Federica Sora1,2, Patrizia Chiusolo1,2, Luca Laurenti1,2, Idanna Innocenti1, Francesco Autore1, Sabrina Giammarco1, Elisabetta Metafuni1, Eleonora Alma1, Alessia Di Giovanni1, Simona Sica1,2 and Andrea Bacigalupo1,2.
1
Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed
Ematologia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma.
2 Sezione di Ematologia, Dipartimento di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Roma.
Correspondence to: Andrea Bacigalupo. Istituto di Ematologia,
Policlinico “A. Gemelli”, Università Cattolica S.Cuore, Largo Gemelli,
8, 00168 Roma. Tel. +39-06-30154278, fax +39- 06-3017319. E-mail:
andrea.bacigalupo@unicatt.it
Published: September 1, 2020
Received: April 30, 2020
Accepted: August 4, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020055 DOI
10.4084/MJHID.2020.055
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
We
have studied the number of days alive outside the Hospital (DAOH) and
the number of readmissions within the first 100 days after transplant
in 185 patients who received an allogeneic hemopoietic stem cell
transplant (HSCT). The donors were matched siblings (SIB; n=61), or
alternative donors (ALT; n=124). The median number of DAOH for SIB
transplants (78 days, range 21-84) was significantly greater than DAOH
for ALT donor grafts (73 days, range 2-87) (p=0.0003). Other positive
predictors of DAOH were the use of reduced-intensity regimens (p=0.01),
grade 0-I acute graft versus host disease (GvHD) (p=0.0006), and a
comorbidity index equal or less than two (p=0.04). Fifty-one patients
required readmission (22%), which was predicted by grade II-IV acute
GvHD (p=0.009), higher comorbidity index (p=0.06), and ALT donors as
compared to SIBS (p=0.08). The CI of readmission was 18% (95%CI 10-31)
for SIB and 30% (95%CI 23-39) for ALT donor grafts. The non relapse
mortality (NRM) for patients re-admitted was 25% (95%CI 15-43%),
compared to 5% (95%CI 2-12%) for patients not readmitted (p=0.0001). In
a multivariate analysis, readmission was the strongest predictor of
non-relapse mortality (NRM) (HR 2.0) (p=0.0006) and survival (HR 3.4)
(p<0.0001).
In conclusion: ALT donor transplants have lower
numbers of DAOH, as compared to SIB grafts, which implies longer stay
in hospital and higher costs. Readmission to hospital within 100 days,
is predicted by acute GvHD, comorbidity index, donor type, and has a
significant strong impact on non-relapse mortality and survival.
|
Introduction
Allogeneic
hemopoietic stem cell transplantation (HSCT) can be performed using
different donor types, including HLA identical siblings (SIB),
unrelated donors (UD), matched unrelated donors (MUD), mismatched
unrelated donors (mmUD), cord blood units (CB), or family HLA
haploidentical donors (HAPLO). Many retrospective studies have compared
the outcome of transplants from different donor types, with endpoints
such as acute and chronic graft versus host disease (GvHD), non-relapse
mortality (NRM), relapse, relapse related death, survival, and
disease-free survival.[1-7] Another less common
outcome is the length of stay in hospitals and the number of
readmissions in the first 100 days. Length of stay in Hospital (LOS)
within 100 days from transplant is a significant component of early
post-transplant costs and is estimated to represents between 75% and
95% of the total transplant cost;[8-11] it also
provides a surrogate marker of early complications. The bias due to
early deaths, leading to a short Hospital stay, can be overcome by
calculating the number of days alive out of Hospital (DAOH), within the
first 100 days,[12] therefore excluding patients who
were never discharged. This parameter gives a rapid evaluation of
tolerance of a given procedure and toxicity, beyond crude NRM,
including readmissions due to cytopenia or GvHD. In a recent paper,[2]
patients receiving CB grafts had a significantly lower number of days
alive outside the hospital, as compared to matched and mismatched UD
grafts.
The primary objective of our present study was to
compare days alive and outside the Hospital (DAOH) in recipients of
HSCT, together with the rate of readmission to the transplant ward, in
the first 100 days after HSCT, in patients receiving grafts from SIB
donors, UD and HLA family HAPLO identical donors.
Material and Methods
Patients.
We retrospectively analyzed medical records of 185 patients who
received an allogeneic transplant for hematological malignancies
between February 2012 and January 2018 in our Department and had been
discharged after transplantation within 100 days. The study was
approved by our Institutional Review Board. Included were consecutive
transplants from different donor types, excluding one unrelated cord
blood graft. Medical records were retrospectively reviewed for
demographic data, diagnosis and disease phase, GVHD prophylaxis, stem
cell source and donor type, CD34+ cell dose in the graft, duration of
hospital stay, time of engraftment, and acute GvHD. When a potential
transplant candidate lacked a suitable HLA-identical sibling donor
(SIB), the search for a matched unrelated donor (UD) was started. A
haploidentical related donor (HAPLO) was chosen as a donor, when
suitable HLA matched sibling or volunteer UD, were either temporarily
or definitively unavailable; when 8/8 HLA matched unrelated donor
clinical characteristics of 185 patients are outlined in Table 1.
GvHD prophylaxis consisted of cyclosporine A and short-course
methotrexate (CsA+MTX) for SIB grafts, with the addition of rabbit
anti-thymocyte globulin (ATG) (Thymoglobulin, Genzyme, Boston USA), 5
mg/kg for UD transplants. Patients receiving bone marrow grafts from
HAPLO related donors received GvHD prophylaxis with CsA, mycophenolate
mofetil, and high dose post-transplant cyclophosphamide on days +3+5
(PT-CY).[14-19]
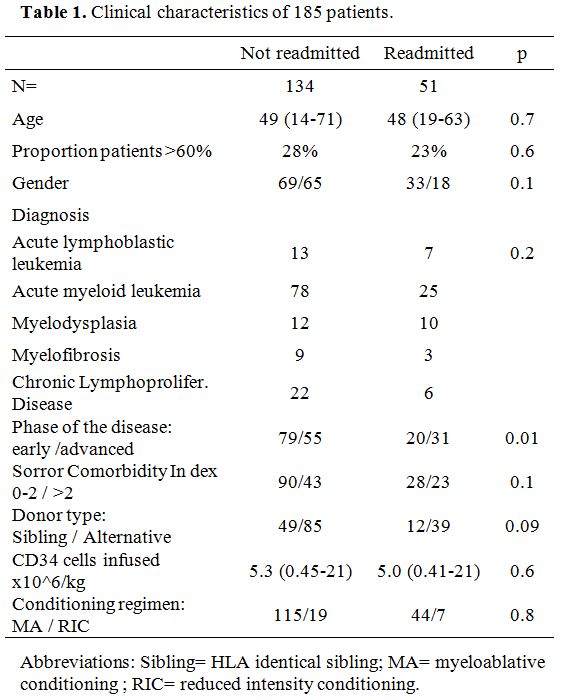 |
Table 1. Clinical characteristics of 185 patients. |
The comorbidity index was calculated as described by Sorror and coworkers.[20]
The number of CD34+ cells infused with the graft was significantly
higher in patients receiving PB cells. In the UD group, 36 patients
received an 8/8 HLA allele matched donor graft, whereas 26 patients
received a graft from a donor mismatched for 1 HLA alleles/antigens.
Endpoints. The primary endpoint of this study was days alive and out of the Hospital (DAOH), as previously reported.[12]
The secondary endpoint was the probability of readmission. Other
endpoints were: time to neutrophil engraftment; time to first
discharge; time and causes of readmission; overall survival;
non-relapse mortality; graft versus host and relapse-free survival
(GRFS).[13] Patients were readmitted either because
of fever, diarrhea, suspected GvHD, respiratory insufficiency,
hemorrhagic cystitis. The attending physicians have not changed in the
study period. First, second and third readmissions were recorded.
Statistical analysis.
The NCSS19 software was used for statistical analysis. Contingency
table analysis and the Chi-square test were used for categorical
variables. Median, mean, and the T-test were used for numerical
variables. The cumulative incidence (CI) of readmission to the hospital
was calculated using death as a competing event, and Gray's test was
used to assess differences between groups. The CI of non-relapse
mortality (NRM) was calculated with relapse as a competing event. A Cox
multivariate analysis was run on the probability of being readmitted to
the hospital, with the following variables: donor and recipients age
and gender, donor type, the intensity of the conditioning regimen
myeloablative (MA) vs. reduced-intensity (RIC), disease phase
(remission vs. non-remission), Comorbidity Index (<=/> 2),
diagnosis (acute vs. chronic disorders), the presence of GvHD grade 0-I
vs. grade II-IV. A second Cox model was run for NRM, which included the
same variables, with the addition of readmission (no vs. yes). Survival
curves were drawn according to Kaplan Meier, and the log-rank test was
used for differences between groups.
Results
DAOH.
The median number of DAOH was 73 days (range 0-88); it was 59 days
(2-82), and 77 (21-87) for patients readmitted or not (p<0.00001) (Table 2).
Patients receiving SIB grafts had significantly more DAOH as compared
to ALT donor grafts: median 78 days (21-78) compared to 73 days (2-78),
p=0.0003. This difference was also seen for SIB vs. MUD grafts (78 vs.
63 days, p=0.00002), vs. HAPLO grafts (78 vs. 72 days, p=0.0008), or
vs. mmUD grafts (78 vs. 73 days, p=0.008). There was no significant
difference in DAOH between HAPLO and UD grafts.
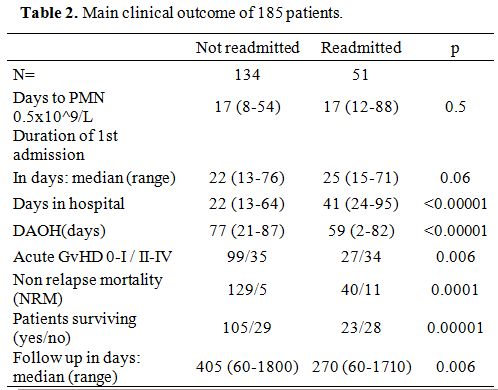 |
Table 2. Main clinical outcome of 185 patients. |
Other
variables predictive of DAOH were the intensity of the conditioning
regimen (79 days, range 24-87, for RIC vs. 74 days, range 2-78, for MA
regimens)(p=0.01), the presence of GvHD grade II-IV (72 days, range
3-87) compared with GvHD grade 0-I (76 days, range 2-78) (p=0.006), and
a comorbidity index of 0-2 (76 days, range 9-87) vs. an index >2 (72
days, range 2-78) (p=0.04). By adding together the positive predictors
(SIB transplant, comorbidity index <=2, RIC regimen and GvHD grade
0-I), the median DAOH ranged from 79 days for patients with all four
positive predictors, with a minimum of 64 DAOH, to 59 DAOH for patients
with none of them, with a minimum
Readmissions.
One hundred and seventy-nine patients were discharged within 100 days.
Fifty-one patients had to be readmitted to the Unit, within 100 days,
because of complications, and the overall cumulative incidence (CI) was
22% (95%CI 18-29%) (Figure 1):
Forty patients had one readmission, eight patients were readmitted
twice, and three patients were admitted three times. The CI of
readmission was 18% (95%CI 10-31%) for SIB grafts and 30% (95%CI
23-39%) for ALT donor grafts (p=0.09): it was 20% for HAPLO, 47% for
MUD 30% for mmUD. A higher risk of readmission was seen in patients
with acute GvHD grade II-IV (35%) compared to patients with acute GvHD
grade 0-I (21%) (p=0.01), and in patients with advanced disease
compared to patients with early disease (35% vs. 20%) (p=0.02). Age did
not impact readmission: in patients aged 14-48, the CI of readmission
was 28%; for the age >48 years, it was 24% (p=0.6).
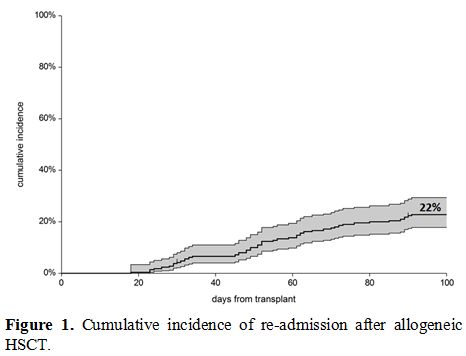 |
Figure 1. Cumulative incidence of re-admission after allogeneic HSCT. |
Other non-predictive variables were the intensity of the conditioning regimen and the number of CD34+ cells infused.
Fever
with or without documented infections was the leading cause of the
first readmission to Hospital after HSCT: 20 for ALT donor grafts and 9
for SIB grafts. Acute GvHD was the cause of readmission in 5 ALT donor
grafts and 1 SIB graft. Other reasons for readmission to the hospital,
were hemorrhagic cystitis, thoracic, or abdominal pain (Table 3).
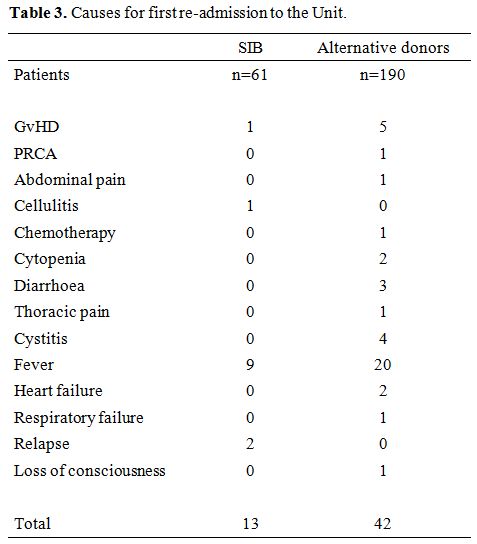 |
Table 3. Causes for first re-admission to the Unit. |
A
second readmission was recorded in 10/124 ALT donor grafts and 2/61 SIB
grafts (p=0.2). Reasons for readmission were GvHD (n=6) and fever
(n=6). A third readmission was recorded in 6 patients receiving ALT
donor grafts and 0 in SIB grafts (p=0.1): reasons for a third
readmission were GvHD in 2 patients, fever in 2 patients, dyspnea in 1
patient and pancytopenia in 1 patient.
In multivariate Cox
analysis, GvHD grade II-IV was the strongest predictor of readmission
(RR 2.2, p=0.009), with a trend for a Sorror risk score of greater than
2 (RR 1.8, p=0.06) and ALT donors compared to SIB grafts (RR 2.0,
p=0.08) (Table 4). Other variables, including stem cell source, were
not significant predictors.
Engraftment and first discharge.
The median time to a neutrophil count of 0.5x10^9/L was 17 days
(12-88). It was similar (17 days) in patients who would subsequently be
readmitted or not (Table 2).
The median time to discharge was 23 days (13-76): it was 25 vs. 22 days
for patients who would subsequently be readmitted or not (p=0.06) (Table 2).
Patients receiving ALT donor grafts were discharged at a median
interval of 25 days (range 13-76) compared to 21 days (range 15-60) for
SIB grafts (p=0.0007). The median day of discharge was 25.5 days (range
9-100) for MUD, 26 days (range 13-56) for mmUD, 25 days (range 7-100)
for HAPLO grafts: there was no significant difference in time to first
discharge, between HAPLO and MUD (p=0.7) and mmUD (p=0.8). Time to
first discharge was significantly delayed in patients grafted with a MA
regimen (24, range 15-71) compared to RIC regimens (20, range 13-76)
(p=0.006). Age (<=48/ vs >48 years) had no effect on the duration
of first admission (p=0.5). Similarly there was no difference in time
to first discharge for patients receiving <=/> 5.3 x10^6/kg CD34
cells in the transplant: 23 days (15-76) vs 23.5 days (13-64) (p=0.2).
Non-relapse mortality (NRM). After discharge, patients who required readmission had a higher risk of non-relapse mortality (NRM), as shown in Figure 2
(5% vs. 25%, p=0.0001). In a Cox model, readmission was the only
predictor of NRM (RR 8.5, p=0.0006) and the strongest predictor of
survival (RR 3.3 p=0.0001) (Table 4).
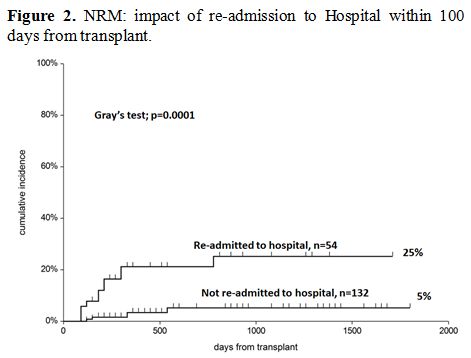 |
Figure 2. NRM: impact of re-admission to Hospital within 100 days from transplant. |
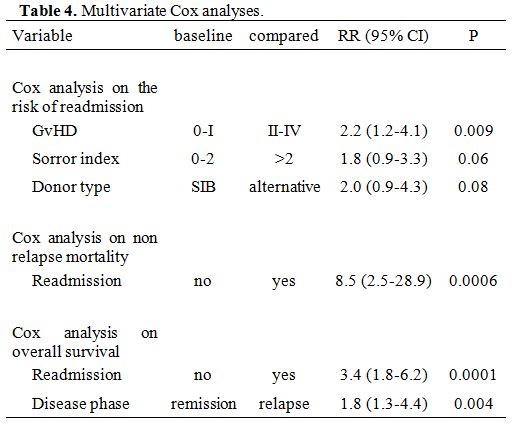 |
Table 4. Multivariate Cox analyses. |
Survival. The actuarial survival of patients who required readmission or not is shown in Figure 3,
with a significant advantage for patients nor readmitted after
transplantation. GRFS for the two groups at five years was 53% (95%CI
44-62) and 33% (95%CI 19-46), respectively (p=0.03).
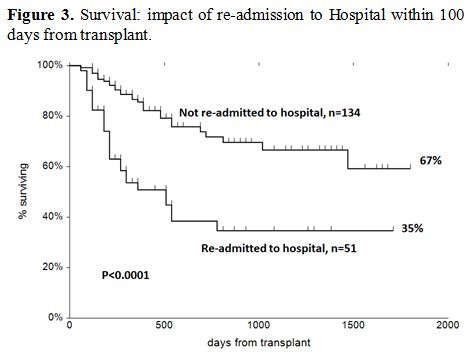 |
Figure 3. Survival: impact of re-admission to Hospital within 100 days from transplant. |
Discussion
The
number of days alive and outside the hospital, which we referred to as
DAOH, can be regarded as a critical surrogate of transplant outcome: it
gives an immediate perception of the clinical course of the patient,
the number and severity of infections, the rate of acute GvHD and the
severity of organ toxicity. When calculating DAOH together with the
number of readmissions, one can analyze an outcome that includes many
of the early transplant complications and also roughly evaluates the
cost of the transplant. In a recent study, DAOH was 65 days for single
cord blood transplants, 63 for double CB transplants, 79 for unrelated
donor transplant, with a significant difference in favor of the latter.[12]
This was true both for pediatric as well as adult patients, primarily
driven by the fact that CB grafts have delayed engraftment when
compared to UD peripheral blood transplants; the difference was less
pronounced when comparing CB vs. mismatched UD marrow grafts.[12]
In the present study, we focus on DAOH as well as on-time of first
discharge and readmissions in patients grafted from different donor
types, including unmanipulated HAPLO transplants.
Time to first
discharge was shorter in SIB transplants as compared to ALT donor
transplants by four days; interestingly there was no difference between
matched UD, mismatched UD, and HAPLO donors, despite the fact that the
latter were grafted with marrow stem cells; alternative donor
transplants were discharged 4-5 days later than SIB grafts, suggesting
a role of alloreactivity in ALT donor transplants on top of cell dose
of inoculum. In keeping with this observation, there was no effect of
the dose of CD34 cells on the duration of the first admission. We would
instead have expected a more prolonged first admission in older
patients, but this was not the case, which contradicts what we think to
be shared in our daily practice. Myeloablative conditioning delayed the
time to first discharge, by four days, when compared to RIC regimens,
and this was statistically significant.
After the patients had
been discharged a first time, we asked what was the cumulative
incidence of readmission within 100 days: this turned out to be 22%
(18-29%), suggesting that 1 in 5 patients, at least in our experience
will be readmitted to the hospital after an allogeneic transplant, the
leading cause being fever (29 patients). Other causes for readmission
were acute GvHD (n=6), diarrhea with or without cytopenia (n=6), and
cystitis (n=4). Then we looked at predictive factor for readmission:
these turned out to be acute GvHD II-IV (p=0.006), advanced disease at
transplant (p=0.02), a graft from an ALT donor (p=0.09) and a Sorror
score greater than 2 (p=0.09. In a multivariate Cox analysis, factors
predicting readmission were acute GvHD, followed by donor type and
comorbidity index greater than 2. Again older age was not a negative
predictor of readmission.
We then looked at DAOH: the median
number of days alive and outside the hospital was 75 days with a wide
range from a minimum of 2 days to a maximum of 78 days. A higher number
of DAOH was predicted by a SIB transplant, acute GvHD grade 0-I, a
Sorror score of <=2, and a RIC regimen. There was no significant
difference in DAOH when comparing different alternative donor sources,
UD, mismatched UD, and HAPLO donors. When we considered the positive
predictors jointly, the patients with at least one of them has a median
of 79 days of DAOH, with a minimum of 64 DAOH, compared to 59 DAOH for
patients with no positive predictor, and a minimum of 2 DAOH
(p=0.00001), with a median difference of 20 days. This suggests that
SIB donor results in more days outside the hospital; however, the
intensity of the conditioning regimen and the occurrence of acute GvHD
also play a major role in determining the early outcome of the
transplant.
How did these events impact NRM and survival? Patients
requiring readmission had a significantly increased risk of NRM, and,
in a multivariate Cox analysis, re-entry was the strongest predictor of
NRM. This may be useful information for transplant programs: a patient
being readmitted within 100 days is at higher risk of NRM, whatever the
cause of readmission, and should, therefore, be considered at high risk
of death. In patients being readmitted, one may tentatively reduce the
risk of death, by the intensification of infection surveillance and
treatment, or possibly prophylaxis. The relevance of readmission on the
outcome is confirmed by the 30% difference in five-year survival, which
can be seen when looking at patients readmitted or not.
There are
limitations to this study, which include the retrospective nature, the
fact that we analyzed patients from one Center, and that the analysis
was limited to the first 100 days post-transplant.
Conclusions
We
see more days alive outside Hospital (DAOH) and fewer readmissions in
SIB grafts, as compared to alternative donor grafts, suggesting a more
favorable transplant course; acute GvHD, and the intensity of the
conditioning regimen also play a role in DAOH. After a first discharge,
readmission to the Transplant Unit is more frequent if the patient
develops acute GvHD and in donors other than HLA identical siblings.
Readmission is a significant predictor of non-relapse mortality and
should call for dedicated procedures.
References
- Eapen M, Rocha V, Sanz G et al. Effect of graft
source on unrelated donor haemopoietic stem-cell transplantation in
adults with acute leukaemia: a retrospective analysis. Lancet Oncol.
2010 ;11:653. https://doi.org/10.1016/S1470-2045(10)70127-3
- Marks
DI, Woo KA, Zhong X et al. Unrelated umbilical cord blood transplant
for adult acute lymphoblastic leukemia in first and second complete
remission: a comparison with allografts from adult unrelated donors.
Haematologica. 2014 ;99:322. https://doi.org/10.3324/haematol.2013.094193 PMid:24056817 PMCid:PMC3912963
- Ruggeri
A, Labopin M, Sanz G et al.Comparison of outcomes after unrelated cord
blood and unmanipulated haploidentical stem cell transplantation in
adults with acute leukemia. Leukemia. 2015 ;299:1891. https://doi.org/10.1038/leu.2015.98 PMid:25882700
- Robin
M, Ruggeri A, Labopin M et al. Comparison of unrelated cord blood and
peripheral blood stem cell transplantation in adults with
myelodysplastic syndrome after reduced-intensity conditioning regimen:
a collaborative study from Eurocord (Cord blood Committee of Cellular
Therapy & Immunobiology Working Party of EBMT) and Chronic
Malignancies Working Party. Biol Blood Marrow Transplant. 2015;21:489. https://doi.org/10.1016/j.bbmt.2014.11.675 PMid:25529382
- Ringdén
O, Labopin M, Beelen DW et al. Bone marrow or peripheral blood stem
cell transplantation from unrelated donors in adult patients with acute
myeloid leukaemia, an Acute Leukaemia Working Party analysis in 2262
patients. J Intern Med. 2012 ;272:472. https://doi.org/10.1111/j.1365-2796.2012.02547.x PMid:22519980
- Nagler
A, Labopin M, Shimoni A , et al. Mobilized peripheral blood stem cells
compared with bone marrow as the stem cell source for unrelated donor
allogeneic transplantation with reduced-intensity conditioning in
patients with acute myeloid leukemia in complete remission: an analysis
from the Acute Leukemia Working Party of the European Group for Blood
and Marrow Transplantation. Biol Blood Marrow Transplant. 2012
;18:1422. https://doi.org/10.1016/j.bbmt.2012.02.013 PMid:22446014
- Ruggeri
A, Ciceri F, Gluckman E et al. Eurocord and Acute Leukemia Working
Party of the European Blood and Marrow Transplant Group.Alternative
donors hematopoietic stem cells transplantation for adults with acute
myeloid leukemia: Umbilical cord blood or haploidentical donors? Best
Pract Res Clin Haematol. 2010 ;23:207. https://doi.org/10.1016/j.beha.2010.06.002 PMid:20837332
- Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120:1545. https://doi.org/10.1182/blood-2012-05-426783 PMid:22700725
- Preussler
JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of
hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;
18:1620. https://doi.org/10.1016/j.bbmt.2012.04.001 PMid:22484549 PMCid:PMC3678555
- Majhail
NS, Mau LW, Denzen EM, et al. Costs of autologous and allogeneic
hematopoietic cell transplantation in the United States: a study using
a large national private claims database. BoneMarrow Transplant. 2013;
48:294. https://doi.org/10.1038/bmt.2012.133 PMid:22773126 PMCid:PMC3469749
- Majhail
NS, Mothukuri JM, Brunstein CG, et al. Costs of hematopoietic cell
transplantation:comparison of umbilical cord blood and matched related
donor transplantation and the impact ofposttransplant complications.
Biol Blood Marrow Transplant. 2009; 15:564. https://doi.org/10.1016/j.bbmt.2009.01.011 PMid:19361748
- Ballen
KK, Joffe S, Brazauskas R et al. Hospital length of stay in the first
100 days after allogeneic hematopoietic cell transplantation for acute
leukemia in remission: comparison among alternative graft sources. Biol
Blood Marrow Transplant. 2014;11:1819. https://doi.org/10.1016/j.bbmt.2014.07.021 PMid:25064747 PMCid:PMC4194253
- Mehta
RS, Holtan SG, Wang T, et al. Composite GRFS and CRFS Outcomes After
Adult Alternative Donor HCT. J Clin Oncol. 2020; 18:2062-2076. https://doi.org/10.1200/JCO.19.00396 PMid:32364845
- Ruggeri
A, Sun Y, Labopin M et al. Post-transplant cyclophosphamide versus
antithymocyte-globulin as graft versus host disease prophylaxis in
haploidentical transplant. Haematologica 2017 ;102:401. https://doi.org/10.3324/haematol.2016.151779 PMid:27758821 PMCid:PMC5286948
- Bacigalupo A, Sica S. HLA Haplotype Mismatch Transplants and Posttransplant Cyclophosphamide. Adv Hematol. 2016;2016:7802967 https://doi.org/10.1155/2016/7802967 PMid:27143973 PMCid:PMC4838781
- Finke
J, Bethge WA, Schmoor C et al. Standard graft-versus-host disease
prophylaxis with or without anti-T-cell globulin in haematopoietic cell
transplantation from matched unrelated donors: a randomised,
open-label,multicentre phase 3 trial. Lancet Oncol. 2009 ;9:855. https://doi.org/10.1016/S1470-2045(09)70225-6
- Walker
I, Panzarella T, Couban S et al.Pretreatment with anti-thymocyte
globulin versus no anti-thymocyte globulin in patients with
haematological malignancies undergoing haemopoietic cell
transplantation from unrelated donors: a randomised, controlled,
open-label, phase 3, multicentre trial. Lancet Oncol. 2016 ;2:164. https://doi.org/10.1016/S1470-2045(15)00462-3
- Fuchs EJ. Related haploidentical donors are a better choice than matched unrelated donors: Point Blood Advances 2017 1:397. https://doi.org/10.1182/bloodadvances.2016002196 PMid:29296954 PMCid:PMC5738988
- De
Jong CN, Meijer E, Bakunina C et al. Post transplantation
cyclophosphamide after allogeneic hematopoietic stem cell
transplantation: results of a prospective randomized HOVON 96 trial in
recipient of HLA matched related and unrelated donors. Blood. 2019 ;
134 (suppl.1 ); https://doi.org/10.1182/blood-2019-124659
- Sorror
ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB et al.
Comorbidity-age index: a clinical measure of biologic age beforen
allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014
:10;:3249. https://doi.org/10.1200/JCO.2013.53.8157 PMid:25154831 PMCid:PMC4178523
[TOP]






