens, Athens, Greece,
7
Pediatrics and Endocrinology Department of Pediatrics, Hamad Medical
Center, Doha, Qatar and Department of Pediatrics, University of
Alexandria, Alexandria, Egypt (Steering Committee);
8
Paediatric Hematology Unit, Child Health Department, College of
Medicine, Sultan Qaboos University Oman and Department of Paediatrics,
Faculty of Medicine, Alexandria University, Egypt,
9 Center of Thalassemia, Baku, Azerbaijan,
10 İstanbul University, Faculty of Medicine, Department of Hematology, İstanbul, Turkey,
11 Uludag University, Medical Faculty, Dept. of Pediatric Hematology, Bursa, Turkey,
12 Istanbul University, Istanbul Faculty of Medicine, Pediatric Hematology / Oncology, Istanbul, Turkey,
13 Archibishop Makarios III Hospital, Thalassaemia Clinic, Nicosia, Cyprus,
14 UOSD Thalassemia, Umberto I° Hospital, Siracusa, Italy,
15
Department of Pediatric Endocrinology Alexandria University, Egypt and
Department of Pediatrics, Sultan Qaboos University, Oman, Qatar,
16 Dr. Behcet Uz Children's Hospital, Izmir, Turkey,
17 Expert Center for Coagulopathies and Rare Anemias, Varna, Bulgaria,
18 Pediatric Hematoncology, University Hospital "Tzaritza Giovanna – ISUL," Sofia, Bulgaria,
19 Pediatric Haematoncology, Pugliese-Ciaccio Hospital, Catanzaro, Italy,
20 Hematology Section, National Center for Cancer Care and Research, Hamad Medical Corporation, (HMC), Doha, Qatar,
21 Pediatric Hematology Oncology, Department of Pediatrics, Dayanand Medical College and Hospital, Ludhiana, India,
22 First Department of Paediatrics, National Kapodistrian University of Athens, Athens, Greece,
23 Pediatric Hematology Department, Çukurova University, Adana, Turkey,
24
Pediatric Endocrinology Unit, Child Health Department, College of
Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman,
25
Paediatric Endocrinologist, Head "Screening and Functional Endocrine
Diagnostics" University Paediatric Hospital, Sofia, Bulgaria,
26 Department of Paediatrics, Pugliese-Ciaccio Hospital, Catanzaro, Italy,
27 Royal Lancaster Infirmary, Lancaster, UK,
28 Pediatrician, Lecce, Italy,
29 Emeritus Director in Pediatrics, "Santobono-Pausilipon" Children's Hospital, Naples, Italy.
Correspondence to: Vincenzo De Sanctis MD, Pediatric and Adolescent
Outpatient Clinic, Quisisana Hospital, 44121 Ferrara, Italy. Tel: +39
0532 770243. E-mail:
vdesanctis@libero.it
Published: July 1, 2020
Received: May 30, 2020
Accepted: June 14, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020046 DOI
10.4084/MJHID.2020.046
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objectives:
This study aims to investigate, retrospectively, the epidemiological
and clinical characteristics, laboratory results, radiologic findings,
and outcomes of COVID-19 in patients with transfusion-dependent β
thalassemia major (TM), β-thalassemia intermedia (TI) and sickle cell
disease (SCD).
Design: A total of 17 Centers, from 10 countries, following 9,499 patients with hemoglobinopathies, participated in the survey.
Main outcome data:
Clinical, laboratory, and radiologic findings and outcomes of patients
with COVID-19 were collected from medical records and summarized.
Results:
A total of 13 patients, 7 with TM, 3 with TI, and 3 with SCD, with
confirmed COVID-19, were identified in 6 Centers from different
countries. The overall mean age of patients was 33.7±12.3 years
(range:13-66); 9/13 (69.2%) patients were females. Six patients had
pneumonia, and 4 needed oxygen therapy. Increased C-reactive protein
(6/10), high serum lactate dehydrogenase (LDH; 6/10), and erythrocyte
sedimentation rate (ESR; 6/10) were the most common laboratory
findings. 6/10 patients had an exacerbation of anemia (2 with SCD). In
the majority of patients, the course of COVID-19 was moderate (6/10)
and severe in 3/10 patients. A 30-year-old female with TM, developed a
critical SARS-CoV-2 infection, followed by death in an Intensive Care
Unit. In one Center (Oman), the majority of suspected cases were
observed in patients with SCD between the age of 21 and 40 years. A
rapid clinical improvement of tachypnea/dyspnea and oxygen saturation
was observed, after red blood cell exchange transfusion, in a young
girl with SCD and worsening of anemia (Hb level from 9.2 g/dl to
6.1g/dl).
Conclusions: The
data presented in this survey permit an early assessment of the
clinical characteristics of COVID 19 in different countries. 70% of
symptomatic patients with COVID-19 required hospitalization. The
presence of associated co-morbidities can aggravate the severity
of COVID- 19, leading to a poorer prognosis irrespective of age.
|
Introduction
The
recent COVID-19 outbreak has been deemed a global health emergency.
From Dec 31, 2019, to May 20, 2020, 4,861,456 cases of COVID-19 (in
accordance with the applied case definitions and testing strategies in
the affected countries), and 322,483 deaths were reported (https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases).
The
clinical presentation of COVID-19 disease is quite variable.[1,2]
A study from the Chinese Center for Disease Control and
Prevention on 72,314 patients with COVID-19 (44,672
laboratory-confirmed, 16,186 suspected, and 10,567 clinically
diagnosed), reported that the clinical severity was mild in 81.4%,
severe in 13.9%, and critical in 4.7%. Most patients were between 30 to
79 years of age (87%), 1% were less than 9 years, 1% between 10
and 19 years and 3% were 80 and above years.[3]
Subjects
at higher risk for severe illness are: adults > 60 years,
patients with chronic diseases [heart, lungs, and end-stage renal
disease neuromuscular disorders, sickle cell disease (SCD), cirrhosis
and diabetes], immunocompromised patients, pregnant women or those
immediately postpartum (<2 weeks), and patients who reside in
nursing homes or long-term care facilities (https://www.england.nhs.uk/coronavirus/wp content/uploads/ sites; Updated:11 April 2020).
In
subjects with SCD, this is probably due to impaired immunity resulting
from impaired function of the spleen, systemic vasculopathy that
predisposes to end-organ dysfunction, and an increased risk of
thrombosis. Patients with thalassemias could have multiple
organ impairment due to iron overload and chronic anemia (chronic
hypoxemia) of the heart, lungs (pulmonary artery hypertension), liver
and endocrine glands. Both groups of patients have been considered
vulnerable to COVID-19 and at potentially higher risk for severe
complications compared to the general young population (especially in
the older age group).[4] Moreover, coexistent immune system
impairment in patients with thalassemia also predisposes to more severe
COVID-19 disease.[4] However, there is limited information available on
COVID-19 infection in patients with hemoglobinopathies.[4-9]
We
report the preliminary results of an International Multicentre Study
(IMS), promoted by the International Network of Clinicians for
Endocrinopathies in Thalassemia and Adolescence Medicine (ICET-A). The
survey aimed to investigate the COVID-19 retrospectively in patients
with hemoglobinopathies, namely β-thalassemias (TM and TI) and SCD,
followed in 17 Centers from 10 countries.
Survey Design and Participants
Questionnaire development.
A. First step. In
April 2020, the Coordinator of the ICET-A (VDS) with DC and JLVC
designed and promoted a survey questionnaire to collect, as a primary
aim, data on confirmed COVID-19 in patients with
hemoglobinopathies and as a secondary aim, the numbers of suspected or
probable COVID-19 cases,[10] without performing the test, registered
from Jan 1, 2020, to 7th June 2020.
For
a uniform collection of data, the diagnosis of β-thalassemias was based
on the definitions proposed by Kattamis et al.[11] in TM
patients, Karimi et al.[12] in TI cases, and by Quinn[13] for the
diagnosis of SCD.
Before the distribution to the ICET-A members,
the questionnaire was revised and discussed with the ICET-A Board (SD,
CK, and MK).
A. Second step. The final questionnaire was sent by mail to 12 Thalassemia Centers of the ICET-A Network.
Each
ICET-A member was free to distribute the questionnaire to other
Thalassemia Centers within their own country. The deadline for
returning the requested data was fixed to 7th June 2020.
Definitions of confirmed COVID-19 and close contact.
A patient was classified as confirmed COVID-19 in the presence of
laboratory confirmation of COVID-19 infection, documented by at least
one nasal/pharyngeal swab specimen positive for SARS-CoV-2 nucleic acid
testing (NAT) using reverse-transcriptase polymerase-chain-reaction
(RT-PCR) technology,[14] irrespective of clinical signs and symptoms,
according to the European Centre for Disease Prevention and Control as
of Mar 2, 2020. Close contact definition was based on the current WHO
available information.[10]
Data collection.
The collected data included: demographic data, medical history,
exposure history, underlying comorbidities, symptoms, signs, laboratory
findings, chest X-ray and/or computed tomography (CT) scans, treatment
schedules, and outcomes. The disease onset was defined as the day when
the first symptoms appeared before the first medical
contact/examination. Time (in days) from the onset of disease to
hospital discharge was also recorded. Laboratory data included:
complete blood cell count (CBC), C-reactive protein (CRP), erythrocyte
sedimentation rate (ESR), liver enzymes and serum creatinine, D-dimer,
levels of procalcitonin, serum lactate dehydrogenase (LDH), and serum
ferritin levels.
Clinical and diagnostic classification.
Patients with a nasal/pharyngeal swab specimen positive for SARS-CoV-2,
fever, respiratory symptoms, and radiologic changes consistent with
diffuse pneumonitis were defined as having pneumonic COVID-19. Positive patients, with fever and respiratory symptoms without radiologic changes, were defined as having non-pneumonic COVID-19. Positive patients, without fever and respiratory symptoms, were defined as asymptomatic.
Furthermore, according to the latest recommendations of the National
Health Commission of the People's Republic of China[14] and
WHO,10 COVID-19 disease was classified into four types: mild, moderate, severe and critical. Type 1 mild: mild clinical symptoms without pneumonia at chest computed tomography; Type 2 moderate: fever and other respiratory symptoms with pneumonia seen at imaging; Type 3 severe:
respiratory distress (≥ 30 breaths per min), hypoxia (oxygen
saturation: ≤ 93%), or abnormal results of blood gas analysis; and type 4 critical:
respiratory failure requiring mechanical ventilation, shock, or other
organ(s) failure requiring intensive care unit monitoring and treatment.
Study approval.
Ethical approval and informed patient or guardian consent were obtained
in accordance with local institutional requirements and with Good
Clinical Practice and the Declaration of Helsinki principles for
ethical research, and its later amendments.
Data presentation.
Descriptive statistics of the participants' baseline characteristics
are provided as mean and standard deviation (SD) for continuous
variables and frequency and percentages for categorical variables. A
detailed description of confirmed COVID-19 patients is also provided.
Results
A
total of 17 Centers, 5 from Turkey, 3 from Italy, 2 from Bulgaria, and
1 each from Azerbaijan, Cyprus, Greece, India, Iran, Oman, and Qatar,
following 9,499 patients with hemoglobinopathies (β- thalassemias and
SCD), participated in the survey. The distribution by age groups, sex,
and type of hemoglobinopathy are listed in table 1. The largest group of patients with TM and TI was reported in Azerbaijan (TM:1,304; TI:605) and for SCD in Oman (SCD: 2,000).
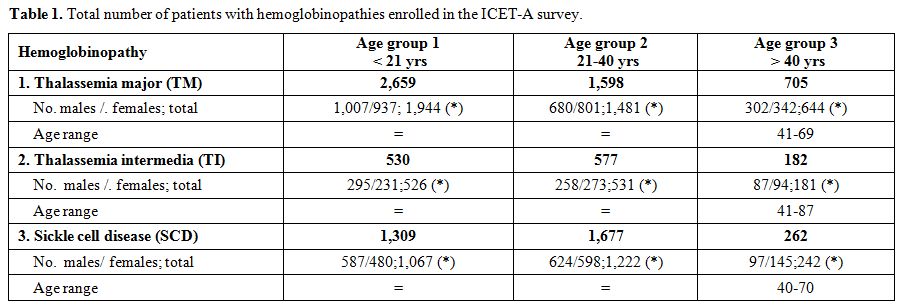 |
Table 1. Total number of patients with hemoglobinopathies enrolled in the ICET-A survey. |
A
total of 13 patients with confirmed COVID-19 by RT-PCR, 7 with TM, 3
with TI, and 3 with SCD, were retrospectively identified from 6 Centers
(Azerbaijan, Cyprus, Iran, Italy, Turkey, and Oman) (Table 2). The overall mean age of patients was 33.7±12.3 years (range:13-66); 9 (69.2%) were females.
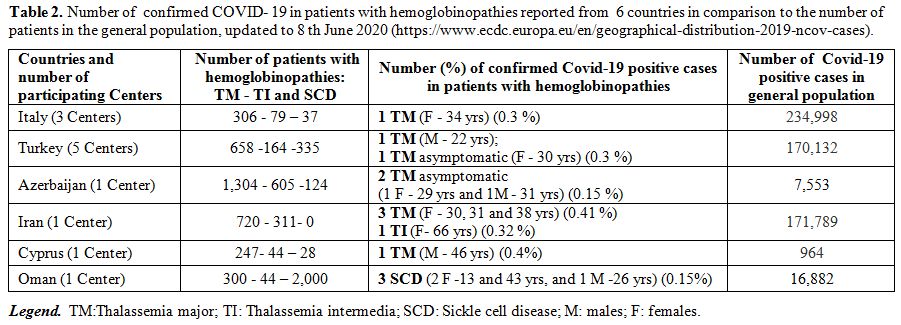 |
Table 2. Number of
confirmed COVID- 19 in patients with hemoglobinopathies reported
from 6 countries in comparison to the number of patients in the
general population, updated to 8 th June 2020
(https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases). |
The general characteristics of patients with thalassemias, SCD, and confirmed COVID- 19 are reported in table 3.
None of them were overweight. The mean serum ferritin level in 12/13
patients was 1,428±1,538 ng/ml. Four patients with thalassemia had been
splenectomised; 4 had endocrine complications: 3 had insulin-dependent
diabetes mellitus (2 with TM and in 1 with TI), and one hypogonadal
female patient had a history of renal disease with hypertension. One
patient had arrhythmia (Table 2).
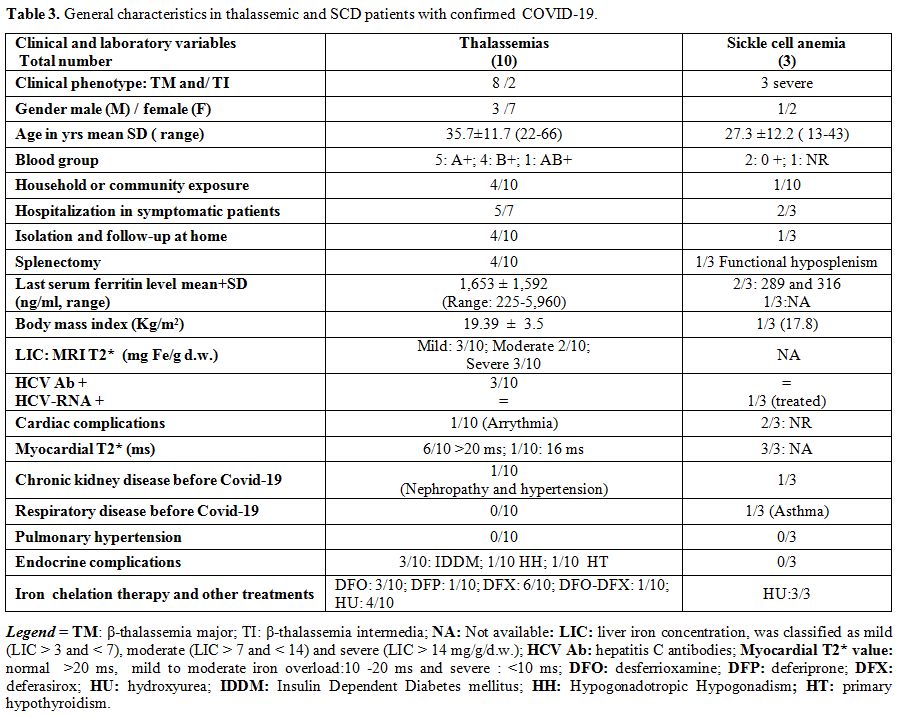 |
Table 3. General characteristics in thalassemic and SCD patients with confirmed COVID-19. |
Three
asymptomatic patients had laboratory-confirmed positive results for
SARS-CoV-2 (based on nucleic acid testing of pharyngeal swab samples).
All had had close contact with an infected family member or community
exposure. These patients remained asymptomatic throughout quarantine
and clinical monitoring.
In symptomatic patients, the mean interval between symptoms onset and first medical evaluation was 5.0 ± 3.4 days (range: 3–14).
Fever
was present in 8 out of 10 (80%) symptomatic patients (peak
38.1°C-39.5°C). Other common signs and symptoms were: cough (70%),
headache (60%), fatigue (60%), gastrointestinal symptoms (diarrhea
/vomiting/abdominal pain; 50%), tachypnea/dyspnea (40%), sore throat
(40%), anosmia/hyposmia (40%), conjunctivitis (30%), rhinorrhea (20%)
and myalgia (10%). Back and chest pain were reported in a patient with
SCD. Six patients had pneumonia (unilateral in 3, bilateral in 2, and
multiple opacities in 1 patient), and four needed oxygen therapy, and
four patients (2 with SCD) had non-pneumonic COVID-19.
In the
majority of patients (6), the course of COVID-19 was defined as
moderate, and severe in 3. Oxygen saturation of ≤ 93% was documented in
3 patients. One of them, a 30-year-old female with TM developed
critical type 4 COVID-19, according to the National Health Commission
of the People's Republic of China[14] and WHO,[10] characterized
by progressive respiratory and renal insufficiency, followed by death
in an Intensive Care Unit.
Increased C-reactive protein (6/10),
high LDH (6/10), high ESR (6/10), and high D-dimers, in 4 out of 5
tested patients, were the most common laboratory findings. Six patients
with confirmed COVID-19 (1 with SCD) had a reduction of hemoglobin
levels, three patients had lymphopenia (low absolute number of
lymphocytes), and 1 SCD patient had thrombocytopenia.
Experimental
treatments for SARS-CoV-2 infection, including hydroxychloroquine (2.5
mg/kg twice daily; 2 patients), azithromycin (10 mg/kg once
daily)/clarithromycin (7.5 mg/kg twice daily) or moxifloxacin (3
patients), were given to 5 of 6 patients with TM. Low molecular
weight heparin and antiviral drugs (2 and 1 out of 10 patients,
respectively) were less commonly used. A rapid clinical improvement of
tachypnea/dyspnea and oxygen saturation was observed, after red blood
cell exchange transfusion, in a 13-year-old girl with SCD and COVID-19.
At hospital admission, she presented with high fever, cough, worsening
of anemia (decreased Hb level from 9.2 g/dl to 6.1g/dl) and elevated
D-Dimers).
None of the SCD patients received hydroxychloroquine or convalescence plasma transfusion.
The average time from the onset of disease to hospital discharge was 12.8±5.4 days.
Seven
Centers did not report cases of suspected or probable COVID-19, and 5
Centers did not respond with the requested information. One Center
reported detailed information (data are presented in Table 4). The majority of cases were observed in patients with SCD, between 21- 40 years (5.5%).
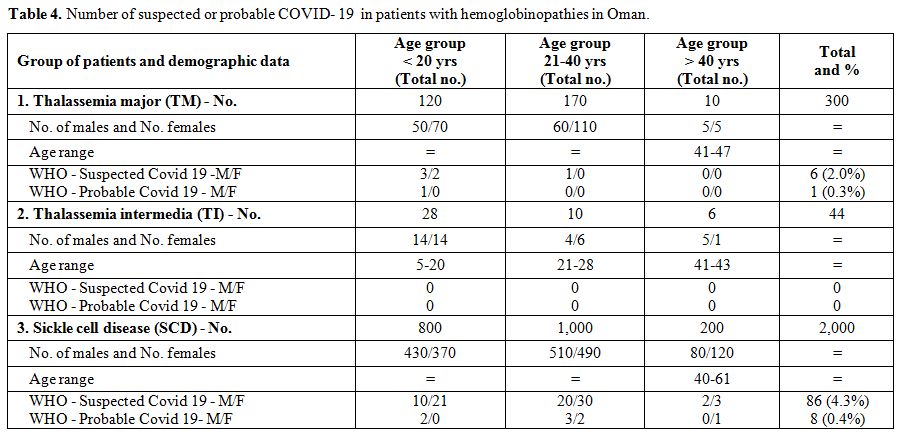 |
Table 4. Number of suspected or probable COVID- 19 in patients with hemoglobinopathies in Oman. |
Discussion
People
of all ages are susceptible to SARS- CoV-19 infection. Clinical
manifestations of SARS-CoV-19 infection range from asymptomatic to
severe pneumonia and respiratory failure. Severe disease can lead to
death. Hospitalization rates are higher for people of advanced age
(> 60 years). Although age and comorbidities have been described as
the main determinants of disease progression towards severe respiratory
distress, the high diversity in clinical severity among patients could
suggest a possible role of the host genetic background contributing to
the observed inter-individual clinical variability associated with
COVID-19 in black and ethnic minority people.
Up to 7th
June 2020, the impact of COVID-19 in thalassaemic or SCD patients had
only been preliminarily evaluated in different countries.[3-9,15]
A study of a small cohort of patients with thalassemia from
Northern Italy, which was the epicenter for coronavirus COVID-19 in
Europe, showed relatively mild to moderate COVID-19 disease in 11
patients (10 with TM and 1 with TI) compared to the general population
with all infected thalassemia patients recovered.[9] The mean age
of this cohort of the thalassemic patients was 44 ± 11 years (range
31-61 years), and 55% (6/11) were females. One patient, with severe
symptoms, required ventilation with continuous positive airway pressure
(CPAP). All patients had associated comorbidities, and 70% were
splenectomised. The likely source of infection was found in 64% of
cases, while the clinical course ranged from 10 to 29 days.[9]
A
multicenter, retrospective, cross-sectional study was obtained across
all comprehensive thalassemia centers in Iran, from January to Apr 29,
2020. All suspected and confirmed COVID-19 cases from a total of 15,950
TM and 2,400 TI patients were evaluated Fifteen confirmed
cases (12 TM and 3 TI; mean age 36.1 ± 12.1; range 22-66 years).
Moreover, eight symptomatic suspected β- thalassemia patients (6 TM and
2 TI) of COVID-19 were detected. Seventeen patients (73.9%) had
mild to moderate symptoms and recovered, while six patients died
(26.1%, 2 TM, and 4 TI). More than 60% of all patients had at least one
comorbidity, and 80% were splenectomised. The prevalence of COVID-19 in
thalassemia patients was less than the general population, but the
mortality rate was significantly higher, also taking into consideration
the lower age.[15] Therefore, these findings provide further objective
evidence to take into account the comprehensive risk assessment and
prognosis among thalassemic patients with COVID-19.
The
clinicopathological features, management, and outcomes of 10 SCD
patients (8 male and 2 female), with COVID-19 infection (6 with
confirmed COVID-19 and 4 with suggestive clinical, laboratory and
radiological features, but a negative swab) with a mean age of 36
years, were reported by McCloskey et al.[5] A 57-year-old patient with
several pre-existing comorbidities including severe neurological
impairment as a result of a previous stroke, died. Another patient with
chronic kidney disease (CKD) stage III developed significant
deterioration of renal function and required temporary peritoneal
dialysis, but otherwise had a full recovery, as did the remaining
patients.[5]
To our knowledge, this is the first
preliminary multicenter study evaluating the COVID-19 in patients with
hemoglobinopathies, namely β-thalassemias and SCD. Approximately 90% of
COVID-19 cases are associated with household or community exposure, and
10% are associated with travel.[1,2] In the present survey the
likely source of infection was detected in only 5/13 (38.4%) of
patients.
A recent review of data from 59,254 patients from 11
countries has shown a positive association between male sex and a
higher mortality rate.[16] Although adult men are more susceptible to
COVID-19 infection and adult females produce more robust inflammatory
responses as compared to men,[17] 10 out of 13 of patients (76.9%) in
our survey were females. Therefore, further studies are needed to
elucidate this particular gender aspect of COVID-19 in
hemoglobinopathies.
Asymptomatic infection at the time of
laboratory confirmation has been reported in many
settings.[18,19] Some of these cases developed symptoms at a
later stage of infection; the proportion of these cases has not yet
thoroughly evaluated.[20] There are also reports of patients remaining
asymptomatic throughout quarantine, as observed in our patients.
Four
of our patients (2 with SCD) had non-pneumonic COVID-19, 6 had
pneumonic COVID-19, and five patients, in addition to fever and cough,
had gastrointestinal symptoms, such as diarrhea, vomiting and/or
abdominal pain.
In the majority of patients (90 %) worldwide, the
outcome of COVID-19 infection has been defined as a mild or
moderate disease, but severe, and especially critical cases are
accompanied by a high mortality rate. Current knowledge has shown that
the mortality rate is high in people with chronic underlying
diseases.[21,22]
Blood groups were known in 12 of our 13 confirmed
COVID-19 patients: 5 were blood group A (41.6%), 33.3% blood group B,
16.6% blood group 0, and 8.3% blood group AB. Therefore, it is
plausible that different blood groups might vary in their
susceptibility to COVID-19, as reported by Zhao et al.[23] However, more
evidence is needed to confirm this observation, taking into
consideration the specific distribution of blood groups among
populations.
Furthermore, in patients with
hemoglobinopathies, several factors may be associated directly or
indirectly with the triggering of a severe outcome of the
COVID-19.[3-6] Both intravascular and extravascular hemolysis can
occur in thalassemia patients. Clinicians should, therefore, closely
monitor blood counts of thalassemia patients with COVID-19, and caution
should be maintained towards the possibility of exacerbated hemolytic
anemia in the setting of acute viral infection.
Moreover, there
is a significant concern that the overlap of lung disease from COVID-19
with acute chest syndrome (ACS) may result in increased complications
among individuals with SCD.[24]
Splenectomy is a common
therapeutic intervention in β-thalassemias, while many SCD patients
have a hypo-functional spleen. Splenectomy was reported in 6/13 of our
patients with thalassemias.
Based on the knowledge of the
immunological functions of the spleen, there is no evidence that
asplenic/ hyposplenic patients are at higher risk of having severe
COVID -19 infection.
Nevertheless, since fever could indicate
bacterial as well as viral infection, all patients should be instructed
to seek medical advice by contacting their clinical team if they
develop fever. Medical consideration should be given to the presence of
superimposed infection, particularly with encapsulated pathogens.[25,26]
In
patients with SCD, hypoxia, dehydration, or acidosis due to respiratory
infection may trigger a vaso-occlusive and hemolytic crisis and acute
chest syndrome (ACS), with a high risk of thrombosis in pulmonary
arteries.[27] Thus, measures to prevent and treat ACS early in the
event of viral infection, require particular alertness by physicians
treating infected patients.[28]
Another relevant aspect for
COVID-19 infection in hemoglobinopathies, mainly SCD and TI, relates to
current therapy with hydroxycarbamide (hydroxyurea), a cytotoxic agent,
with possible immune-compromising effects contributing to an adverse
outcome of these patients.[28] Risk stratification
recommendations for children and adults hospitalized with COVID are
available from the American Society of Hematology (https://hematology.org/covid-19/covid-19-and-vte-anticoagulation).
The
current study has some limitations. First, only 13 patients with
confirmed COVID-19 infection were identified. However, the data
presented in this study permit an early assessment of the clinical
characteristics of COVID-19 in different countries. Second, though the
sample of patients with COVID-19 was small, we observed a prevalence of
females versus males. These data contrast with the reduced
susceptibility of females, probably linked to the X chromosome and sex
hormones, which play an important role in innate and adaptive
immunity.[29] Nevertheless, this is a preliminary report of a rapidly
evolving condition, as the parameters discussed here are changing
quickly with time. Third, our survey included mainly young adult
patients with an age range between 20 and 40 years. Lastly, the number
of reported COVID-19 cases has certainly underestimated the real burden
of disease, given the widespread unavailability and accuracy of tests,
and also the significant proportion of infected persons, who develop
asymptomatic or mild unidentified forms of the disease, remain
undiagnosed. The high number of suspected or probable COVID-19 in
patients with SCD sustain this hypothesis. Therefore, we should be
careful when measuring the prevalence of confirmed COVID-19,
acknowledging that the rate will be likely higher once the denominator
is adjusted to the correct number of individuals who acquired the
infection.
Conclusions
It
is reasonable to say that few cases of COVID-19 have so far been
reported in thalassemias and SCD patients in the literature. Is this
due to a lack of testing or a real lack of infection/susceptibility? In
our survey, a total of only 13 patients with confirmed COVID-19 were
identified in 17 Centers, from 10 countries, following 9,499 patients
with hemoglobinopathies. However, our provisional data should be
interpreted cautiously because only 20% of patients with thalassemias
and 8.7% of SCD patients were in the higher age group (> 40 years)
for SARS-CoV-2 infection. Despite their age, 70% of symptomatic
COVID-19 patients required hospitalization, and the clinical outcome in
one patient confirmed that associated comorbidities could aggravate the
severity of infection, leading to death. Automated exchange transfusion
improved the outcome of COVID-19 respiratory failure in a young girl
with SCD. In the forthcoming weeks, we will continue to monitor the
epidemiology of the COVID-19 outbreak collecting data from the
participating Centers. Any further international participation is
welcomed.
References
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu
Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological
and clinical characteristics of 99 cases of 2019 novel coronavirus
pneumonia in Wuhan, China: a descriptive study. Lancet.
2020;395:507-513. https://doi.org/10.1016/S0140-6736(20)30211-7
- Wichmann
D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A,
Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G,
Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de
Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S,
Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy
Findings and Venous Thromboembolism in Patients With COVID-19. Ann
Intern Med. 2020;M20-2003. https://doi.org/10.7326/M20-2003 PMid:32374815 PMCid:PMC7240772
- Wu
Z, McGoogan JM. Characteristics of and Important Lessons From the
Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a
Report of 72 314 Cases From the Chinese Center for Disease Control and
Prevention [published online ahead of print, 2020 Feb 24]. JAMA.
2020;10.1001/ jama.2020. 2648. https://doi.org/10.1001/jama.2020.2648 PMid:32091533
- Karimi
M, De Sanctis V. Implications of SARSr-CoV 2 infection in thalassemias:
Do patients fall into the "high clinical risk" category? Acta Biomed.
2020;91:50-56. https://doi.org/10.23750/abm.v91i2.9592
- McCloskey KA, Meenan J, Hall R,
Tsitsikas DA. COVID-19 Infection and Sickle Cell Disease: A UK Centre
Experience. Br J Haematol. 2020;10.1111/bjh.16779.
https://doi.org/10.1111/bjh.16779 PMid:32369606
- Hussain
FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. COVID-19
infection in patients with sickle cell disease. Br J Haematol.
2020;10.1111/bjh.16734. https://doi.org/10.1111/bjh.16734 PMid:32314798
PMCid:PMC7264585
- Nur E, Gaartman AE, van Tuijn CFJ, Tang
MW, Biemond BJ. Vaso-occlusive crisis and acute chest syndrome in
sickle cell disease due to 2019 novel coronavirus disease (COVID-19).
Am J Hematol. 2020; 95:725‐726. https://doi.org/10.1002/ajh.25821
PMid:32267016 PMCid:PMC7262303
- Odièvre MH, de Marcellus
C, Ducou Le Pointe H, Allali S, Romain AS, Youn J, Taytard J, Nathan N,
Corvol H. Dramatic improvement after tocilizumab of severe COVID-19 in
a child with sickle cell disease and acute chest syndrome. Am J
Hematol. 2020;10.1002/ajh.25855. https://doi.org/10.1002/ajh.25855
PMid:32358817 PMCid:PMC7267654
- Motta I, Migone De Amicis
M, Pinto VM, Balocco M, Longo F, Bonetti F, Gianesin B, Graziadei G,
Cappellini MD, De Franceschi L, Piga A, Forni GL. SARS-CoV-2 infection
in beta thalassemia: Preliminary data from the Italian experience. Am J
Hematol. 2020;10.1002/ajh.25840. https://doi.org/10.1002/ajh.25840
PMid:32311145 PMCid:PMC7264660
- WHO. Clinical management
of severe acute respiratory infection when novel coronavirus (nCoV)
infection is suspected. 2020;
https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratoryinfection-when-novel-coronavirus-(ncov)-infection-is-suspected
- Kattamis C, Metaxotou-Mavromati A, Ladis VH. Tsiarta HS,
Laskari S, Kanavakis E. The clinical phenotype of β and δβ thalassemias
in Greece. Eur J Pediatr.1982;139:135-138.
https://doi.org/10.1007/BF00441497 PMid:7151834
- Karimi M,
Cohan N, De Sanctis V, Mallat NS, Taher A. Guidelines for diagnosis and
management of Beta-thalassemia intermedia. Pediat Hematol Oncol.
2014;31:583-96. https://doi.org/10.3109/08880018.2014.937884
PMid:25247665
- Quinn CT. Minireview: Clinical severity in
sickle cell disease: the challenges of definition and prognostication.
Exp Biol Med (Maywood). 2016;241:679-688.
https://doi.org/10.1177/1535370216640385 PMid:27013545 PMCid:PMC4871738
- National
Health Commission of the People's Republic of China. National
recommendations for diagnosis and treatment of respiratory infections
caused by 2019-nCoV (the 6th edition). 18-20 February 2020. Available
from: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d
329df351d7da8aefc2/ files/ b218cfeb1bc54639af227f922b f6b817.pdf
- Karimi
M, Haghpanah S, Azarkeivan A, Zahedi Z, Zarei T, Akhavan Tavakoli M,
Bazrafshan A, Shirkavand A, De Sanctis V. Prevalence and Mortality due
to Outbreak of Novel Coronavirus Disease (COVID-19) in β-Thalassemias:
The Nationwide Iranian Experience [published online ahead of print,
2020 Jun 2]. Br J Haematol. 2020. https://doi.org/10.1111/bjh.16911
PMid:32484906 PMCid:PMC7300954
- Borges do Nascimento IJ,
Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I,
Esfahani MA, Civile VT, Marusic A, Jeroncic A, Carvas Junior N, Pericic
TP, Zakarija-Grkovic I, Meirelles Guimarães SM, Luigi Bragazzi N,
Bjorklund M, Sofi-Mahmudi A, Altujjar M, Tian M, Arcani DMC, O'Mathúna
DP, Marcolino MS. Novel Coronavirus Infection (COVID-19) in Humans: A
Scoping Review and Meta-Analysis. J Clin Med. 2020 Mar
30;9(4):941.https://doi.org/10.3390/jcm9040941 PMid:32235486
PMCid:PMC7230636
- Klein SL, Flanagan KL. Sex differences
in immune responses. Nat Rev Immunol. 2016;16(10):626-638.
https://doi.org/10.1038/nri.2016.90 PMid:27546235
- Mizumoto
K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic
proportion of coronavirus disease 2019 (COVID-19) cases on board the
Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill.
2020;25(10):2000180.
https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000180 PMid:32183930
PMCid:PMC7078829
- Ki M; Task Force for 2019-nCoV.
Epidemiologic characteristics of early cases with 2019 novel
coronavirus (2019-nCoV) disease in Korea. Epidemiol Health.
2020;42:e2020007. https://doi.org/10.4178/epih.e2020007 PMid:32035431
- Cereda
D, Tirani M, Rovida F, Demicheli V, Ajelli M, Poletti P, Trentini F,
Guzzetta G, Marziano V, Barone A, Magoni M, Deandrea S, Diurno G,
Lombardo M, Faccini M, Pan A, Bruno R, Pariani E, Grasselli G, Piatti
A, Gramegna M, Baldanti F, Melegaro A, Merler S. The early phase of the
COVID-19 outbreak in Lombardy, Italy 2020. Available from:
https://arxiv.org/abs/2003.09320v1
- Sun K, Chen J,
Viboud C. Early epidemiological analysis of the coronavirus disease
2019 outbreak based on crowd sourced data: a population-level
observational study. The Lancet Digital Health. 2020.
https://doi.org/10.1016/S2589-7500(20)30026-1
- Emami A,
Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of Underlying Diseases
in Hospitalized Patients with COVID-19: a Systematic Review and
Meta-Analysis. Arch Acad Emerg Med. 2020;8(1):e35. Published 2020 Mar
24.
- Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X , Zhang Z,
Liu L, Liu T, Liu Y, He Y, Sun B, Wei M, Yang G, Wang X, Zhang L, Zhou
X, Xing M, Wang PG. Relationship between the ABO blood group and the
COVID-19 susceptibility. [Accessed on: 23/03/2020.]medRxiv 2020.03.11.
20031096 https://doi.org/10.1101/2020.03.11.20031096
- Ali
T. Taher, Rayan Bou‐Fakhredin, Firas Kreidieh, Irene Motta, Lucia De
Franceschi, Maria Domenica Cappellini. Care of patients with hemoglobin
disorders during the COVID‐19 pandemic: An overview of
recommendations.Am J Hematol. 2020 May 21:10.1002/ajh.25857.
https://doi.org/10.1002/ajh.25857 PMid:32394480 PMCid:PMC7272998
- Leone
G, Pizzigallo E. Bacterial Infections Following Splenectomy for
Malignant and Nonmalignant Hematologic Diseases Mediterr J Hematol
Infect Dis. 2015;7(1): e2015057. Published online 2015 Oct 13.
https://doi.org/10.4084/mjhid.2015.057 PMid:26543526 PMCid:PMC4621170
- Haemoglobinopathy
HCCs: Advice on COVID-19 in patients with Sickle Cell Disease and
Thalassaemia Haemoglobinopathy Co-ordinating Centres V9 Apr 20 2020.
https://b-s-h.org.uk/media/18244/hbp-hccs-response-to-covid-v9-200420.pdf
- Heilbronner C, Berteloot L, Tremolieres P, Dupic L, De
Saint Blanquat L, Lesage F, Odièvre MH, de Marcellus C, Fourgeaud J, de
Montalembert M, Grimaud M, Moulin F, Renolleau S, Allali S, Oualha M.
Patients with Sickle cell disease and suspected COVID-19 in a pediatric
ICU. Br J Haematol. 2020; 10.1111/ bjh.16802.
https://doi.org/10.1111/bjh.16802 PMid:32420608 PMCid:PMC7276717
- Vives
Corrons JL, De Sanctis V. Rare Anaemias, Sickle-Cell Disease and
COVID-19. Acta Biomed. 2020;91(2):216‐217.Published 2020 May
11.doi:10.23750/abm.v91i2.9532.
- Jaillon S, Berthenet K,
Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy
Immunol. 2019;56: 308-321. https://doi.org/10.1007/s12016-017-8648-x
PMid:2896361
TOP]



