Naveen Gupta1, Manoranjan Mahapatra2, Tulika Seth2, Seema Tyagi2, Sudha Sazawal2 and Renu Saxena2.
1 Department of Medical Oncology, Mahatma Gandhi Medical College and Hospital, Jaipur, India
2 Department of Hematology, All India Institute of Medical Sciences, New Delhi, India.
Correspondence to:
Manoranjan Mahapatra, Professor & Head. Department of Hematology,
All India Institute of Medical Sciences, New Delhi, India. E-mail:
mrmahapatra@hotmail.com
Published: January 1, 2021
Received: August 7, 2020
Accepted: December 7, 2020
Mediterr J Hematol Infect Dis 2021, 13(1): e2021004 DOI
10.4084/MJHID.2021.004
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
Outcomes in chronic myeloid leukemia (CML) have vastly improved after
introducing tyrosine kinase inhibitors. However, patients in low and
middle-income countries (LMICs) face many challenges due to social and
financial barriers.
Objective:
This study was conducted to understand socio-economic hindrances,
knowledge-attitudes-practices, and assessing nonadherence to treatment
in chronic phase CML patients taking imatinib.
Materials and Methods:
Patients of chronic phase CML, aged 15 and above, taking imatinib for
six months or more were included in the study. A questionnaire (in the
Hindi language) was administered, inquiring about the nature of the
disease and its treatment, how imatinib was obtained, drug-taking
behavior, and the treatment's economic and social burden. Nonadherence
was assessed by enquiring patients for missed doses since the last
hospital visit and for any treatment interruptions of ≥7 days during
the entire course of treatment (TIs).
Results:
Four hundred patients were enrolled (median age 37 years, median
duration on imatinib 63 months). Patients hailed from 16 different
Indian states, and 29.75% had to travel more than 500 kilometers for
their hospital visit. Scheduled hospital visits were missed by 14.75%.
A third of the patients were unaware of the lifelong treatment
duration, and 41.75% were unaware of the risks of discontinuing
treatment. Treatment was financed by three different means- 61.75%
received imatinib via the Glivec International Patient Assistance
Program (GIPAP), 14.25% through a cost-reimbursement program, and 24%
self-paying. 52.75% of patients felt financially burdened due to
the cost of drugs (self-paying patients), cost of investigations, the
expenditure of the commute and stay for the hospital visit, and loss of
working days due to hospital visits. 41.25% of patients reported missed
doses in the last three months, and 9% reported missing >10% doses.
16.5% of patients reported TIs. Nonadherence>10% and TIs were
significantly higher in self-paying patients (15.6% and 25%
respectively).
Conclusion:
We observed that patient awareness about the disease was suboptimal.
Patients felt inconvenienced and financially burdened by the treatment.
Nonadherence and treatment interruptions were observed in 41.25% and
16.5%, respectively. These issues were prevalent in self-paying
patients.
|
Introduction
The
long-term prognosis of chronic myeloid leukemia (CML) underwent a
revolutionary change since the introduction of tyrosine kinase
inhibitors (TKIs). These agents have altered CML's natural history and
changed it from a fatal disease into a chronic disease with lifelong
treatment. Thousands of CML patients across the globe are currently
taking one of the TKIs. However, treating CML in low and middle-income
countries (LMICs) is still challenging owing to issues with patient
awareness, delayed diagnosis, and poor access to treatment. The current
study was conducted to understand knowledge-attitudes-practices of
patients of CML who are taking imatinib.
Study Methodology
This study was a single-center cross-sectional observational study conducted from 1st May 2017 to 31st
July 2018 at the Hematology clinic of a public sector tertiary hospital
in North India. Consecutive patients of chronic phase CML, aged 15
and above, who had been taking imatinib for six months or more, were
enrolled in the study. Patients in accelerated phase or blast phase and
those who were taking treatment other than imatinib were excluded.
Prior approval from the Institutional Ethics Committee was obtained.
All procedures followed were in accordance with the responsible
committee's ethical standards on human experimentation (institutional
and national) and with the Helsinki Declaration of 1975, as revised in
2008. Informed consent was obtained from all patients for being
included in the study.
Clinical history and examination
findings, along with demographic data and treatment procedures, were
recorded. The investigator administered a questionnaire (in the Hindi
language); wherein patients were asked about their perceptions of the
nature of the disease and its treatment, how imatinib was obtained,
drug-taking behavior, the economic and social burden of the treatment.
The patient reported nonadherence was recorded by enquiring the
percentage of missed doses since the last hospital visit and episodes
of treatment interruptions (TIs) of ≥7 days (at any point during
treatment).
Categorical variables were presented in number and
percentage (%), and continuous variables were presented as mean ± SD
and median. The normality of data was tested by the Kolmogorov-Smirnov
test. If the normality was rejected, then the non parametric test was
used. Quantitative variables were compared using the Kruskal Wallis
test for more than two groups. Qualitative variables were correlated
using the Chi-Square test. A p-value of <0.05 was considered
statistically significant. The data was entered in MS EXCEL
spreadsheet, and analysis was done using Statistical Package for Social
Sciences (SPSS) version 21.0.
Results
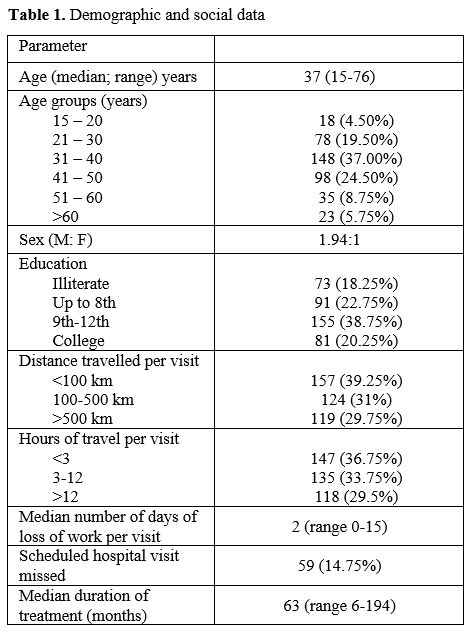
A total of 400 patients were enrolled. Demographic data is presented in Table 1.
The median age of the study group was 37 years, with a higher number of
male patients. The median duration on imatinib was 63 months. The study
group comprised patients from varied educational backgrounds, and
18.25% of the patients were illiterate. Patients hailed from 16
different states, with the largest numbers hailing from Uttar Pradesh,
Delhi, Haryana, and Bihar. Roughly one-third of the patients had to
travel more than 500 kilometers (each side) for their hospital visit
with a travel duration of >12 hours each side. Patients lost a
median of 2 workdays for each hospital visit. Scheduled hospital
visits were missed by 14.75% of patients.
 |
Table
1. Demographic and social data .
|
Patient awareness about the disease and treatment is described in Table 2.
The disease's nature was thought to be a "blood infection" by 23
patients (5.75%). A third of the patients were unaware of the lifelong
nature of the treatment. One hundred sixty-seven patients (41.75%) were
unaware of the risks of interrupting treatment. Drug-taking practices
are mentioned in Table 3. A
fixed routine for taking the drug was followed by 94.25% of the
patients, and nearly two-thirds preferred to take the drug at bedtime.
27% of the patients relied on reminders from family members to take the
drug every day. One hundred eighteen (29.5%) patients felt
inconvenienced by the treatment, and that was due to a combination of
adverse drug effects, treatment financial burden and to the need
for regular lifelong follow-up and treatment.
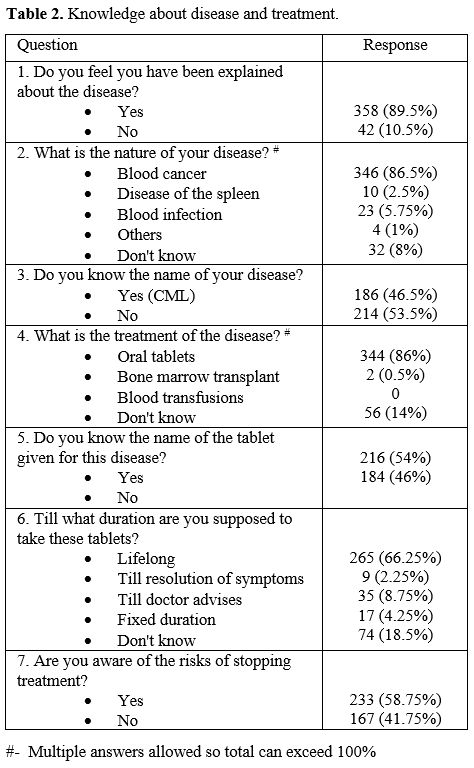 |
Table 2. Knowledge about disease and treatment. |
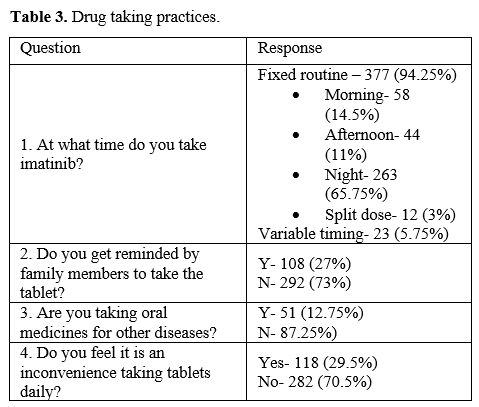 |
Table 3. Drug taking practices. |
Imatinib was obtained through three different means (Table 4).
The majority (61.75%) obtained imatinib under the Glivec International
Patient Assistance Program (GIPAP). This group of patients received
imatinib free of cost from a designated GIPAP center in Delhi. They had
to bear the cost of investigations by themselves. The second group
(14.25%) of patients obtained imatinib through a cost-reimbursement
program which covered all treatment-related expenses. The third
group (24%) were self-paying patients who had to bear the entire
treatment cost themselves. The GIPAP group received Glivec, and the
other two groups of patients received generic imatinib. The median
annual treatment related expenditure was highest in the self-paying
group of patients, followed by the GIPAP group. The majority of
self-paying patients felt that the treatment was a significant
financial burden. 44.4% of patients in the GIPAP group also felt the
treatment was a financial burden due to the cost of investigations, the
expenditure of the commute for the visit, and loss of employment due to
hospital visits. Monitoring BCR-ABL IS by quantitative PCR (at any
point during follow-up) was done by 76% of patients. Among the three
groups, the GIPAP group had the maximum number of patients (30%) who
had not tested even once during follow-up.
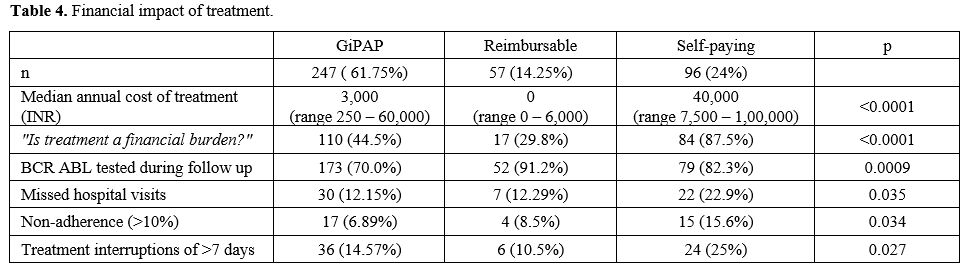 |
Table 4. Financial impact of treatment.
|
One
hundred sixty-five patients (41.25%) reported missing a dose of
imatinib since the last hospital visit. The frequency of hospital
visits was once in 3 months. Thirty-six patients (9%) had missed more
than 10% of doses. Sixty-six patients (16.5%) reported treatment
interruptions of 7 days or more (at any time during treatment). The
self-paying patients had significantly higher nonadherence rates
(15.6%) and treatment interruptions (25%). (Table 4)
Most
common treatment-related adverse effects were gastrointestinal (nausea,
vomiting, and decreased appetite), followed by skin hypopigmentation
and fatigue. (Figure 1)
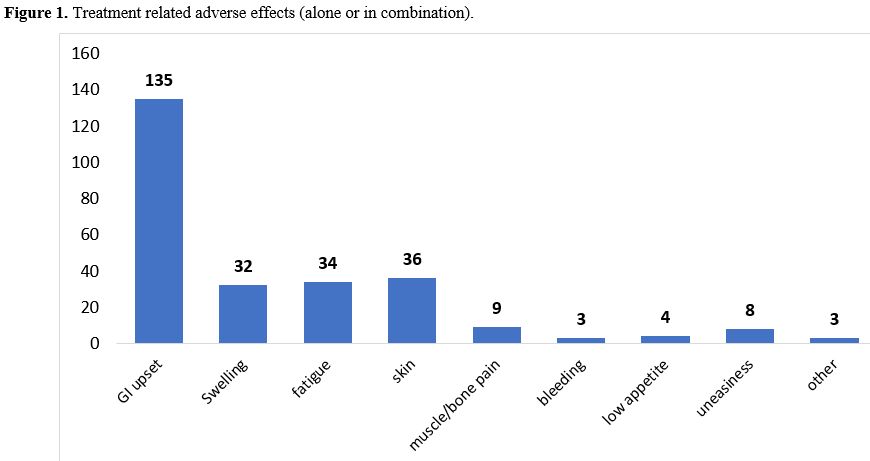 |
Figure 1. Treatment related adverse effects (alone or in combination).
|
Discussion
Majority
of the patients at public sector hospitals in LMICs hail from the lower
socioeconomic strata in whom education levels are low, as reflected by
a large number of illiterate patients in our study group. Our study
group's education status was similar to that observed in another Indian
study[1] and lower than those from Italy[2] and Brazil.[3] The
study population comprised patients of 16 states, and a large number of
them had to undertake lengthy travel for each hospital visit.
Hamerschlak et al. made similar observations in a study from Brazil.[4]
The lack of availability of hematology/oncology specialists in smaller
towns coupled with imatinib's unavailability at these centers leads to
aggregation of patients at tertiary hospitals in metro cities. The long
duration of travel leads to loss of work, and the cost of travel and
accommodation further adds to treatment-related expenditure and leads
to patient dissatisfaction. The costly and
cumbersome nature of hospital visits also leads to the patient missing
their scheduled hospital visits.
Patient awareness regarding disease and treatment has been suboptimal in studies from India[1] and Brazil,[4] whereas it was much better in studies from Europe.[2,5]
We observed low patient awareness regarding the nature of the disease
and treatment, particularly regarding treatment duration. This poor
information is a peculiar challenge faced during the treatment of CML
in low and middle-income nations, particularly in the public sector,
where many patients belong to the lower socioeconomic strata and are
less educated. Patient awareness is a critical component in ensuring
optimum treatment as lack of adequate knowledge about the disease adds
to patient anxiety, hampers adherence to treatment, and creates a trust
deficit between the patient and the physician.[6]
These findings reiterate the need for focused and easy-to-understand
counseling at diagnosis and its repeated reinforcement during
subsequent visits.
Patients tend to adopt various practices to
make the daily intake of drugs regular and convenient. We observed that
most patients followed a regular routine, and many relied on reminders
from family members. Similar practices have been reported previously as
well.[2,7] Studies from India have reported a lower incidence of comorbid ailments[8] than what is observed in developed nations.[9] and that can be attributed to a younger CML patient population in India.
Many
of our patients reported that the treatment caused them inconvenience
due to a combination of various factors-adverse drug effects, the
financial burden of treatment, and the need for regular lifelong
follow-up and treatment. This vital issue can get these patients
demotivated and may induce them to discontinue treatment.
The
financial impact of cancer treatment is immense, and it remains one of
the most important issues that patients have to take into consideration
while going for treatment.[10] The GIPAP program,
launched in 2001, provides free of cost Glivec to thousands of patients
across the globe. It has been a boon for patients of CML in low-income
countries. The greatest beneficiaries of the program have been from
India.[11] New patients were being enrolled in the
program till 2016, and almost all CML patients at our center prior to
this got their drug through GIPAP. This group comprises the major bulk
of CML patients at our center and it is reflected in the study
population with 62% enrolled under GIPAP. We observed that treatment
led to a substantial financial burden in our study group. The median
annual treatment related expenditure was highest in the self-paying
patients, for whom the cost of imatinib made up the bulk of the
treatment expenditure. A large number of GIPAP patients also felt
financially burdened by the treatment accessories related to the cost
of investigations, travel and accommodation for the hospital visit, and
loss of employment. The expenses are comparatively lower than other
countries[10] but still substantial for a country with an average annual per capita income of INR 92,565.[12]
The
cost of BCR-ABL quantitative estimation by PCR is around INR
6,000-7,000. This is almost two times the cost of monthly generic
imatinib. The unaffordability of repeated BCR-ABL estimations is
reflected by the high number of patients who did not get even a single
estimation done in the follow-up. This tendency is true even in
developed countries, and regular disease assessment either by
cytogenetics or molecular methods is infrequently seen outside the
setting of clinical trials.[13,14]
Nonadherence to TKI therapy is a major hindrance to obtaining favorable long term outcomes in patients with CML.[15,16]
The nonadherence patient-reported is a less sensitive methodology for
assessing nonadherence as it may underestimate the actual prevalence.
Despite this, we observed that a large number of patients were
non-adherent to imatinib, and also that many patients reported lengthy
treatment interruptions. Previous studies from India have observed
nonadherence rates of 25% to 55%.[1,8,15]
The proportion of nonadherence >10% and TIs was significantly higher
in the self-paying patients, concerning the financial difficulties
faced by these patients.
Managing CML in low and middle-income
countries requires careful titration of the treatment according to the
patients' socioeconomic status. All avenues of financial support from
both government and non-government schemes must be pursued to ensure
uninterrupted treatment.[17] The excellent survival
rates of patients under the GIPAP program are a testament to the fact
that by improving accessibility to TKIs in LMICs, we can produce
results comparable to high-income countries.[18] The
availability of TKIs must be coupled with better penetrance of
hematology/oncology services to smaller towns and cities and an
emphasis on better patient education and treatment adherence.
Our
study has several limitations. Patients were assessed at only a single
time point without follow up. The disease's awareness would depend upon
the initial patient counselling and education that might not be uniform
for all patients. The patient-reported nonadherence was assessed over a
3-month duration, which is a less sensitive method and underestimates
the actual nonadherence.
Conclusions
This
study highlights the major challenges encountered in TKI-based
treatment of CML in low and middle-income countries. Inadequate patient
education status contributes to suboptimal awareness about disease and
treatment. Lack of hematology/oncology services in most parts of the
country, costs of drugs and investigations pose a significant financial
burden on the patients. Nonadherence (>10% of doses) and treatment
interruptions were observed in 9% and 16.5% of patients respectively.
These were significantly higher in self-paying patients.
References
- Unnikrishnan R, Veeraiah S, Mani S, Rajendranath R,
Rajaraman S, Vidhubala Elangovan GS, et al. Comprehensive Evaluation of
Adherence to Therapy, Its Associations, and Its Implications in
Patients With Chronic Myeloid Leukemia Receiving Imatinib. Clin
Lymphoma Myeloma Leuk. 2016 Jun;16(6):366-371.e3. https://doi.org/10.1016/j.clml.2016.02.040 PMid:27052853
- Breccia
M, Efficace F, Sica S, Abruzzese E, Cedrone M, Turri D, et al.
adherence and future discontinuation of tyrosine kinase inhibitors in
chronic phase chronic myeloid leukemia. A patient-based survey on 1133
patients. Leukemia Research. 2015 1st October;39(10):1055-9. https://doi.org/10.1016/j.leukres.2015.07.004 PMid:26282944
- de
Almeida MH, Pagnano KBB, Vigorito AC, Lorand-Metze I, de Souza CA.
Adherence to tyrosine kinase inhibitor therapy for chronic myeloid
leukemia: a Brazilian single-center cohort. Acta Haematol.
2013;130(1):16-22. https://doi.org/10.1159/000345722 PMid:23363706
- Hamerschlak
N, Souza C de, Cornacchioni AL, Pasquini R, Tabak D, Spector N, et al.
Patients' perceptions about diagnosis and treatment of chronic myeloid
leukemia: a cross-sectional study among Brazilian patients. Sao Paulo
Med J. 2015 Dec;133(6):471-9. https://doi.org/10.1590/1516-3180.2014.0001306 PMid:25388686
- Noens
L, van Lierde M-A, De Bock R, Verhoef G, Zachée P, Berneman Z, et al.
Prevalence, determinants, and outcomes of nonadherence to imatinib
therapy in patients with chronic myeloid leukemia: the ADAGIO study.
Blood. 2009 28th May;113(22):5401-11. https://doi.org/10.1182/blood-2008-12-196543 PMid:19349618
- Ruddy
K, Mayer E, Partridge A. Patient adherence and persistence with oral
anticancer treatment. CA: A Cancer Journal for Clinicians. 2009 1st
January;59(1):56-66. https://doi.org/10.3322/caac.20004 PMid:19147869
- Eliasson
L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia
patients' reasons for not adhering to the oral anticancer drug imatinib
as prescribed. Leuk Res. 2011 May;35(5):626-30. https://doi.org/10.1016/j.leukres.2010.10.017 PMid:21095002
- Kapoor
J, Agrawal N, Ahmed R, Sharma SK, Gupta A, Bhurani D. Factors
influencing adherence to imatinib in Indian chronic myeloid leukemia
patients: a cross-sectional study. Mediterr J Hematol Infect Dis.
2015;7(1):e2015013. https://doi.org/10.4084/mjhid.2015.013 PMid:25745540 PMCid:PMC4344173
- Efficace
F, Baccarani M, Rosti G, Cottone F, Castagnetti F, Breccia M, et al.
Investigating factors associated with adherence behaviour in patients
with chronic myeloid leukemia: an observational patient-centered
outcome study. Br J Cancer. 2012 4th September;107(6):904-9. https://doi.org/10.1038/bjc.2012.348 PMid:22871884 PMCid:PMC3464760
- Jiang
Q, Yu L, Gale RP. Patients' and hematologists' concerns regarding
tyrosine kinase-inhibitor therapy in chronic myeloid leukemia. J Cancer
Res Clin Oncol. 2018 Apr;144(4):735-41. https://doi.org/10.1007/s00432-018-2594-8 PMid:29380058
- Garcia-Gonzalez
P, Boultbee P, Epstein D. Novel Humanitarian Aid Program: The Glivec
International Patient Assistance Program-Lessons Learned From Providing
Access to Breakthrough Targeted Oncology Treatment in Low- and
Middle-Income Countries. J Glob Oncol. 2015 23rd September;1(1):37-45. https://doi.org/10.1200/JGO.2015.000570 PMid:28804770 PMCid:PMC5551649
- Mospi.gov.in.
(2020). Press note on provisional estimates of annual national income,
2018-19 and quarterly estimates of gross domestic product for the
fourth quarter (q4) of 2018-19. [online] Available at: http://www.mospi.gov.in/sites/default/files/press_release/Press%20Note%20PE%202018-19-31.5.2019-Final.pdf
- Goldberg
SL. Monitoring Chronic Myeloid Leukemia in the Real World: Gaps and
Opportunities. Clinical Lymphoma Myeloma and Leukemia. 2015 1st
December;15(12):711-4. https://doi.org/10.1016/j.clml.2015.08.088 PMid:26433907
- Geelen
IGP, Thielen N, Janssen JJWM, Hoogendoorn M, Roosma TJA, Willemsen SP,
et al. Treatment outcome in a population-based, 'real-world' cohort of
patients with chronic myeloid leukemia. Haematologica. 2017
Nov;102(11):1842-9. https://doi.org/10.3324/haematol.2017.174953 PMid:28860339 PMCid:PMC5664388
- Ganesan
P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S, et al.
Nonadherence to imatinib adversely affects event free survival in
chronic phase chronic myeloid leukemia. Am J Hematol. 2011
Jun;86(6):471-4. https://doi.org/10.1002/ajh.22019 PMid:21538468
- Ibrahim
AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Szydlo R, et al. Poor
adherence is the main reason for loss of CCyR and imatinib failure for
chronic myeloid leukemia patients on long-term therapy. Blood. 2011 7th
April;117(14):3733- 6. https://doi.org/10.1182/blood-2010-10-309807 PMid:21346253 PMCid:PMC6143152
- Malhotra
H, Radich J, Garcia-Gonzalez P. Meeting the needs of CML patients in
resource-poor countries. Hematology Am Soc Hematol Educ Program. 2019
6th December;2019(1):433-42. https://doi.org/10.1182/hematology.2019000050 PMid:31808889 PMCid:PMC6913442
- Umeh
CA, Garcia-Gonzalez P, Tremblay D, Laing R. The survival of patients
enrolled in a global direct-to-patient cancer medicine donation
program: The Glivec International Patient Assistance Program (GIPAP).
EClinicalMedicine. 2020 Feb;19:100257. https://doi.org/10.1016/j.eclinm.2020.100257 PMid:32140674 PMCid:PMC7046500
[TOP]




