In contrast, COVID -19 infection is often associated with uncontrolled inflammatory response and cytokine release syndrome. There are limited data describing the impact of COVID-19 on MM patients. Herein, we report the outcome of six patients with MM and concomitant COVID-19 infection diagnosed between April 15, 2020, and June 30, 2020, admitted to tertiary care hospital in the state of Qatar. Two patients have newly diagnosed MM, and four were known myeloma cases who acquired the infection during the disease course. Four patients had a mild infection, and two patients had a severe and critical infection that required intensive care unit (ICU) admission; both cases were treated with Tocilizumab with variable response and outcome.
The first patient is a-42- year-old who presented with fever, cough, and sore throat for five days. Polymerase chain reaction (PCR) nasopharyngeal swab came positive for SARS-CoV-2. Laboratory workup revealed moderate anemia with Hemoglobin at 9.8 gm/dL (13.0-17.0 gm/dl) and increase in serum creatinine at 360 umol/L (62-124 umol/L) and total protein at 96 gm/L (66-87 gm/L) with low albumin of 27 gm/L (35-52 gm/L) and normal calcium level. Further workup including serum protein electrophoresis (SPEP) showed monoclonal band typed as IgG lambda (28 g/L), light chain analysis revealed free light chain lambda at 14,170 mg/L (5.7-26.3 mg/L), with kappa/lambda ratio of <0.01 (0.26-1.65). Bence-Jones protein (BJP) was detected at 3.9 g/L and high Beta-2 microglobulin (B2M) at 20 mg/L (0.8-2.2 mg/L). The patient was treated with Hydroxychloroquine, Azithromycin and Dexamethasone 40 mg for four days (for 2 cycles) to treat MM. He was hospitalized for 21 days with an uneventful course. Bone marrow (BM) aspiration and biopsy were done and showed a 70% plasma cell infiltrate consistent with MM. Subsequently, the patient was started on Bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11, Cyclophosphamide 500 mg, and Dexamethasone 40mg weekly every 21 days cycle.
The second patient was a 41-year-old female with no chronic illness presented with fever, cough, and shortness of breath. A chest x-ray showed diffuse bilateral lung infiltrates (Figure 1).
 |
Figure
1. Chest x-ray for case (2) showing diffuse bilateral lung infiltrates. |
COVID-19 infection was suspected and confirmed by PCR from a nasopharyngeal swab. Laboratory workup revealed severe anemia with Hgb of 6 g/dL (12-15 g/dL) and impaired renal function with a serum creatinine of 322 umol/L (62-124 umol/L). SPEP revealed a small monoclonal band, while light chain analysis showed high free light chain kappa at 10,291 mg/L (3.3-19.4 mg/L), with a kappa/lambda ratio of 910 (0.26-1.65). The patient was started on Hydroxychloroquine and Azithromycin. On day 3 of admission, she was transferred to ICU) due to an increase in her oxygen requirement. Serum IL-6 was found to be as high as 3,611 pg/mL (≤7 pg/mL). Tocilizumab, 400 mg, was started. Subsequently, her IL-6 dropped to 330 pg/mL. Ten days later, the IL-6 level was increased to 4,800 pg/mL. Another dose of Tocilizumab 400 mg was given, resulting in a drop of IL-6 level to 211 pg/mL. Dexamethasone 40mg (2 cycles for four days) was started on which the patient showed significant improvement in her clinical situation and biochemical parameters with a significant reduction in free light chain kappa from 10,291 mg/L to 1,1470 mg/L (Figure 2). BM aspiration and biopsy were performed subsequently and revealed 20% plasma cell infiltration consistent with MM diagnosis. The patient was started on Bortezomib 1.5 mg/m2 (day 1, 8, 15, 22) with Cyclophosphamide 500 mg and Dexamethasone 20mg weekly) every 28 days cycle.
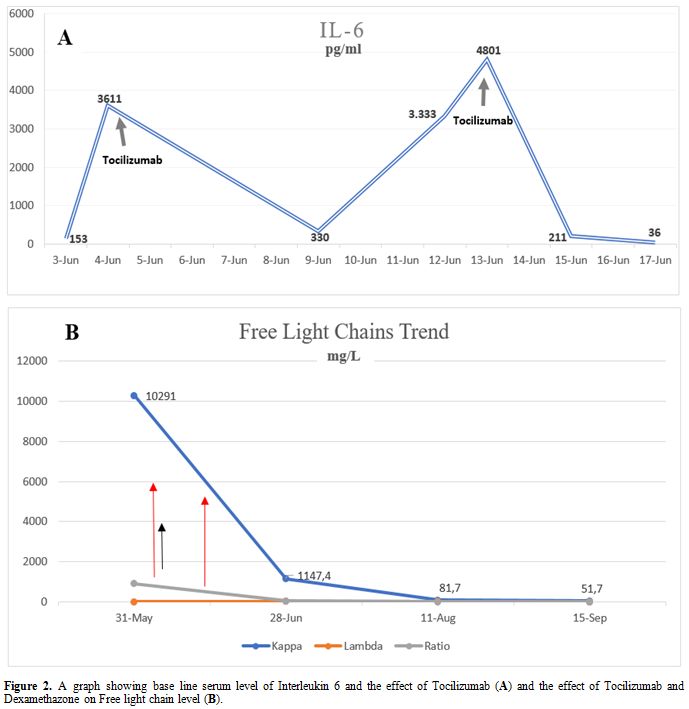 |
Figure 2. A graph showing base line serum level of Interleukin 6 and the effect of Tocilizumab (A) and the effect of Tocilizumab and Dexamethazone on Free light chain level (B). |
The third patient is a 56-year-old male, known case of diabetes mellitus type 2 (DM2), and was diagnosed with IgG Kappa MM in 2015 and was treated previously with VCD protocol and autologous stem cell transplant (ASCT), followed by lenalidomide maintenance. He presented with mild COVID-19 pneumonia, which required hospitalization for ten days; he was treated with Hydroxychloroquine and Azithromycin and recovered completely from COVID-19 with an uneventful hospital course.
The fourth patient is a 70-year-old male with a background of DM2, dyslipidemia, and morbid obesity. He was diagnosed in 2005 with IgA Kappa MM and heavily pretreated previously with multiple lines of therapy, including Bortezomib, Immunomodulatory drugs (IMIDs), Daratumumab, Carfilzomib based therapy, and two autologous stem cell transplant. He presented with severe pneumonia secondary to COVID-19 infection, which required mechanical ventilation at day seven. COVID-19 treatment included Hydroxychloroquine, Azithromycin, Lopinavir/Ritonavir, and convalescent plasma. Tocilizumab at 8mg/kg was given for 3 doses for elevated IL6 level with rapid resolution of inflammatory markers but without significant clinical impact or response. The patient had a complicated ICU course with prolonged intubation, renal failure requiring hemodialysis, secondary bacterial infection, and encephalopathy. He died after 80 days after admission as result of COVID-19 related ARDS and significant comorbidities.
The fifth case is a 64-year-old male diagnosed in 2018 with smoldering myeloma. He presented with mild upper respiratory tract symptoms secondary to COVID-19 infection; his symptoms resolved after five days of Hydroxychloroquine and Azithromycin.
The sixth patient was a 55-year-old male diagnosed with MM IgG Kappa three months before presentation while receiving ongoing induction chemotherapy, consisting of Bortezomib, Lenalidomide, and Dexamethasone (VRD). He presented with mild upper respiratory tract symptoms secondary to COVID-19 that did not require hospitalization, and the patient resumed his chemotherapy 14 days after full recovery.
Herein, we report a heterogeneous group of multiple myeloma patients (in different phases) who had a concomitant COVID-19 with different severity. Out of the six patients with COVID-19 infection, two were newly diagnosed as MM at their initial hospital admission, and four patients were already known myeloma patients who acquired the infection during the disease course.
The latter group (four patients) includes one patient who got COVID-19 infection post ASCT while on maintenance therapy; a second was on induction Bortezomib, Lenalidomide, and Dexamethasone (VRD) therapy. The third patient was a case of relapsed/refractory myeloma, who failed multiple previous lines of therapy. The fourth case was smoldering myeloma under observation. Detailed patients' and disease characteristics and laboratory findings were listed in tables 1 and 2.
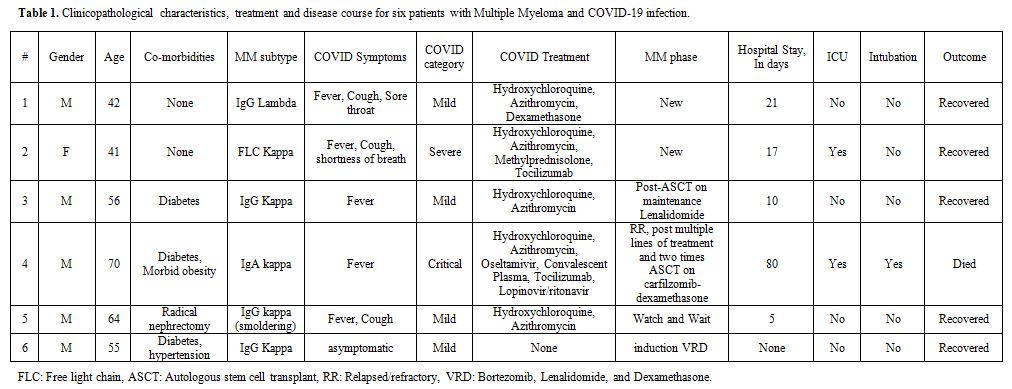 |
Table 1. Clinicopathological characteristics, treatment and disease course for six patients with Multiple Myeloma and COVID-19 infection. |
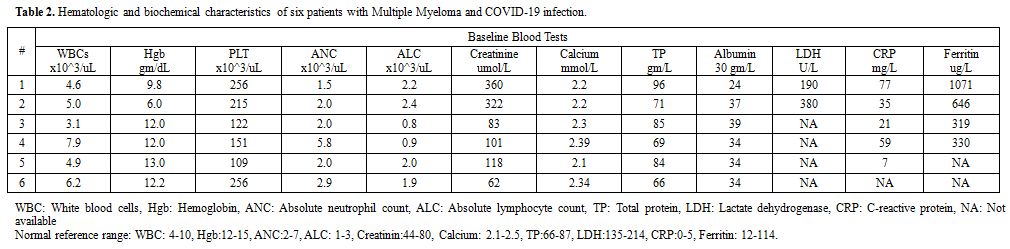 |
Table 2. Hematologic and biochemical characteristics of six patients with Multiple Myeloma and COVID-19 infection. |
Our cohort included four patients who had mild COVID-19 infection and two patients with severe critical disease. Only one patient died secondary to COVID-19 after a prolonged ICU course (80 days). These findings are contrary to those reported by Dhakal et al. in a recently published case series, which included seven patients with MM and COVID-19 infection, where all patients had an unfavorable disease course, three of the reported patients died, and four-needed ICU admission.[5]
Interlukin6 (IL-6) has an essential role in MM pathogenesis by interaction with adhesion molecules, tumor suppressor genes, oncogenes, and apoptosis inhibition in myeloma cells.[6] The studies addressing Tocilizumab use, an interleukin-6 (IL-6) receptor antagonist, are still rather controversial. While some centers reported a significant effect in patients with COVID-19 infection,[7] preliminary data from phase III clinical trials did not show any significant benefit.[8]
Two of our patients (case 2 and case 4) had received Tocilizumab; one of them had a dramatic response both in a clinical situation and biochemical parameters. The successful use of Tocilizumab in MM patients with COVID-19 was reported by Chandos et al..[9] It is noteworthy that the high mortality rate in COVID-19 was suggested to be secondary to cytokine storm and inflammatory response, and it was noted that levels of IL-6 were associated with the severe COVID-19 pneumonia.[10] These cases may emphasize the importance of using IL-6 antagonists in treating patients with COVID-19, especially in those diagnosed with MM.
There is no specific treatment for COVID-19 infection right now; however, many approaches have been tried, including a combination of antiviral, hydroxychloroquine, dexamethasone, and convalescent plasma with varying success rates. In this report, we present our experience in the management of MM in the era COVID-19 pandemic. The current data are still insufficient and rather contradictory. Further multicenter studies on a larger number of patients are needed to draw a definite conclusion regarding the role of using Tocilizumab in patients with COVID-19, generally and in Multiple Myeloma patients with associated COVID-19 infection.