Chiara Agrati1, Veronica Bordoni1, Alessandra Sacchi1, Nicola Petrosillo1, Emanuele Nicastri1, Franca Del Nonno1, Gianpiero D’Offizi1, Fabrizio Palmieri1, Luisa Marchioni1, Maria Rosaria Capobianchi1, Andrea Antinori1, Giuseppe Ippolito1 and Michele Bibas1.
1 National Institute for Infectious Diseases, "Lazzaro Spallanzani" Rome, Italy.
Correspondence to:
Michele Bibas, MD. National Institute for Infectious Diseases, L
Spallanzani, Via Portuense 292, 00149 Rome, Italy. Tel. +393934305748,
Fax+39065517064.
Published: March 1, 2021
Received: September 17, 2020
Accepted: February 3, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021016 DOI
10.4084/MJHID.2021.016
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Coronavirus disease 2019 (COVID-19) is mainly a respiratory tract
disease and acute respiratory failure with diffuse microvascular
pulmonary thrombosis are critical aspects of the morbidity and
mortality of this new syndrome.
Purpose:
The aim of our study was to investigate, in severe COVID-19
hospitalized patients, the P-selectin plasma concentration as a
biomarker of endothelial dysfunction and platelet activation.
Methods:
46 patients with severe or critical SARS-CoV-2 infection were included
in the study. Age-matched patients then were divided in those requiring
admission to the intensive care unit (ICU, ICU cases) vs those not
requiring ICU hospitalization (non-ICU cases). Blood samples of severe
COVID-19 patients were collected at the time of hospital admission. The
quantification of soluble P-selectin was performed by ELI, assay.
Results:
Our study showed a higher P-selectin plasma concentration in patients
with Covid-19, regardless of ICU admission, compared to the normal
reference values and compared to ten contextually sampled healthy
donors (HD); (COVID-19): median 65.2 (IQRs: 45.1-81.1) vs. HD: 40.3
(IQRs: 24.3-48.7), p=0023. Moreover, results showed a significant
reduction of P-sele din after platelets removal in HD, in contrast,
both ICU and non-ICU COVID-19 patients showed similar high levels of
P-selectin with and without platelets.
Conclusion:
Elevation of P-selectin suggests a central role of platelet endothelium
interaction as part of the multifaced pathogenic mechanism of COVID-19
leading to the local activation of hemostatic system forming pulmonary
thrombi. Further work is necessary to determine the therapeutic role of
antiplatelets agents or of the anti P-selectin antibody Crizanlizumab.
|
Introduction
Despite
a worldwide spread of severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) infection approaching, in January 2021, one hundred
million cases and two million deaths, this disease's pathophysiology
remains inadequately defined and largely ununderstood.
COVID-19 is
mainly a respiratory tract disease, and acute respiratory failure and
diffuse microvascular pulmonary thrombosis are critical aspects of the
morbidity and mortality of the coronavirus disease 2019 (Covid-19).[1]
However, both autopsy findings and clinical observations have described
vascular damages and thrombotic complications in a wide range of organs.
Available
published data suggest that from one-third to one-half of patients
hospitalized with COVID-19 have hemostatic laboratory parameters
suggestive of a pro-thrombotic state leading to a coagulopathy. These
patients also manifest a hyperinflammatory state characterized by
elevated inflammatory markers, strongly associated with severe
pneumonia and a high mortality rate.[3]
SARS-CoV-2
enters human cells by binding to the angiotensin-converting-enzyme 2
(ACE2) receptor, expressed on respiratory epithelial cells and other
cell types, including endothelial cells.[2]
Direct
infection of endothelial cells, as well as the inflammatory
environment, might result in an endothelial activation that drives the
expression of P-selectin and tissue factor (TF), thus promoting
platelet recruitment and aggregation.[4] Subsequent
accumulation of mononuclear cells provides a platform for the
initiation of plasma coagulation by triggering prothrombin's cleavage
to thrombin and fibrin formation.[5]
The
molecular interaction between P-selectin expressed in platelets and
endothelial cells rapidly triggers TF exposure on monocytes,[6]
and this may represent a mechanism by which platelets and mononuclear
cells contribute to disproportionate intravascular micro-thrombosis in
SARS-CoV-2.
The aim of our study was to investigate, in COVID-19
hospitalized patients compared to healthy adult human controls, the
ex-vivo P-selectin plasma concentration as a biomarker of endothelial
dysfunction and platelet activation. The association between this
parameter at the time of hospital admission and the severity and the
outcomes of the disease with subsequent admittance into the intensive
care unit (ICU) was finally assessed.
Study Population
A
group of 46 patients with confirmed SARS-CoV-2 infection, admitted to
our Institute between March and April 2020, was included in the study.
All enrolled patients had severe illness (respiratory rate >30, SpO2 <93% on room air at sea level, PaO2/FiO2
< 300, or lung infiltrates >50%), or critical illness
(association of acute respiratory distress syndrome (ARDS), septic
shock, cardiac dysfunction, cytokine storm and/or exacerbation of
underlying co-morbidities. Age-matched patients were then divided into
those requiring admission to the intensive care unit (ICU, ICU cases)
vs. those non requiring ICU hospitalization (non-ICU cases). A
significant effort was made to exclude from the study population those
with prior administration of anti-platelet agents or anticoagulant
drugs.A
group of ten age-matched healthy donors (HD) were enrolled in the study
as controls. Characteristics of enrolled patients are described in Figure 1.
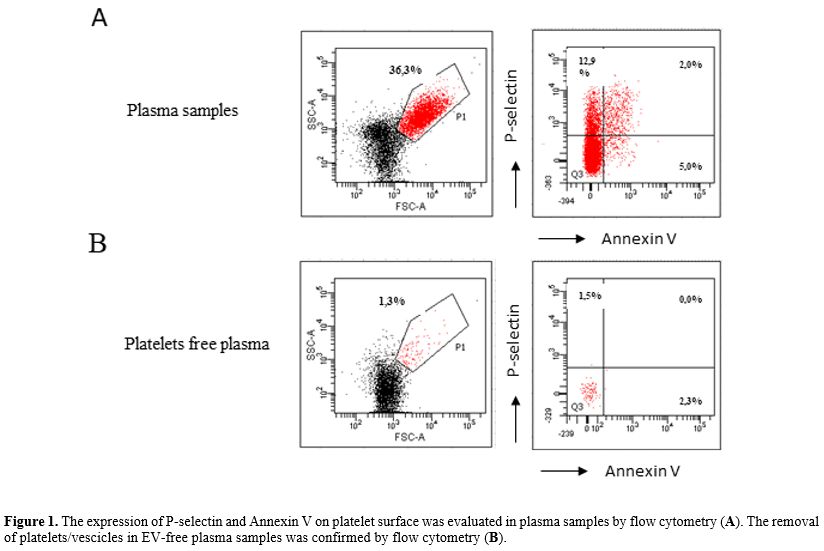 |
Figure 1. The expression of P-selectin and Annexin V on platelet surface was evaluated in plasma samples by flow cytometry (A). The removal of platelets/vescicles in EV-free plasma samples was confirmed by flow cytometry (B).
|
Material and Methods
Blood
samples of severe COVID-19 patients were collected at the time of
hospital admission. Heparin peripheral blood was centrifuged at 1200
rpm for 10 minutes at room temperature to obtain plasma samples
containing extracellular vesicles and platelets (Plasma). After that,
500 ul of plasma samples were further centrifuged at 5000 rpm for 5
minutes at room temperature to eliminate platelets and extracellular
vesicles (EV-free plasma). To verify the removal of platelets/vesicles
in EV-free plasma, we performed a flow cytometry analysis.
Specifically, plasma and EV-free plasma were stained with P-selectin
and Annex V for 15 minutes at room temperature and then acquired to a
FACS Canto II cytometer. (Figure 2).
The quantification of soluble P-selectin was performed by ELISA assay
(R&D system; average value in heparin plasma: mean 39 ng/ml (range:
25-53).
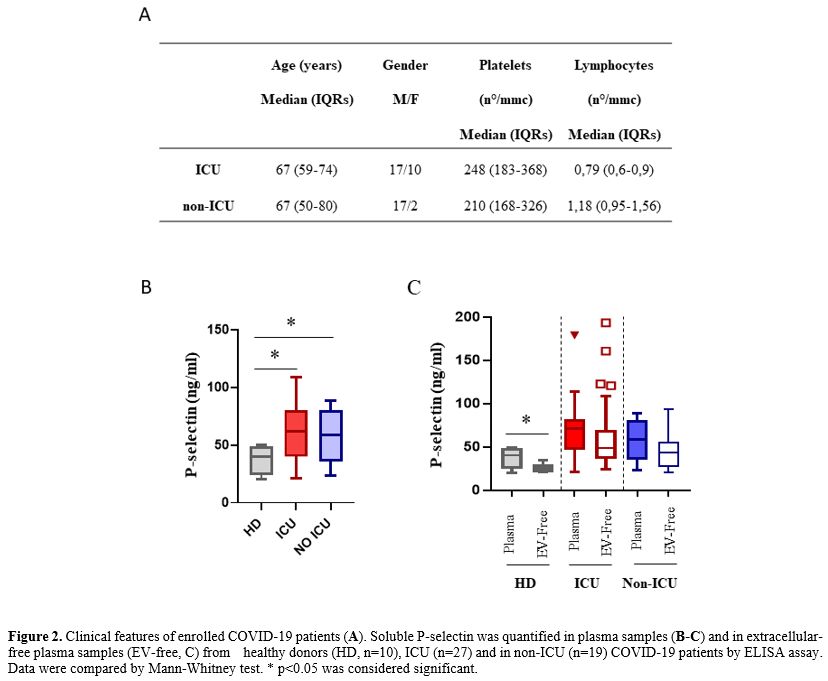 |
Figure 2. Clinical features of enrolled COVID-19 patients (A). Soluble P-selectin was quantified in plasma samples (B-C)
and in extracellular-free plasma samples (EV-free, C) from healthy
donors (HD, n=10), ICU (n=27) and in non-ICU (n=19) COVID-19 patients
by ELISA assay. Data were compared by Mann-Whitney test. * p<0.05
was considered significant.
|
Results
Our study showed a
higher P-selectin plasma concentration in patients with Covid-19,
regardless of ICU admission, compared to the normal reference values
and compared to contextually sample healthy donors; (COVID-19): median
65.2 (IQRs: 45.1-81.1) vs. HD: 40.3 (IQRs: 24.3-48.7), p=0.0023).
Moreover, results showed a significant reduction of P-selectin after
platelet removal in HD, suggesting that most of this molecule was
trapped in the platelets. In contrast, both ICU and non-ICU COVID-19
patients showed similar P-selectin levels with and without platelets,
suggesting that Covid-19 induced a release of these molecules from
activated platelets/cells. A similar platelet count has been observed in the two groups ranging
within the standard value (150-400/mmc). More significantly lower
lymphocyte count was observed in ICU patients, confirming an
association between lymphocytopenia and disease severity.[6,7]
Discussion
Our
results suggest a central role of platelet endothelium interaction as
part of the multifaced pathogenic mechanism of COVID-19, leading to the
local activation of the hemostatic system forming pulmonary
thrombi. More, these interactions amplify the leukocyte
recruitment, increasing chemokine expression on the endothelial surface
with extensive adhesion, activation, and leukocyte trafficking across
the endothelial wall.[8]
It will be interesting
to examine whether therapies inhibiting platelet-endothelium
interaction or inhibiting platelet function might improve microvascular
perfusion, reduce thrombo-inflammation, and finally reduce COVID-19
morbidity and mortality.
In this perspective, we suggest
studying, in the early phases of COVID-19 disease, the role of
anti-platelet agents, acetylsalicylic acid, GPIIb, GPIIIa antagonists,
and P2Y12 antagonists, not only
in de novo therapy initiation but also in patients previously in
prophylaxis or in treatment for cardiovascular disorders. The suggested
mechanism to study is not only the direct P-selectin/platelet
interaction but also the neutrophil extracellular trap (NET) production
as described in sepsis and transfusion-related acute lung injury
(TRALI).[9,10,11] Further, Crizanlizumab-tmca, a
selectin blocker humanized IgG2 kappa monoclonal antibody that binds to
P-selectin, and approved to reduce the frequency of vasoocclusive
crises (VOCs) in adult and pediatric patients, might be evaluated in
severe cases not responding or in combination to anti-platelet therapy.[12,13]
Acknowledgements
Supported
by The Italian Ministry of Health (Ricerca Corrente Linea 1,
COVID-2020-12371735 and COVID-2020-12371817). All Authors have reviewed
and approved the manuscript. All authors have reviewed the authorship
policy. No author has any conflicts of interest related to this work.
We gratefully acknowledge the Collaborators Members of INMI COVID-19 study group: Maria
Alessandra Abbonizio, Amina Abdeddaim, Chiara Agrati, Fabrizio
Albarello, Gioia Amadei, Alessandra Amendola, Mario Antonini, Tommaso
Ascoli Bartoli, Francesco Baldini, Raffaella Barbaro, Bardhi Dorian,
Barbara Bartolini, Rita Bellagamba, Martina Benigni, Nazario
Bevilacqua, Gianlugi Biava, Michele Bibas, Licia Bordi, Veronica
Bordoni, Evangelo Boumis, Marta Branca, Donatella Busso, Marta Camici,
Paolo Campioni, Maria Rosaria Capobianchi, Alessandro Capone, Cinzia
Caporale, Emanuela Caraffa, Ilaria Caravella, Fabrizio Carletti,
Concetta Castilletti, Adriana Cataldo, Stefano Cerilli, Carlotta Cerva,
Roberta Chiappini, Pierangelo Chinello, Carmine Ciaralli, Stefania
Cicalini, Francesca Colavita, Angela Corpolongo, Massimo Cristofaro,
Salvatore Curiale, Alessandra D’Abramo, Cristina Dantimi, Alessia De
Angelis, Giada De Angelis, Maria Grazia De Palo, Federico De Zottis,
Virginia Di Bari, Rachele Di Lorenzo, Federica Di Stefano, Gianpiero
D’Offizi, Davide Donno, Francesca Faraglia, Federica Ferraro, Lorena
Fiorentini, Andrea Frustaci, Matteo Fusetti, Vincenzo Galati, Roberta
Gagliardini, Paola Gallì, Gabriele Garotto, Saba Gebremeskel Tekle,
Maria Letizia Giancola, Filippo Giansante, Emanuela Giombini, Guido
Granata, Maria Cristina Greci, Elisabetta Grilli, Susanna Grisetti,
Gina Gualano, Fabio Iacomi, Giuseppina Iannicelli, Giuseppe Ippolito,
Eleonora Lalle, Simone Lanini, Daniele Lapa, Luciana Lepore, Raffaella
Libertone, Raffaella Lionetti, Giuseppina Liuzzi, Laura Loiacono,
Andrea Lucia, Franco Lufrani, Manuela Macchione, Gaetano Maffongelli,
Alessandra Marani, Luisa Marchioni, Raffaella Marconi, Andrea Mariano,
Maria Cristina Marini, Micaela Maritti, Alessandra Mastrobattista,
Giulia Matusali, Valentina Mazzotta, Paola Mencarini, Silvia Meschi,
Francesco Messina, Annalisa Mondi, Marzia Montalbano, Chiara Montaldo,
Silvia Mosti, Silvia Murachelli, Maria Musso, Emanuele Nicastri,
Pasquale Noto, Roberto Noto, Alessandra Oliva, Sandrine Ottou, Claudia
Palazzolo, Emanuele Pallini, Fabrizio Palmieri, Carlo Pareo, Virgilio
Passeri, Federico Pelliccioni, Antonella Petrecchia, Ada Petrone,
Nicola Petrosillo, Elisa Pianura, Carmela Pinnetti, Maria Pisciotta,
Silvia Pittalis, Agostina Pontarelli, Costanza Proietti, Vincenzo Puro,
Paolo Migliorisi Ramazzini, Alessia Rianda, Gabriele Rinonapoli, Silvia
Rosati, Martina Rueca, Alessandra Sacchi, Alessandro Sampaolesi,
Francesco Sanasi, Carmen Santagata, Alessandra Scarabello, Silvana
Scarcia, Vincenzo Schininà, Paola Scognamiglio, Laura Scorzolini,
Giulia Stazi, Fabrizio Taglietti, Chiara Taibi, Roberto Tonnarini,
Simone Topino, Francesco Vaia, Francesco Vairo, Maria Beatrice Valli,
Alessandra Vergori, Laura Vincenzi, Ubaldo Visco-Comandini, Pietro
Vittozzi, Mauro Zaccarelli.
References
- Guan WJ, Ni ZY, Hu Y, Liang WH, et al .:Clinical
Characteristics of Coronavirus Disease 2019 in China. N Engl J Med.
2020 Apr 30;382(18):1708-1720. https://doi.org/10.1056/NEJMoa2002032 PMid:32109013 PMCid:PMC7092819
- Hamming
I, Timens W, Bulthuis ML, Lely AT et al.: Tissue distribution of ACE2
protein, the functional receptor for SARS coronavirus. A first step in
understanding SARS pathogenesis. J Pathol. 2004 Jun;203(2):631-7. https://doi.org/10.1002/path.1570 PMid:15141377 PMCid:PMC7167720
- Kreidieh
F., Temraz S.SARS-CoV-2: infected patient: from a hematologist's
perspective. Mediterr J Hematol Infect Dis 2020, 12(1): e2020078, https://doi.org/10.4084/mjhid.2020.078 PMid:33194152 PMCid:PMC7643802
- Varga Z, Flammer AJ, Steiger P et al.: Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2 https://doi.org/10.1016/S0140-6736(20)30937-5
395(10234):1417-1418. Epub 2020 Apr 21.
- Jackson
SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of
therapeutically targeting coagulation and other host defense
mechanisms. Blood. 2019 Feb 28;133(9):906-918. https://doi.org/10.1182/blood-2018-11-882993 PMid:30642917
- Ivanov
II, Apta BHR, Bonna AM, Harper MT. Platelet P-selectin triggers rapid
surface exposure of tissue factor in monocytes. Sci Rep. 2019 Sep
16;9(1):13397. https://doi.org/10.1038/s41598-019-49635-7 PMid:31527604 PMCid:PMC6746844
- Tan
Li, Wang Qi, Zhang D. et al.: Lymphopenia predicts disease severity of
COVID-19: a descriptive and predictive study. Signal Transduct Target
Ther. 2020 Mar 27;5(1):33. https://doi.org/10.1038/s41392-020-0148-4 PMid:32296069 PMCid:PMC7100419
- Gu
SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, Kwan JM, Krause DS, Lee AI,
Halene S, Martin KA, Chun HJ, Hwa J. Thrombocytopathy and
endotheliopathy: crucial contributors to COVID-19 thromboinflammation.
Nat Rev Cardiol. 2020 Nov 19:1-16. https://doi.org/10.1038/s41569-020-00469-1 PMid:33214651 PMCid:PMC7675396
- Zuo Y, Yalavarthi S, Shi H et al.: Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 Apr 24. pii: 138999. https://doi.org/10.1172/jci.insight.138999
- Du
F, Jiang P, He S, Song D, Xu F. Antiplatelet Therapy for Critically Ill
Patients: A Pairwise and Bayesian Network Meta-Analysis. Shock. 2018
Jun;49(6):616-624. https://doi.org/10.1097/SHK.0000000000001057 PMid:29176404
- Semple
JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and
transfusion-related acute lung injury. Blood. 2019 Apr
25;133(17):1840-1853 https://doi.org/10.1182/blood-2018-10-860809 PMid:30808638
- Blair HA. Crizanlizumab: First Approval. Drugs. 2020 Jan;80(1):79-84. https://doi.org/10.1007/s40265-019-01254-2 PMid:31933169
- Neri
T, Nieri D, Celi A. P-selectin blockade in COVID-19-related ARDS. Am J
Physiol Lung Cell Mol Physiol. 2020 Jun 1;318(6):L1237-L1238 https://doi.org/10.1152/ajplung.00202.2020 PMid:32464083 PMCid:PMC7276981
[TOP]