Fayez Hanna1,2, Annemarie Hyppa2,3, Ajay Prakash1,2, Usira Vithanarachchi1,2, Hizb U Dawar4, Zar Sanga4, George Olabode5, Hamish Crisp5 and Alhossain A. Khalafallah1,2.
1 Faculty of Health Sciences, Launceston, University of Tasmania, Tasmania, 7249, Australia.
2 Department of Haematology, Specialist Care Australia, Launceston, Tasmania, 7250 Australia.
3 Medical School, University of Saarland, Homburg, Germany.
4 Augusta Medical Centre, Lenah Valley 7008, Tasmania, Australia.
5 Launceston General Hospital, Launceston, Tasmania, 7250 Australia.
Correspondence to:
Professor Alhossain A. Khalafallah. University of Tasmania, Australia.
Tel: +61367791300, Fax: +61367791301. E-mail:
a.khalafallah@utas.edu.au
Published: March 1, 2021
Received: November 4, 2020
Accepted: February 5, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021017 DOI
10.4084/MJHID.2021.017
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objective:
To study patients receiving anticoagulants with or without antiplatelet
therapy presenting at a regional Australian hospital with bleeding. The
main aims are to explore: (1) patients' characteristics and management
provided; (2) association between the type of anticoagulant and
antiplatelet agent used and the requirement of reversal; (3) and the
length of hospital stay (LoS) in conjunction with bleeding episode and
management.
Methods: A
prospective cross-sectional review of medical records of all patients
who presented at a tertiary referral centre with bleeding while
receiving anticoagulation therapy between January 2016 and June 2018.
Data included: patients, demographics, investigations (kidney and liver function
tests, coagulation profile, FBC), LoS, bleeding site, type of and
reason for anticoagulation therapy, and management provided. Data
analysis included descriptive statistics, χ2 association, and
regression models.
Results: Among the 144 eligible patients, 75 (52.1%) were male, and the mean age was 76 years (SD=11.1). Gastrointestinal tract bleeding was the most common (n=48, 33.3%), followed by epistaxis (n=32, 22.2%). Atrial fibrillation was the commonest reason for anticoagulation therapy (n=65, 45.1%). Warfarin was commonly used (n=74, 51.4%), followed by aspirin (n=29, 20.1%), rivaroxaban (n=26, 18.1%), and apixaban (n=12, 8.3%). The majority had increased blood urea nitrogen (n=67,
46.5%), while 58 (40.3%) had an elevated serum creatinine level, and 59
(41.0%) had a mild reduction in eGFR. Thirty-five of the warfarinised
patients (47.3%) had an INR above their condition's target range
despite normal liver function. Severe anaemia (Hb<80g/L) was
reported in 88 patients (61.1%). DOACs were associated with a reduced
likelihood of receiving reversal (B= -1.7, P=<.001), and with a shorter LoS (B= -4.1, P=.046) when compared with warfarin, LMWH, and antiplatelet therapy.
Conclusion:
Warfarin use was common among patients who presented with acute
bleeding, and the INR in many warfarinised patients exceeded the target
for their condition. DOACs were associated with a reduced likelihood of
receiving reversal and a shorter LoS than warfarin, LMWH, which might
support a broader application of DOACs into community practice.
|
Introduction
One
in every 20 patients will suffer from venous thromboembolism (VTE),
either in the form of DVT alone or in combination with PE.[1]
VTE is associated with high morbidity and mortality rates and
significantly harms the quality of life. Furthermore, it has negative
financial consequences. All of this highlights the need for proper
prevention[2] and treatment. VTE management requires a challenging risk assessment,[2] and measures which may be pharmacological, mechanical, surgical, or a combination.[3] Pharmacological anticoagulation therapy is most common and includes anticoagulants[4] and antiplatelet agents.[5]
Anticoagulants are classified into vitamin K antagonists (such as
warfarin), unfractionated heparin, low molecular weight heparin (LMWH,
e.g., enoxaparin), and the novel direct oral anticoagulants (DOACs), including rivaroxaban,
apixaban, and dabigatran.[4] Anticoagulants work by targeting steps in the coagulation cascade.[4]
DOACs achieve an equivalent anticoagulant effect to classical
anticoagulants (warfarin, heparin, and its derivatives) with equal or
reduced bleeding risk.[6-8] While more specific,[9] the
DOACs are more costly than warfarin, which may hinder widespread use in
the community, even though they do not need a specific monitoring test.[10]
Balancing the benefits of anticoagulants against the associated risks
is a concern for clinical practice and requires further real-world
evidence to support decision-making.
The rate of major bleeding
resulting from receiving anticoagulants in Australia is high (seven out
of every 100 patients per year),[11] suggesting the
need for pragmatic, evidence-based guidelines for their use. While the
DOACs have relatively low bleeding risk when compared with warfarin,[8,12,13] clinicians do not tend to use DOACs because they are difficult to monitor and no standard reversal agent is available.[14,15]
The
treatment of patients presenting with bleeding while receiving
anticoagulants with or without antiplatelet agents is based on many
factors, such as the source of bleeding, hemodynamic stability of the
patient, and the severity of blood loss.[16] In major
bleeding, the management provided might include interventions such as
reversing the effect of a therapeutic agent, a surgical achievement of
homeostasis, or a combination of both.[17,18] Since reversal is indicated for severe and life-threatening haemorrhage among such patients,[19]
it might be acceptable to consider reversal-receiving as an indicator
of a severe bleeding episode. There are currently limited studies of
the real-world association between pharmacological anticoagulation
therapy and reversal being implemented in severe and life-threatening
bleeding events. Further, patients who receive a reversal of
anticoagulant therapy often require hospitalisation and recommencement
of anticoagulation therapy. This decision could be challenging, given
the lack of evidence-based guidelines in the selection of therapy.[20,21]
LoS is used extensively in the literature to indicate the severity of a condition and the efficacy and cost of treatment.[22] Moreover, LoS is used as an outcome measure for health services,[23] including quality improvement.[24]
It is worth noting that the use of LoS as an outcome measure should be
taken into account for other individual factors as an indicator of both
bleeding severity and management cost.[24]
Currently, only a few studies have explored the LoS associated with
bleeding events among patients receiving anticoagulation therapy in
Australia.
The aim of this study was to explore the gap between
VTE assessment and management guidelines on the one hand and clinical
practice on the other. This purpose was achieved by investigating
patients receiving anticoagulants with or without antiplatelet agents
who presented with an acute bleeding episode at a regional tertiary
referral hospital, Launceston General Hospital Emergency Department
(LGH-ED). This exploration included the patients' characteristics,
organ function in correlation with their bleeding presentation and the
management provided, type of anticoagulation therapy, the severity of
bleeding, and LoS. Translating the findings into real-world clinical
practice might bridge the knowledge–practice gap in this field.
Methods
Ethics approval.
The project was approved by the Human Research Ethics Committee, the
University of Tasmania (H0016734). Because this study was a clinical
audit where patient management was not affected, and patients were not
actively participating, consent was not required from patients; thus,
the Ethics Committee agreed to waive the consent requirement for this
low-risk audit.
Data sampling.
Data sampling was limited to patients who presented to the LGH-ED with
acute bleeding and at the same time were receiving anticoagulation
therapeutic agent(s). The LGH is a tertiary regional referral centre in
Northern Tasmania, and it has the only Emergency Department within a
100-kilometer radius in the region. The LGH has an electronic/digital
medical record (DMR) for all patients presenting to the Emergency
Department. Thus, we conducted an
electronic search for all patients who presented with bleeding in the
period between January 2016 and June 2018. The Pharmacy Department then
checked the records at the LGH to determine whether those patients were
receiving anticoagulation-therapeutic agent(s) in the form of an
anticoagulant with or without antiplatelet agents. Accordingly, only
records that satisfied our selection criteria – presentation with acute
bleeding while receiving anticoagulation therapeutic agent in the form
of DOACs plus/minus antiplatelet agents – were included in the
analysis.
Data collection.
Data were extracted from the LGH patients' DMR. The LGH electronic
patient file and computerised records provided basic demographics such
as age, gender, ethnic group, and language. Further, the system data
provided information about the bleeding episode, including the source
of bleeding, admission/discharge details, management provided, and LoS
in days. The DMR offers data about the indications for administering
anticoagulation therapeutic agent(s) and their doses and results of
routine blood tests carried out on admission for each patient
presenting with bleeding. These tests included full blood count (FBC),
renal function (blood urea nitrogen, serum creatinine, eGFR), liver
function (ALT, AST albumin, bilirubin), bleeding and coagulation
profile (INR, APTT, PT, platelet count), and intervention
provided.
Data analysis. Data were analysed using SPSS V26.1.[25]
The values of laboratory tests were categorised after adjusting for
gender, as per the reference intervals published by the Royal College
of Pathologists of Australasia or the World Health Organization.[26]
The anticoagulation therapeutic agent(s) were re-categorised based on
the mechanism of action (antiplatelet or anticoagulants). Participants'
characteristics, count, and valid percentages (for non-missing values)
were calculated for categorical variables, and means with standard
deviation (SD) were calculated
for continuous variables. The association between the reason for
administering anticoagulation therapeutic agent(s) and the medication
used was evaluated by a multinomial logistic regression. A Firth
logistic regression model was used to overcome the small sample size to
explore the association between the type of anticoagulation therapeutic
agent(s) and receiving reversal. Finally, an adjusted linear regression
model was used to explore the association between the type of
anticoagulation therapeutic agent(s) used and LoS.
Results
Participant characteristics.
Among the 1501 patients presenting to the ED at the LGH in the period
between January 2016 and June 2018 with a diagnosis of acute bleeding,
only 144 (14.4%) were identified by the Pharmacy Department as
receiving anticoagulation therapeutic agent(s) in the form of
anticoagulants or antiplatelet agents and therefore were eligible for
inclusion in our study. Just over half of patients were males n=75 (52.1%), and the mean age was 76 years (SD=11.1).
Gastrointestinal tract (GIT) bleeding was the most common site of
bleeding in 48 patients (33.3%), followed by epistaxis (n=32,
22.2%), while haematuria was present in 14 patients (9.7%). The most
common reason for administering anticoagulation therapeutic agent(s)
was atrial fibrillation (AF) (n=65,
45.1%), while PE/VTE treatment was documented in 19 patients (13.2%),
and other reasons for anticoagulation therapy were also given (n=27,
18.8%). In a multiple response descriptive analysis for the type of
anticoagulation therapeutic agent(s) used among the patients presented
with acute bleeding, warfarin was the most common (n=74, 51.4%), followed by aspirin, which was used by 29 patients (20.1%), then rivaroxaban (n=26, 18.1%). Patients' characteristics are detailed in Table 1.
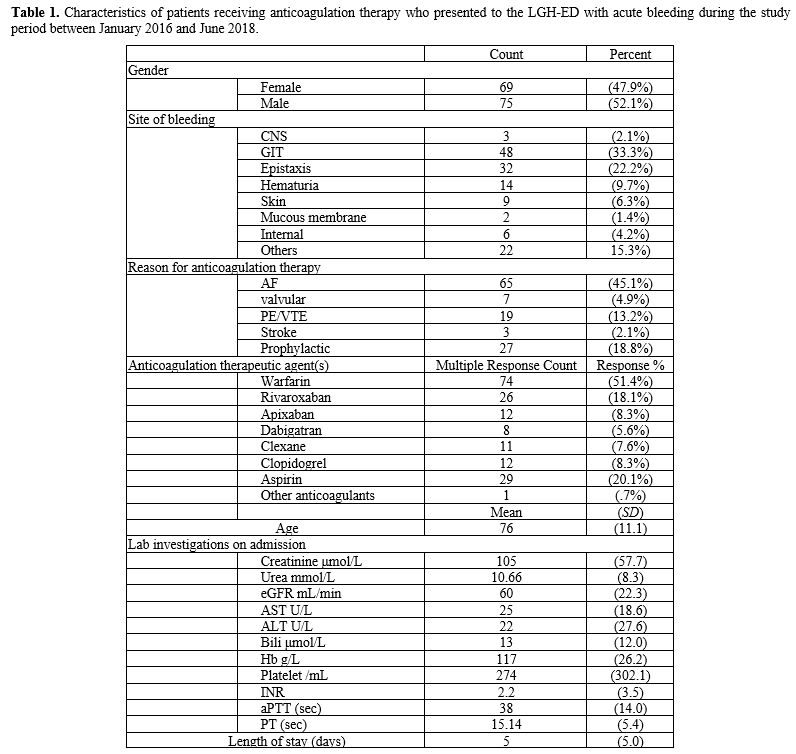 |
Table
1. Characteristics of patients receiving anticoagulation therapy who
presented to the LGH-ED with acute bleeding during the study period
between January 2016 and June 2018.
|
According
to a multinomial logistic regression model for the type of
anticoagulation therapeutic agent(s) used and the reason for
administration, DOACs were more likely to be used with AF patients (OR=9.6, P=.016) than warfarin (OR=6.1, P=.044). Also, for DVT/PE treatment, LMWH was significantly used (OR=30.0, P=.003) than DOACs when compared with warfarin.
Patient management.
Laboratory investigations.
On patients' presentation at the LGH-ED with acute bleeding, routine
laboratory investigations were carried out, including coagulation
profile, FBC, and kidney and liver function for all patients. It was
found that a majority (n=67,
46.5%) had an increased blood urea nitrogen level (>3.0-8.0 mmol/L),
while 59 (41.0%) had a mild reduction in eGFR (60–89 mL/min). The liver
function test showed that most patients had normal AST (n=103, 71.5%), all of them had a normal ALT test (n=144, 100%), and normal bilirubin levels (<20 mmol/L) (n=131, 91.0%). Among those under the vitamin K antagonist warfarin) (n=74), many (n=35, 47.3%) had their INR above the target range (adjusted for the reason of administration),[27] despite a minority having elevated AST (n=14,
18.9%), while all (100%) had normal ALT without a known liver disease.
It is worth noting that no data were available on assays used for
measuring DOACs activities. Most patients (n=112, 77.8%) had a normal platelet count (150-400 x109/L), but a few (n=18, 12.5%) had thrombocytopenia (<150 x109/L), and among them 13 (72.2%) had severe thrombocytopenia (less than 30 x109/L). Thrombocythemia (>400 x109/L) was reported in 14 (9.7%) patients. However, most patients (n=88, 61.1%) were found to have severe anaemia (Hb: female <80g/L, male <80g/L), which was based on the PenaRosas, et al.[26]
guidelines on haemoglobin concentration for diagnosis and assessment of
anaemia severity published by the World Health Organization. Details of
laboratory investigations are found in Table 2.
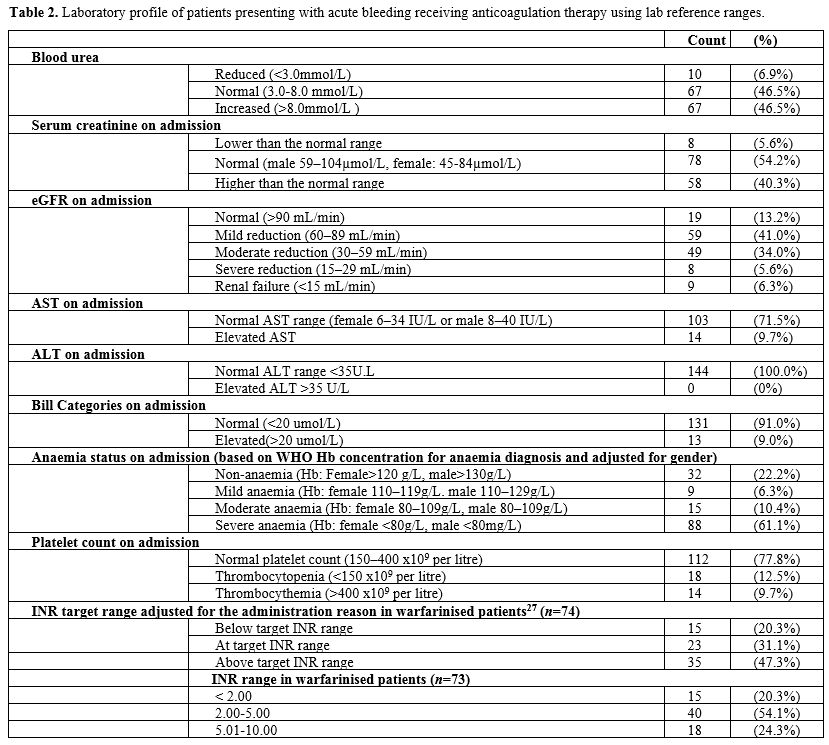 |
Table
2. Laboratory profile of patients presenting with acute bleeding receiving anticoagulation therapy using lab reference ranges.
|
Treatment provided.
Among those patients who presented with bleeding while receiving
anticoagulation therapy, 128 patients (88.9%) were admitted for
management, and 81 patients (56.3%) received an intervention. Among
those patients who received an intervention, medical management was the
most common (n=44, 54.3%) followed by surgical intervention (n=28, 34.6%), such as ligation/cautery of the bleeding vessel, while a few received a combined medical and surgical management (n=9, 11.1%). Among those who received reversal (n=47), by using multiple response descriptive, vitamin K was the most frequent (n=23, 48.9%), while 17 (36.2%) received prothrombin (Table 3).
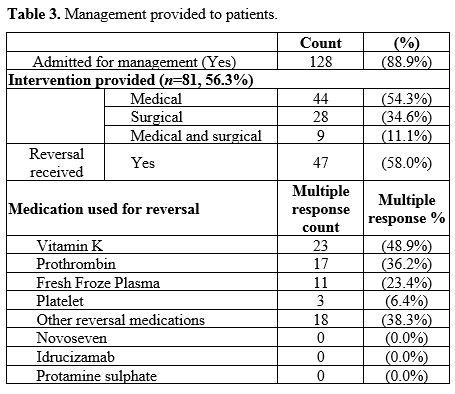 |
Table 3. Management provided to patients.
|
The choice of anticoagulation therapeutic agents on recommencement was similar to the pre-admission agent: warfarin (OR=17.5, P=<.001), rivaroxaban (OR=60.7, P=<.001), apixaban (OR=22.2, P=<.001), clexane (OR=8.1, P=<.033), clopidogrel (OR=61.9, P=<.001), and aspirin (OR=64.0, P=<.001). For more detail, see Table 4.
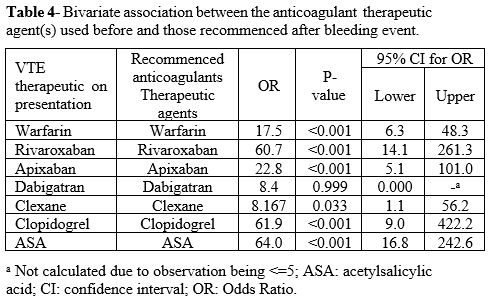 |
Table 4. Bivariate
association between the anticoagulant therapeutic agent(s) used
before and those recommenced after bleeding event.
|
Type of anticoagulation therapeutic agent(s) associated with receiving reversal. Based on a χ2 association for receiving reversal and the type of anticoagulation therapeutic agent(s), vitamin K antagonist (χ2 =24.2, P=<.001) and DOACs (χ2=12.7, P=<.001) were significantly associated with receiving reversal (Table 5).
Using a Firth logistic regression, DOACs use was associated with a
reduced likelihood of receiving reversal compared with vitamin K
antagonists (B=-1.7, P=<.001), as shown in Table 5.
It is worth noting that idarucizumab is the approved reversal for
dabigatran in Australia. We observed the use of other options[19] for reversing the effect of DOACs in some cases, such as prothrombin complex and fresh frozen plasma.
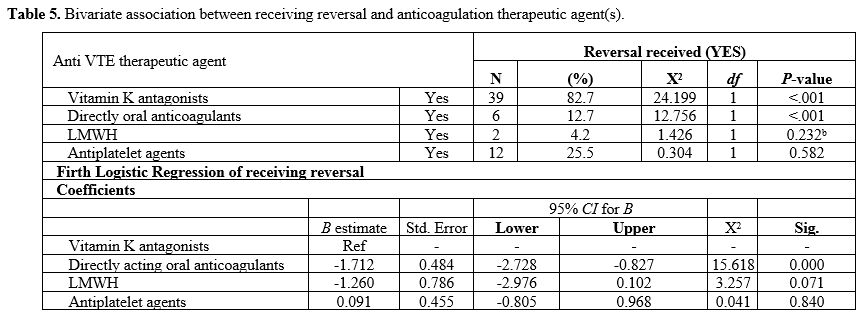 |
Table 5. Bivariate association between receiving reversal and anticoagulation therapeutic agent(s).
|
Association
between the type of anticoagulation therapeutic agent and LoS due to
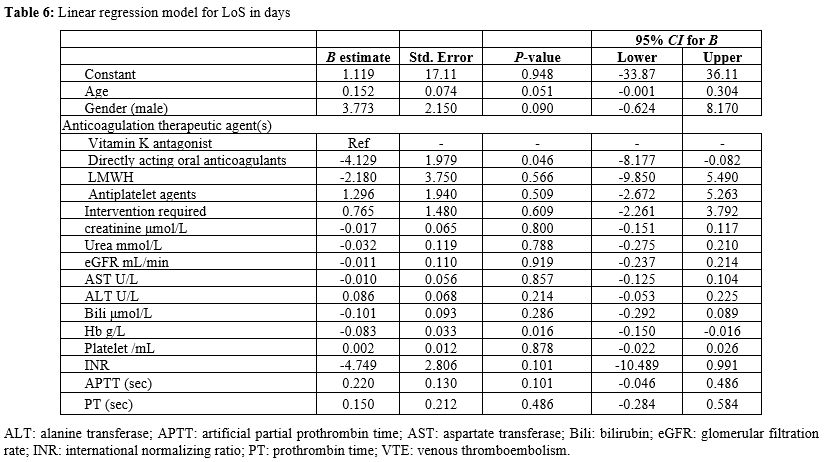
the acute bleeding event in an adjusted linear regression model. In an adjusted linear regression model for LoS in days, DOACs were associated with a significantly shorter LoS (B=-4.1, 95% CI: -8.177, -0.082, P=0.046)
when compared with vitamin K antagonist (warfarin); additionally, a
higher haemoglobin concentration on admission was associated with a
shorter LoS (B=-0.083, 95% CI: -0.150- -0.016, P=0.016) (Table 6).
 |
Table
2. Linear regression model for LoS in days
|
Discussion
This
study illustrates the characteristics and profile of patients receiving
different anticoagulation therapy – in the form of oral anticoagulants
including DOAC and antiplatelet agents – who presented with acute
bleeding at a regional tertiary hospital in Tasmania, Australia. The
associations between the type of anticoagulation therapeutic agent on
the one hand and the severity of bleeding and receiving reversal
agent(s) on the other, in conjunction with LoS, were studied. The study
showed that warfarin was a frequent anticoagulation therapeutic agent
among patients who presented with bleeding. Additionally, many of those
warfarinised patients had INRs above the desired target range for the
condition being administered. While conventional coagulation profile
tests were requested for most patients, no agent-specific laboratory
tests were requested for patients receiving DOACs. When compared with
warfarin, DOACs use was more common in patients with AF. It is worth
noting that the Therapeutic Goods Administration (TGA) approves
dabigatran in non-valvular AF patients only; rivaroxaban and apixaban
are approved for both non-valvular AF and anticoagulation-treatment and
prophylaxis. While most patients were admitted for management, many had
already received medical management to reverse the effect of the
anticoagulation therapeutic agent(s). The reversal agents were less
likely to be used with DOACs than warfarin and other anticoagulation
therapeutic agents (s). On exploring the association between LoS and
individual agents (in an adjusted analysis), the use of DOACs was
associated with a shorter LoS than LMWH or antiplatelets compared to
warfarin. It is worth mentioning that LoS was longer when the patient
had lower haemoglobin concentration on admission.
The study findings might enhance the use of DOACs,[28]
which were introduced about ten years ago in Australia and had a steady
prescription pattern at the present study time. However, because the
study did not weigh the prevalence of these medications' prescription
rates, caution should be exercised. Overall, the recommended target INR
range was not achieved in many patients who received warfarin and
presented with bleeding.[29] This suggests the need
for continued educational development on pharmacological
anticoagulation therapy and clear guidelines and decision aids for
medical professionals. While most patients had global coagulation
tests, it is argued that these tests are not reliable in patients
receiving DOACs.[30] Some assays are currently available for DOACs, such as ecarin clotting time (ECT) and chromogenic anti-FXa,[18]
but they were seldom requested by the ED physicians in this study. This
finding might suggest the need to improve medical practitioners'
knowledge about more reliable tests for measuring DOACs activity.
The majority (n=128,
88.9%) of patients who had bleeding because of anticoagulation
therapeutic agents were admitted for management. Thirty-one patients
(24%) needed reversal. However, this study was able to identify that
patients on DOACs were less likely to receive reversal when compared
with those who were on warfarin or LMWH. This finding supports the
wider implementation of DOACs[28] when compared with
warfarin and other anticoagulation therapeutic agents. The real-world
association between receiving reversal in patients who presented with
life-threatening bleeding due to anticoagulation therapy is very
difficult to obtain using other research designs, considering that
prolonged cohort studies require substantial resources. However, the
present study arrived at the same inference using a cross-sectional
design.
Furthermore, the present study was able to find a
significant association between pre-and post-bleeding pharmacological
anticoagulation therapeutic agents. In contrast, dabigatran and clexane
were less likely to be used on the resumption of pharmacological
anticoagulation therapy when compared with other agents. It is worth
noting that, in Australia, dabigatran and antiplatelet agents are not
indicated for the treatment of VTE. Accordingly, this finding ought to
be explored in future research.
Using an adjusted analysis[24] for LoS, it was found that DOACs were associated with a shorter LoS (P=0.046) compared with warfarin, LMWH, and antiplatelets. This finding was consistent with two recent studies.[31,32] These studies have concluded that DOACs were significantly associated with a shorter LoS compared to warfarin.[31,32]
Furthermore,
there is evidence that DOACs cost significantly less than warfarin for
hospitalisation due to a specific bleeding event with blunt traumatic
intracranial hemorrhage.[22] However, what is novel
in the current study was the wide variety of the bleeding sites and the
wide range of anticoagulation therapeutic agents used for various
reasons, such as VTE prophylaxis or treatment, in correlation with
coagulation profile and kidney and liver function and management and or
interventions that were conducted at the time of presentation. Although
it might be argued that upfront costs for warfarin administration are
cheaper when compared with DOACs,[10] our finding
suggests that DOACs are more cost-effective overall in the long run when
compared with warfarin or other agents, considering the reduced
likelihood of patients' presentations to ED and receiving reversal and
the significantly shorter LoS.
Recent literature showed that
DOACs have a better safety profile than warfarin, particularly
intracranial and subarachnoid haemorrhage.[33]
Moreover, rivaroxaban appears to be better than warfarin in limitation
of blood-brain barrier disruption after intracranial haemorrhage.[34]
In addition to the VTE prophylaxis effect, DOACs show non-inferior
results and superior results compared to warfarin in the management of
non-valvular atrial fibrillation and prevention of stroke, especially
after the availability of reversal agents such as idarucizumab.[35] In this regard, Coons et al. demonstrated in an extensive study of 1840 patients with morbid obesity (BMI>40 kg/m2) and VTE that DOACs are more effective and less risky than warfarin.[36]
In another study, there was no advantage of warfarin over DOACs as VTE
prophylaxis in patients who have cancer or atrial fibrillation.[37]
It
is worth noting that in our hands that the use of DOACs in the studied
cohort with renal impairment was not associated with excessive bleeding
as occurs with warfarin. In comparison to warfarin, the safety of DOACs
in case of chronic kidney disease (CKD) was always a concern among
clinicians. A recent study by Weber, found that apixaban is safer than warfarin in CKD.[38]
Nonetheless, careful consideration of anticoagulation's desired level
and anticoagulant dose to achieve the best possible anticoagulation
effect and outcome is warranted.[39] It is worth
noting that there are no reliable, up-to-date guidelines for
recommending DOACs in different doses in case of impaired renal
function.[40] However, in practice, DOACs are
considered to have a similar or safer profile compared to warfarin in
mild to moderate renal impairment, but this is not the case in severe
renal impairment, especially in the renal transplant setting.[41]
This
study's main limitation was the small sample size yielded from our
perspective cross-sectional sampling of patients during the sampling
timeframe. However, the same approaches were used to overcome the small
sample size by re-categorising anticoagulation therapeutic agents and
using statistical methods such as the Fisher's exact test and Firth
logistic regression. It may be worth noting that some studies, such as
the one conducted by Lamb et al.[22] in the USA, have
investigated a closely-related topic but relied on smaller sample size.
On the other hand, the present study has several strengths: it included
all patients who had bleeding secondary to anticoagulant with or
without antiplatelet agents in an entire regional population in mid and
north of Tasmania. The LGH is the only tertiary referral hospital in
this area, and any patient with acute bleeding would be referred to it.
The study contributes to clinical practice by showing the need for
better control and effective monitoring of patients on pharmacological
anticoagulation therapeutic agents based on administration.
Additionally,
our study contributes to research on health services cost-effectiveness
by showing that the use of DOACs is associated with a reduced
likelihood of receiving reversal and shorter LoS in the absence of
life-threatening bleeding compared with warfarin. Furthermore, this
study contributes to clinical decision-making with respect to selecting
anticoagulation therapeutic agents by showing that reduced morbidity
was associated with the use of DOACs compared with warfarin. This study
contributed to translational medical research by obtaining real-world
evidence on risk assessment and management in patients receiving
anticoagulation therapy who presented with bleeding while considering
the available guidelines and practice information.
Conclusions
Despite
the limitations of our study, it is suggested that the application of
DOACs is associated with fewer bleeding complications compared to
warfarin. Further, bleeding in DOAC was shown to be less severe in our
cohort study with reduced LoS that encourage DOACs' use, although the
costs due to the fact of their pharmacodynamic reversal is not often
required.
DOACs were associated with a reduced likelihood of
receiving reversal, a shorter LoS, and better overall clinical
outcomes. The guidelines should probably address and include better
indicators for DOACs bleeding risk, such as ECT and Chromogenic
anti-FXa. Therefore, ECT and chromogenic anti-FXa should be better
understood and utilised in the context of bleeding associated with
DOACs among clinicians, especially in the Emergency Department.
References
- Bungard TJ, Ritchie B, Bolt J, Semchuk WM.
Anticoagulant therapies for acute venous thromboembolism: a comparison
between those discharged directly from the emergency department versus
hospital in two Canadian cities. BMJ Open 2018;8(10):e022063. https://doi.org/10.1136/bmjopen-2018-022063 PMid:30385438 PMCid:PMC6224720
- Noel SE, Millar JA. Current state of medical thromboprophylaxis in Australia. The Australasian Medical Journal 2014;7(2):58-63. https://doi.org/10.4066/AMJ.2014.1915 PMid:24611073 PMCid:PMC3941577
- Kakkos
SK, Warwick D, Nicolaides AN, Stansby GP, Tsolakis IA. Combined
(mechanical and pharmacological) modalities for the prevention of
venous thromboembolism in joint replacement surgery. The Journal of
Bone and Joint Surgery 2012;94(6):729-734. https://doi.org/10.1302/0301-620X.94B6.28128 PMid:22628585
- Moheimani
F, Jackson DE. Venous thromboembolism: Classification, risk factors,
diagnosis, and management. ISRN Hematology 2011;2011:e124610. https://doi.org/10.5402/2011/124610 PMid:22084692 PMCid:PMC3196154
- Watson
HG, Chee YL. Aspirin and other antiplatelet drugs in the prevention of
venous thromboembolism. Blood Reviews 2008;22(2):107-116. https://doi.org/10.1016/j.blre.2007.11.001 PMid:18226435
- Harter
K, Levine M, Henderson SO. Anticoagulation drug therapy: a review.
Western Journal of Emergency Medicine 2015;16(1):11-17. https://doi.org/10.5811/westjem.2014.12.22933 PMid:25671002 PMCid:PMC4307693
- Kimachi
M, Furukawa TA, Kimachi K, Goto Y, Fukuma S, Fukuhara S. Direct oral
anticoagulants versus warfarin for preventing stroke and systemic
embolic events among atrial fibrillation patients with chronic kidney
disease. Cochrane Database of Systematic Reviews
2017;2017(11):CD011373. https://doi.org/10.1002/14651858.CD011373.pub2 PMid:29105079 PMCid:PMC6485997
- Gomez-Outes
A, Terleira-Fernandez AI, Lecumberri R, Suarez-Gea ML,
Vargas-Castrillon E. Direct oral anticoagulants in the treatment of
acute venous thromboembolism: a systematic review and meta-analysis.
Thrombosis Research 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020 PMid:25037495
- TGA.
New oral anticoagulants - apixaban (Eliquis), dabigatran (Pradaxa) and
rivaroxaban (Xarelto) Australia: The Australian Government Theraputic
Goods Administration; 2015 [Available from: https://www.tga.gov.au/node/705240
- Ortiz-Cartagena
L, Gotay AO, Acevedo J. Cost effectiveness of oral anticoagulation
therapy for non-valvular atrial fibrillation patients: Warfarin versus
the new oral anticoagulants rivaroxaban, dabigatran and apixaban.
Journal of the American College of Cardiology 2018;71(11):A490. https://doi.org/10.1016/S0735-1097(18)31031-3
- Shoeb
M, Fang MC. Assessing bleeding risk in patients taking anticoagulants.
Journal of Thrombosis and Thrombolysis 2013;35(3):312-319. https://doi.org/10.1007/s11239-013-0899-7 PMid:23479259 PMCid:PMC3888359
- Hamidi
M, Zeeshan M, Sakran JV, Kulvatunyou N, O'Keeffe T, Northcutt A,
Zakaria ER, Tang A, Joseph B. Direct oral anticoagulants vs
low-molecular-weight heparin for thromboprophylaxis in nonoperative
pelvic fractures. Journal of the American College of Surgeons
2018;228(1):89-97. https://doi.org/10.1016/j.jamcollsurg.2018.09.023 PMid:30359834
- Bungard
TJ, Ritchie B, Bolt J, Semchuk WM. Management of acute venous
thromboembolism among a cohort of patients discharged directly from the
emergency department. BMJ Open 2018;8(10):e022064. https://doi.org/10.1136/bmjopen-2018-022064 PMid:30385439 PMCid:PMC6224769
- Scheres
LJJ, Lijfering WM, Middeldorp S, Cheung YW, Barco S, Cannegieter SC,
Coppens M. Measurement of coagulation factors during rivaroxaban and
apixaban treatment: results from two crossover trials. Research and
Practice in Thrombosis and Haemostasis 2018;2(4):689-695. https://doi.org/10.1002/rth2.12142 PMid:30349888 PMCid:PMC6178718
- Ebner
M, Birschmann I, Peter A, Hartig F, Spencer C, Kuhn J, Rupp A,
Blumenstock G, Zuern CS, Ziemann U, Poli S. Limitations of specific
coagulation tests for direct oral anticoagulants: a critical analysis.
Journal of the American Heart Association 2018;7(19):e009807. https://doi.org/10.1161/JAHA.118.009807 PMid:30371316 PMCid:PMC6404908
- Dhakal
P, Rayamajhi S, Verma V, Gundabolu K, Bhatt VR. Reversal of
anticoagulation and management of bleeding in patients on
anticoagulants. Clinical and Applied Thrombosis/Hemostasis
2017;23(5):410-415. https://doi.org/10.1177/1076029616675970 PMid:27789605
- Anderson
I, Cifu AS. Management of bleeding in patients taking oral
anticoagulants. JAMA Journal of American Medical Association
2018;319(19):2032-2033. https://doi.org/10.1001/jama.2018.3504 PMid:29800198
- Eikelboom
J, Merli G. Bleeding with direct oral anticoagulants vs warfarin:
clinical experience. The American Journal of Emergency Medicine
2016;34(11S):S33-S40. https://doi.org/10.1016/j.amjmed.2016.06.003 PMid:27586367
- Almegren M. Reversal of direct oral anticoagulants. Vascular Health and Risk Management 2017;13:287-292. https://doi.org/10.2147/VHRM.S138890 PMid:28769570 PMCid:PMC5529093
- Qureshi
W, Mittal C, Patsias I, Garikapati K, Kuchipudi A, Cheema G, Elbatta M,
Alirhayim Z, Khalid F. Restarting anticoagulation and outcomes after
major gastrointestinal bleeding in atrial fibrillation. The American
Journal of Cardiology 2014;113(4):662-668. https://doi.org/10.1016/j.amjcard.2013.10.044 PMid:24355310
- Witt
DM. What to do after the bleed: resuming anticoagulation after major
bleeding. American Society of Hematology Education Program
2016;2016(1):620-624. https://doi.org/10.1182/asheducation-2016.1.620 PMid:27913537 PMCid:PMC6142471
- Lamb
LC, DiFiori M, Comey C, Feeney J. Cost analysis of direct oral
anticoagulants compared with warfarin in patients with blunt traumatic
intracranial hemorrhages. American Surgeon 2018;84(6):1010-1014. https://doi.org/10.1177/000313481808400657
- Sarkies
MN, Bowles KA, Skinner EH, Mitchell D, Haas R, Ho M, Salter K, May K,
Markham D, O'Brien L, Plumb S, Haines TP. Data collection methods in
health services research: hospital length of stay and discharge
destination. Applied Clinical Informatics 2015;6(1):96-109. https://doi.org/10.4338/ACI-2014-10-RA-0097 PMid:25848416 PMCid:PMC4377563
- Brasel
KJ, Lim HJ, Nirula R, Weigelt JA. Length of stay: an appropriate
quality measure? Archives of Surgery 2007;142(5):461-465. https://doi.org/10.1001/archsurg.142.5.461 PMid:17515488
- IBM SPSS Statistics for Windows [program]. 26.1 version. NY, USA: IBM Corporation, 2020.
- PenaRosas
JP, Cusick S, Lynch S. Haemoglobin concentrations for the diagnosis of
anaemia and assessment of severity. World Health Organization. 2011.
Available from: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?ua=1
- Liew
J, Mathers S. Guideline for the Prevention of Venous Thromboembolism
(VTE) in Adult Hospitalised Patients. Queensland Health. Queensland,
Australia, 2018. Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0031/812938/vte-prevention-guideline.pdf
- Raschi
E, Bianchin M, Ageno W, De Ponti R, De Ponti F. Risk-benefit profile of
direct-acting oral anticoagulants in established therapeutic
indications: an overview of systematic reviews and observational
studies. Drug Safety 2016;39(12):1175-1187. https://doi.org/10.1007/s40264-016-0464-3 PMid:27696300 PMCid:PMC5107188
- Wallace
R, Anderson MA, See K, Gorelik A, Irving L, Manser R. Venous
thromboembolism management practices and knowledge of guidelines: a
survey of Australian haematologists and respiratory physicians.
Internal Medicine Journal 2017;47(4):436-446. https://doi.org/10.1111/imj.13382 PMid:28150371
- Adcock DM, Gosselin R. Direct Oral Anticoagulants (DOACs) in the laboratory: 2015 review. Thrombosis Research 2015;136(1):7-12. https://doi.org/10.1016/j.thromres.2015.05.001 PMid:25981138
- Badreldin
H. Hospital length of stay in patients initiated on direct oral
anticoagulants versus warfarin for venous thromboembolism: a real-world
single-center study. Journal of Thrombosis and Thrombolysis
2018;46(1):16-21. https://doi.org/10.1007/s11239-018-1661-y PMid:29626281
- Charlton
B, Adeboyeje G, Barron JJ, Grady D, Shin J, Redberg RF. Length of
hospitalization and mortality for bleeding during treatment with
warfarin, dabigatran, or rivaroxaban. PLoS One 2018;13(3):e0193912. https://doi.org/10.1371/journal.pone.0193912 PMid:29590141 PMCid:PMC5874024
- Paravattil
B, Elewa H. Approaches to Direct Oral Anticoagulant Selection in
Practice. J Cardiovasc Pharmacol Ther 2018:1074248418793137. https://doi.org/10.1177/1074248418793137 PMid:30092658
- Sawada
S, Ono Y, Egashira Y, Takagi T, Tsuruma K, Shimazawa M, Iwama T, Hara
H. In Models of Intracerebral Hemorrhage, Rivaroxaban is Superior to
Warfarin to Limit Blood Brain Barrier Disruption and Hematoma
Expansion. Curr Neurovasc Res 2017;14(2):96-103. https://doi.org/10.2174/1567202613666161216150835 PMid:27993122
- Rawal
A, Ardeshna D, Minhas S, Cave B, Ibeguogu U, Khouzam R. Current status
of oral anticoagulant reversal strategies: a review. Ann Transl Med
2019;7(17):411. https://doi.org/10.21037/atm.2019.07.101 PMid:31660310 PMCid:PMC6787376
- Coons
JC, Albert L, Bejjani A, Iasella CJ. Effectiveness and Safety of Direct
Oral Anticoagulants versus Warfarin in Obese Patients with Acute Venous
Thromboembolism. Pharmacotherapy 2020;40(3):204-210. https://doi.org/10.1002/phar.2369 PMid:31968126
- Shah
S, Norby FL, Datta YH, Lutsey PL, MacLehose RF, Chen LY, Alonso A.
Comparative effectiveness of direct oral anticoagulants and warfarin in
patients with cancer and atrial fibrillation. Blood Adv
2018;2(3):200-209. https://doi.org/10.1182/bloodadvances.2017010694 PMid:29378726 PMCid:PMC5812321
- Weber
J, Olyaei A, Shatzel J. The efficacy and safety of direct oral
anticoagulants in patients with chronic renal insufficiency: A review
of the literature. Eur J Haematol 2019;102(4):312-318. https://doi.org/10.1111/ejh.13208 PMid:30592337
- Shrestha
S, Baser O, Kwong WJ. Effect of Renal Function on Dosing of Non-Vitamin
K Antagonist Direct Oral Anticoagulants Among Patients With Nonvalvular
Atrial Fibrillation. Ann Pharmacother 2018;52(2):147-153. https://doi.org/10.1177/1060028017728295 PMid:28856898
- Padrini
R. Clinical Pharmacokinetics and Pharmacodynamics of Direct Oral
Anticoagulants in Patients with Renal Failure. Eur J Drug Metab
Pharmacokinet 2019;44(1):1-12. https://doi.org/10.1007/s13318-018-0501-y PMid:30167998
- Kcükköylü S, Rump LC. DOAC use in patients with chronic kidney disease. Hamostaseologie 2017;37(4):286-294. https://doi.org/10.5482/HAMO-17-01-0003 PMid:29582930
[TOP]





