Jemila James1, Vishal Vishnu Tewari2 and Naveen Jain3.
1 Neonatal Intensive Care Unit, King Hamad University Hospital, Al Muharraq, Kingdom of Bahrain.
2 Department of Pediatrics, Command Hospital (SC) and Armed Forces Medical College, Pune, India.
3 Department of Neonatology, Kerala Institute of Medical Sciences, Trivandrum, India.
Correspondence to: Dr
Vishal Vishnu Tewari, Neonatologist, Professor. Department of
Pediatrics, Command Hospital (SC) and Armed Forces Medical College,
Pune – 411040, India. Tel: 8826118889, 7391044489. E-mail:
docvvt_13@hotmail.com
Published: March 1, 2021
Received: November 8, 2020
Accepted: February 10, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021019 DOI
10.4084/MJHID.2021.019
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Antibiotic
therapy is initiated in neonates on suspicion of sepsis. Optimizing
therapy is a felt need of clinicians as prolonged injudicious use
increases mortality and morbidity risk.
Objective: To
evaluate the diagnostic accuracy of clinical tool 'STOPS' and serum
procalcitonin (PCT) for identifying neonates with early-onset neonatal
sepsis (EONS) or late-onset neonatal sepsis (LONS) and early
discontinuation in those with no sepsis.
Methods: The
study had a prospective analytical design conducted at a tertiary care
hospital. Consecutively admitted neonates with suspected EONS or LONS
were enrolled. The 'STOPS' tool comprising sensorium, temperature,
oxygenation, perfusion, skin color, and blood sugar was applied at 6
and 12 hours of enrollment. Serum PCT was sent at 12 hr. The
sensitivity, specificity, positive and negative predictive value (PPV
and NPV), positive and negative likelihood ratio (PLR and NLR) were
estimated.
Results: The
study enrolled 380 neonates, of which 330 were given antibiotics for
EONS and 50 for LONS. Temperature disturbance in the EONS group at 12
hr showed a PPV of 100% and a PLR of 9.1 (7.7 – 18). Perfusion
assessment at 12 hr had a PPV of 77% and PLR of 8.25 (2.3 – 29). Skin
color assessment at 12 hr had a PPV of 100% and PLR of 13.5 (9.7 – 27).
The diagnostic accuracy of PCT in the EONS group was unremarkable. In
the LONS group, skin color at 12 hr had a PPV of 100% and PLR of 11.2
(8.6 – 19.5). The diagnostic accuracy of PCT in the LONS group showed a
PPV of 82% and PLR of 7 (1.7 – 29).
Conclusion: Identifying
abnormal STOPS parameters was superior to PCT alone in EONS and as good
as PCT in LONS. The 'STOPS' tool allows early identification of
neonates with no sepsis, thereby optimizing antibiotic use.
|
Introduction
Neonatal
sepsis imposes a high burden on healthcare services in developing
nations. Prompt diagnosis and treatment are crucial to prevent severe
morbidity and mortality.[1,2] The limited repertoire
of clinical features in neonates with sepsis overlaps with
non-infectious conditions, and low sensitivity and positive predictive
value of biomarkers lead to antibiotic therapy initiation based on
clinical suspicion alone.[3] Prolonged empirical
antibiotics in extremely low birth weight neonates for sepsis is
associated with increased risk of death, necrotizing enterocolitis,[4] and intestinal dysbiosis with long-term health effects.[5]
Optimizing antibiotic therapy and minimizing injudicious use is a felt
need of neonatal clinicians and researchers. Blood culture remains the
gold standard for diagnosing sepsis despite the limitation of
availability of results by 48-72 hours. Even then, blood culture does
not identify most infected neonates with frequent false-negative
results due to neonatal sepsis's pauci-bacterial nature, a small volume
of blood available for inoculating culture bottles, and prior maternal
antibiotic exposure in early-onset neonatal sepsis (EONS) setting.[6,7]
Several acute phase reactants have been evaluated for optimizing
antibiotic usage but do not have high sensitivity if measured early in
the course of sepsis.[8] Among the biomarkers in
clinical application, serum procalcitonin (PCT) is useful in reducing
antibiotics' duration in suspected EONS.[9] Cost and
availability constraints in developing countries do not allow repeated
testing with decision making based mainly on clinical signs.[10]
For reliable identification of sepsis, a bedside clinical tool, quick,
repeatable, not laboratory dependent, inexpensive, and demanding
minimal training, is needed. Our unit has been using a combination of
clinical signs in a simple bedside assessment tool, which includes
sensorium, temperature, oxygenation, perfusion, skin color, and blood
sugar (STOPS) for early identification of neonatal sepsis and
optimizing antibiotic therapy. This study evaluated the accuracy of the
'STOPS' tool and serum PCT, individually and in combination for
diagnosing early and late-onset neonatal sepsis (LONS) for early
discontinuation of antibiotics in those with no sepsis.
Materials and Methods
The
study was an observational analytical study conducted prospectively
over 19 months at the neonatal unit of a tertiary care teaching
hospital in Southern India. The study was approved by the institutional
ethics committee. All neonates with maternal risk factors for EONS and
asymptomatic at birth, all symptomatic neonates at birth or within 72
hours of birth with or without maternal risk factors for EONS, and all
neonates with suspected LONS were eligible for enrollment.
Consecutively admitted neonates were enrolled after informed consent
from the parents and started on empirical intravenous (IV) antibiotics
for maternal risk factors for early-onset sepsis or presence of
clinical signs indicating EONS or LONS. Maternal risk factors included
prolonged rupture of membranes in term gestation (PROM >24 hrs),
maternal fever (>38˚C) from the onset of labor to delivery,
spontaneous preterm (< 37wks) onset of labor (SPTOL), preterm
(<37wks) pre-labor rupture of membranes (pPROM), maternal sepsis or
urinary tract infection within past seven days, and clinical
chorioamnionitis defined as fever >38˚C, maternal tachycardia
(>100 beats/min), fetal tachycardia (>160 beats/min), uterine
fundal tenderness, foul-smelling liquor, maternal leukocytosis (TLC
>15000/mm3) or positive C-reactive
protein (CRP) with no other site of infection. Given the inclusion of a
very preterm population (28-32 weeks gestation) and high EONS incidence
due to gram-negative Enterobacteriaceae, empiric antibiotics were
initiated.[11] Neonates below 28 weeks gestation and
1000 grams birth weight, those with congenital anomalies, confirmed
sepsis, or multi-organ dysfunction were excluded as multiple
morbidities in this population result in symptom overlap with sepsis,
precluding the use of clinical prediction models.[12]
Clinical signs prompting antibiotic therapy included fever (>38°C),
respiratory distress (tachypnea, retractions, grunting, oxygen
requirement), persistent tachycardia (>180 per min), excessive
cry/irritability, depressed sensorium, poor feeding, recurrent apnea,
seizures, abdominal distention, vomiting, gastrointestinal or per
rectal bleeding, or signs of local infection such as omphalitis. The
aim of our study was to estimate the diagnostic accuracy of the
clinical tool 'STOPS', serum PCT, the combination of 'STOPS' tool with
PCT in identifying neonates with EONS or LONS for early discontinuation
of antibiotics in neonates with no sepsis. Following initial
stabilization for one hour, all enrolled neonates initiated on IV
antibiotics for suspect sepsis underwent clinical assessment of 'STOPS'
twice at six hourly intervals within the first 12 hour period of
enrolment. The 'STOPS' tool has been developed, applied, and refined
for over a decade in our unit by process of meticulous bedside
observation, participative discussion of stakeholders, and revisiting
the clinical parameter for its consistency, reliability, and easy
assessment. Description of the 'STOPS' parameters and the score
allotted is as given (Table 1).
During the first three months of the study, 'STOPS' was recorded
independently by the neonatal fellows and nurses, confirmed by the
principal investigator, and the inter-observer reliability was
evaluated. The neonatal intensive care unit (NICU) temperature was kept
at 26°C, and the temperature in skin mode of servo-controlled radiant
warmers was set at 36.5°C. The neonates' skin temperature was
cross-checked by temperature recording using a digital thermometer
placed in the axilla. Temperature <36.5°C was taken as hypothermia,
and >38°C was used to define fever. Oxygenation was assessed
by Downes score and a fraction of inspired oxygen requirement (FiO2)
for maintaining oxygen saturation (SpO2) between 91-95%. Downes score
of ≤3 was normal, 4-6 suggested respiratory distress, and ≥7 indicated
an impending respiratory failure. Blood glucose estimation was done
using heel prick estimation by a Glucometer (OneTouch, LifeScan Inc).
Blood glucose <45 mg/dl was defined as hypoglycemia, and >180
mg/dl indicated hyperglycemia. Serum PCT estimation was done once
between [12-14] hours of enrolment using the
Enzyme-Linked Fluorescent Assay (ELFA) technique (VIDAS BRAHMS
automated PCT bioMerieux). A cut-off of > 2 ng/ml was considered as
a positive test.[13,14]
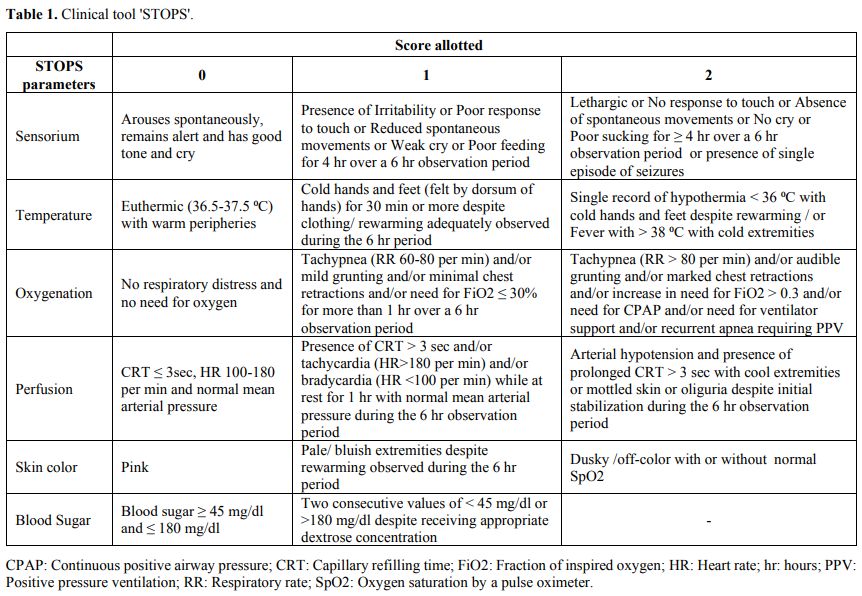 |
Table
1. Clinical tool 'STOPS'.
|
The case definitions of definite, clinical, and no sepsis in the study used as the reference standard[15,16] were adapted for our setting and were based on a combination of clinical signs, serum CRP and blood culture (Table 2).
Serum PCT estimation was not used for this purpose. Serum CRP assays
were done at 48 hours and repeated at 72 hours. Quantitative
determination of CRP was done on Roche/Hitachi Cobas C systems on
plasma using particle enhanced immune turbidimetric assay. A cut-off of
≥ 15 mg/L was considered as a positive test.[14] In
asymptomatic neonates with sterile blood culture and negative CRP at 48
and 72 hours, antibiotic therapy was stopped. Symptomatic neonates with
a positive blood culture received antibiotics for 7 days, and if
asymptomatic by the 7th day with a
negative CRP, antibiotics were discontinued. Lumbar puncture (LP) for
cerebrospinal fluid (CSF) examination for meningitis in neonates with
EONS was done only in those with a positive blood culture (definite
sepsis), while in LONS, it was done in all neonates with definite or
clinical sepsis.
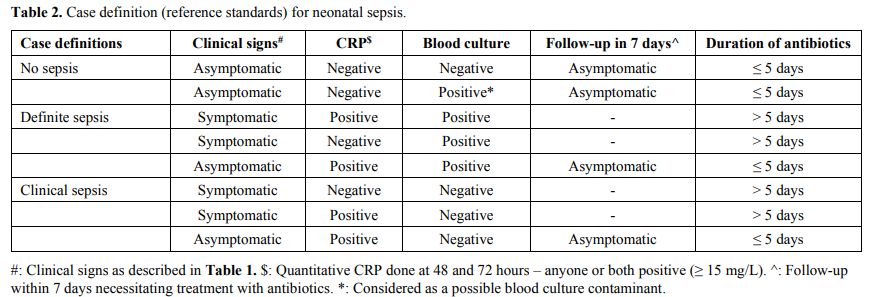 |
Table
2. Case definition (reference standards) for neonatal sepsis.
|
The
BACTEC or BacT/Alert microbial detection system was used to detect
aerobic and facultative anaerobic microorganisms from the blood.
Vitek-2 automated sensitivity and identification system was used for
the identification of organisms. Pediatric blood culture bottles were
inoculated with approximately 1 ml of the blood sample as per study
protocol. Positive blood culture reports were alerted as soon as
indicated by the microbial detection system, and a final report was
made available by 48 hours as positive or negative.
All mothers
with risk factors for EONS received IV Cefuroxime before delivery. EONS
was defined as the onset of symptoms within 72 hrs of age (< 72
hrs), while LONS was defined as the onset of symptoms after 72 hrs of
age (≥ 72hrs).[14,17]
Sample size calculation and statistical methods.
We estimated the prevalence of definite EONS initiated on IV
antibiotics to be 0.3/1000 hospitalized neonates based on a study
conducted at our unit earlier.[18] Using the nomogram for sample size calculation proposed by Carley et al.[19]
with a required confidence interval of 0.05 and desired sensitivity and
specificity of 95%, the requisite sample size was 300 neonates. The
data were entered in a Microsoft excel sheet, and the results were
analyzed using statistical software SPSS version [17]
The index test variables were changed to dichotomous variables and were
tested using the Chi-square test or Fischer exact test with the
reference standard. All p values reported are two-tailed, and a
p-value of ≤ 0.05 is considered statistically significant. The
sensitivity, specificity, PPV, NPV, PLR, and NLR were calculated to
find the index test's diagnostic accuracy with the reference standard.
An NLR of <0.2 was considered relevant. A PLR of 5-10 was taken as
moderately useful and >10 as very useful. The area under the
receiver operating characteristic (ROC) curve (AUC) was calculated for
diagnostic accuracy of PCT in EONS and LONS. Kappa statistics were used
to find out the agreement between observations recorded by the neonatal
fellows and nurses.
Results
The
total number of neonates initiated on IV antibiotics during the study
period was 545, of whom 165 were excluded. There were 38 neonates <
28 weeks/ < 1000 gram birth weight, 75 neonates with surgical
anomalies, 19 with proven sepsis, 9 with multi-organ dysfunction, 12
discharged before completion of the study, and 12 missed cases amongst
those excluded. Three hundred eighty neonates were enrolled in the
study, of whom 330 neonates were started on IV antibiotics for
suspected EONS; 330/380 (86.8%). The mean gestation of neonates in the
EONS group was 34.15 ± 2.8 weeks, and birth weight was 2203 ± 711 gm.
In the EONS group, 48/330 (14.6%) had definite sepsis, and 47/330
(14.2%) had clinical sepsis. There were 203/330 (61.5%) neonates in the
EONS group delivered with maternal risk factors for EONS, with preterm
premature rupture of membranes (pPROM) being the most frequent risk
factor [123/203 (60.5%)], followed by the spontaneous preterm onset of
labor (SPTOL) [66/203 (32.5%)]. There were 226/330 (68.5%)
neonates in the EONS group, who were symptomatic, and 104/330 (31.5%)
who were asymptomatic; of them, only 6/104 (5.8%) had evidence of
definite (1/104) or clinical (5/104) sepsis. Bacteria were isolated on
blood culture in 10/330 (3%) neonates in the EONS group (Table 3). There were no cases of meningitis in the neonates with definite EONS.
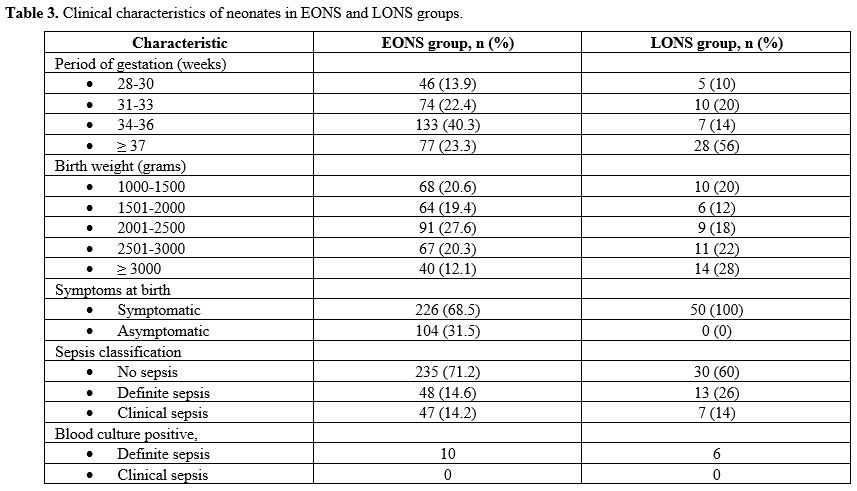 |
Table 3. Clinical characteristics of neonates in EONS and LONS groups.
|
There
were 50/380 (13.2%) neonates in the LONS group. Of these, 5/50 (10%)
were between 28-30 weeks gestational age and 10/50 (20%) between 1000
to 1500 gram bodyweight category. The LONS group's neonates were
heterogeneous, with median gestation of 37.5 weeks (IQR 32.5 – 38) and
a birth weight of 2550 gm (IQR 1751 – 3105). In the LONS group, 13/50
(26%) had definite sepsis, and 7/50 (14%) had clinical sepsis.
Bacterial growth on blood culture was seen in 6/50 (12%) neonates with
LONS (Table 3). One neonate with definite LONS was diagnosed with meningitis on cytology and biochemistry examination of the CSF.
Diagnostic
accuracy of temperature recording in the EONS group at 12 hr showed a
PPV of 100% and a PLR of 9.1 (7.7 – 18). Perfusion assessment at 12 hr
had a PPV of 77% and PLR of 8.25 (2.3 – 29). Skin color assessment at 6
hr had a PPV of 68% and PLR of 5.77 (2.29 – 15), while at 12 hr it had
a PPV of 100% and PLR of 13.5 (9.7 – 27) (Table 4).
The diagnostic accuracy of PCT in the EONS group done at 12 hr showed a
PPV of 33% and an NPV of 86%. The PLR was 1.3, and the NLR was 0.45 (Table 4) (Figure 1). In the LONS group, skin color's diagnostic accuracy at 12 hr had a PPV of 100%, NPV of 61%, and PLR of 11.2 (8.6 – 19.5) (Table 5). The diagnostic accuracy of PCT in the LONS group showed a PPV of 82%, NPV of 74%, PLR of 7 (1.7-29), and NLR of 0.54 (Table 5) (Figure 2).
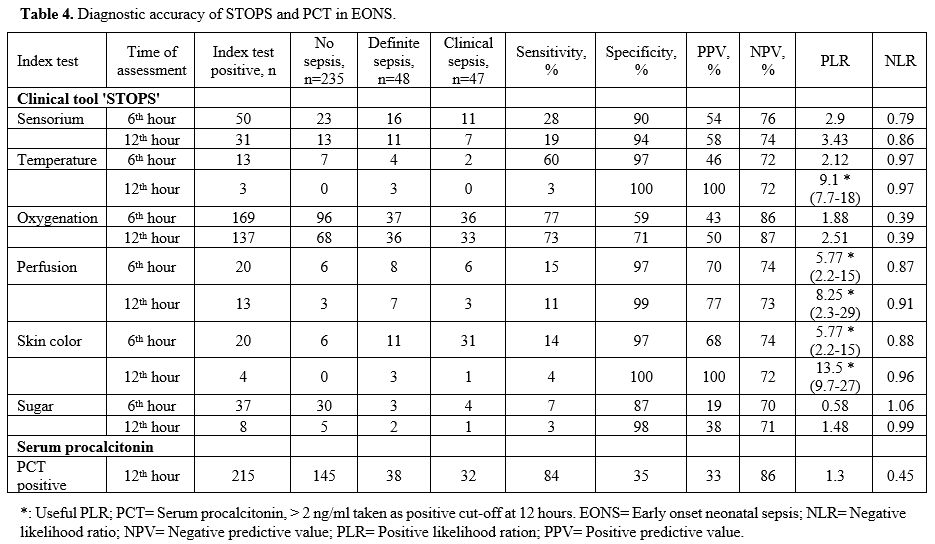 |
Table 4. Diagnostic accuracy of STOPS and PCT in EONS. |
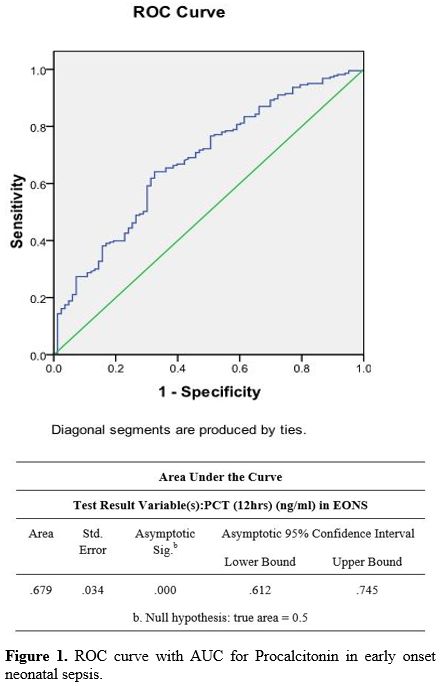 |
Figure 1. ROC curve with AUC for Procalcitonin in early onset neonatal sepsis. |
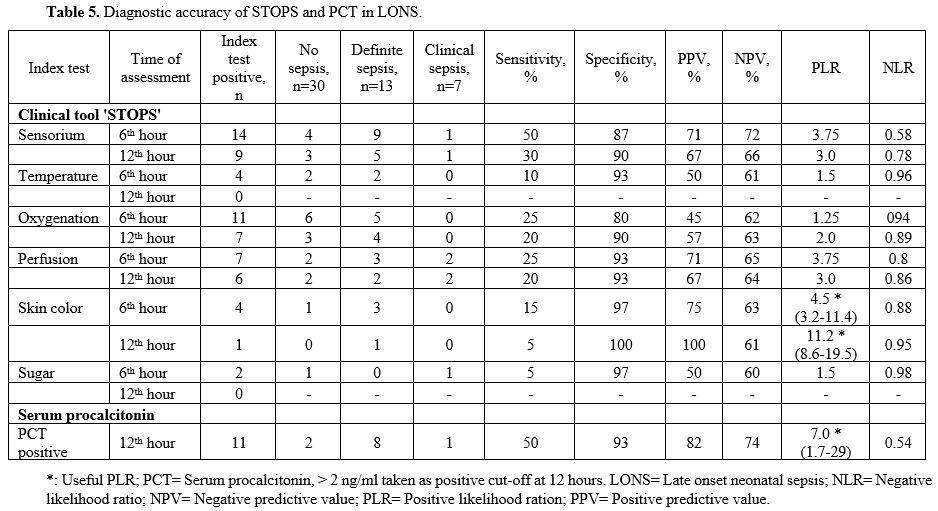 |
Table 5. Diagnostic accuracy of STOPS and PCT in LONS. |
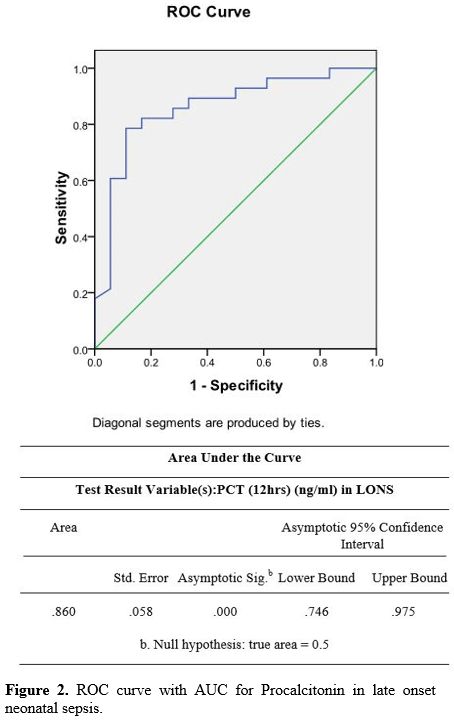 |
Figure
2. ROC curve with AUC for Procalcitonin in late onset neonatal sepsis. |
In the EONS group,
combining positive 'STOPS' and PCT at 12 hr revealed 289 (87.5%)
neonates, with a positive index test indicating antibiotics use. Of
these, there was definite sepsis in 48, clinical sepsis in 45, and no
sepsis in 196 with a low miss rate of 2 neonates. In LONS, combining
'STOPS' and PCT at 12 hr showed 23 (46%) neonates, with a positive
index test where antibiotics were indicated. Of these, 13 had definite
sepsis, 7 clinical sepsis, and no sepsis in 3 with no missed case (Table 6).
Kappa statistics for the STOPS variables ranged between 0.810-1.0 showing perfect agreement with p=0.000.
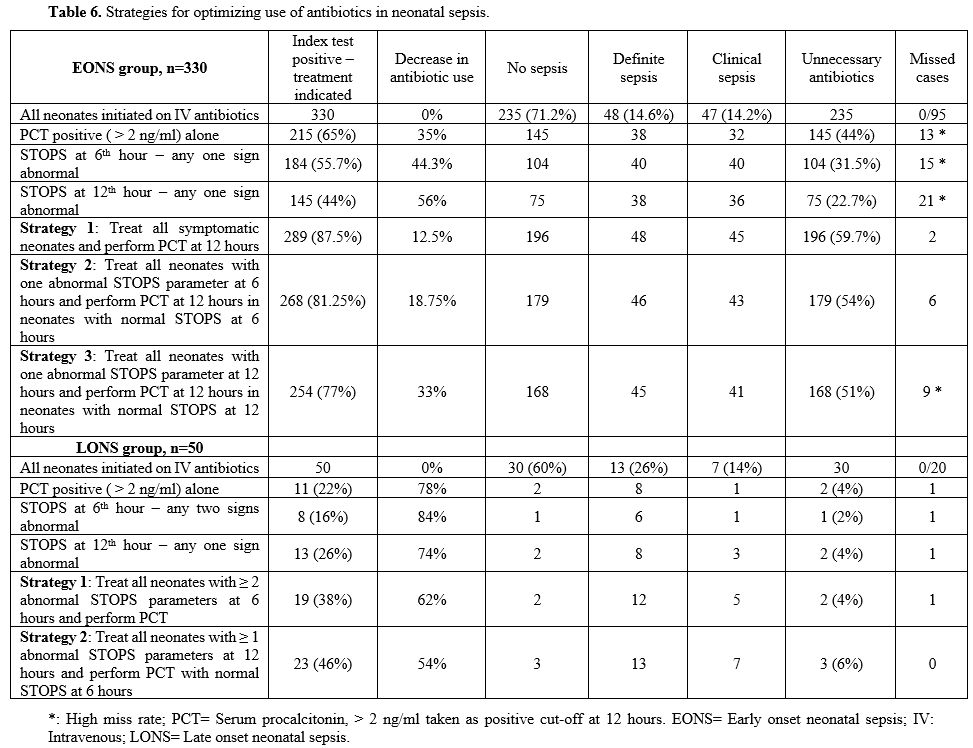 |
Table 6. Strategies for optimizing use of antibiotics in neonatal sepsis.
|
Discussion
This
study examined the diagnostic accuracy of the clinical tool 'STOPS,'
lab estimation of serum PCT, and a combination of the 'STOPS' tool with
PCT for identifying neonates with early or late-onset sepsis for early
discontinuation of antibiotics in neonates with no sepsis. Our study
found a significant number of neonates in the EONS group where
antibiotics were indicated to have no infection (235/330; 71.2%). Only
10/95 (10.5%) neonates had a blood culture isolate, underscoring the
importance of symptoms at or soon after birth and clinical signs in the
form of abnormal 'STOPS' in identifying sepsis requiring continued
antibiotic therapy (Table 4).
All 'STOPS' parameters excluding oxygenation had a specificity of
95-100% for identifying EONS. Temperature disturbance, perfusion
abnormalities, and skin color change were very or moderately useful
based on the PLR (Table 4).
Oxygenation
did not perform well understandably, as a significant number of preterm
neonates had respiratory distress syndrome (RDS). Serum PCT alone had a
very low specificity of 35%, a sensitivity of 84%, and a PLR, which was
not useful. It had an NPV of 86%, which was as good as any of the STOPS
parameters (Table 4). The study
shows that by combining the clinical tool 'STOPS' and PCT as a
treatment strategy, i.e., treating all symptomatic neonates and
performing PCT at 12 hours, a nearly 60% reduction in unnecessary
antibiotics use could be achieved, and which was superior to either by
using STOPS or using PCT (Table 6).
For diagnosing neonatal sepsis presence of clinical symptoms and signs are essential.[14]
Only considering lab results may be erroneous as they may be incomplete
(blood culture-negative sepsis) and misleading (positive CRP or
hematological indices without clinical correlate).[17]
We used the clinical parameters' STOPS', which were predefined, easy to
learn, and interpret, with good inter-observer reliability. Most
clinical studies evaluating sepsis have used similar clinical
parameters for early sepsis diagnosis.[20-23] Ohlin
et al. to evaluate the clinical signs most predictive of sepsis in
neonates in the NICU, used, amongst others, altered sensorium,
perfusion disturbance, abnormal skin color, and increasing oxygen
requirement, which are similar to our 'STOPS' parameters. It showed a
statistically significant association of perfusion disturbance and
abnormal skin color in identifying neonatal sepsis, similar to our
observation. Increasing oxygen requirement was not predictive, which is
also similar to our observation.[23] Prediction tools for EONS as calculators have been studied by many authors and are the subject of a systematic review.[24]
The review concluded that the neonatal EOS calculator is associated
with a substantial reduction in empirical antibiotics for suspected
EOS. However, there are several limitations to this approach in our
setting. Firstly, all the included studies were from developed
countries where the rate of EONS is lower compared to the developing
countries. Secondly, it does not allow application in neonates below 34
weeks. In our study, we included neonates from 28 weeks gestation
upwards. Maternal group-B streptococcus (GBS) status and GBS specific
intrapartum antibiotics used for risk prediction in the calculator are
not relevant in our setting. The calculator allows the inclusion of
clinical findings, including parameters related to sensorium,
perfusion, and oxygenation similar to our 'STOPS'.
As regards
LONS, our study found no sepsis in 30/50 (60%) neonates. All 'STOPS'
parameters had specificity between 90-100% and PPV of 70%, with
abnormal skin color having a valuable predictive ability (Table 5).
Serum PCT alone had high specificity of 93% and was found to be
moderately useful based on the PLR. Combining 'STOPS' and PCT did not
further improve the diagnostic ability to exclude infection with surety
and stop antibiotics (Table 6).
Several studies have evaluated clinical and laboratory parameters in LONS prediction models.[21,25,26] Arayici et al. introduced base excess for early diagnosis of neonatal sepsis in preterm newborns.[27] A retrospective study by Tollner[21]
evaluated clinical and hematological parameters to create a scoring
system. The study identified skin color changes, prolonged capillary
refill time, and hypotonia (indicating altered sensorium) to be most
predictive in the early part and later at the illness's peak. Another
study by Singh et al. evaluating clinical signs in LONS for their
predictive value included lethargy, temperature disturbances, and
central cyanosis. The study found hyperthermia to have a specificity of
90% for definite or probable sepsis. The PPV for the 12 reported
clinical signs was between 50 and 60%, except for grunting (75%) and
intercostal retractions (100%). However, the study did not look at skin
color changes, which in our study had the highest PLR.[25]
A prospective case-control study describing a predictive model for LONS
found clinical parameters of lethargy, poor feeding, temperature
disturbance, abnormal heart rate, respiratory insufficiency, and
hypoxemia to have high predictive ability. The adjusted odds ratio (OR)
identified poor feeding and temperature disturbance to be the most
useful.[26] Unlike earlier studies, we have included
blood glucose estimation as it is routinely monitored in sick neonates,
and both hyperglycemia and hypoglycemia are encountered in neonatal
sepsis.[28,29]
Our study's strength is the easy
interpretation of the 'STOPS' parameter, high inter-observer agreement,
and optimum testing for ruling out sepsis by hematological indices,
quantitative CRP, and blood culture. We have in our study proposed
treatment strategies that allow early discontinuation of antibiotics.
The most optimum strategy in EONS would be to treat neonates with one
abnormal 'STOPS' parameter at 6 hours and perform PCT at 12 hours in
those with normal STOPS at 6 hours. In LONS, the most optimum strategy
would be to treat all neonates with two or more abnormal 'STOPS'
parameters at 6 hours or a positive PCT test using a cut-off of > 2
ng/ml done at 12 hours. Being a single-center experience is a
limitation of this study. Also, in temperature and skin color
assessment at 12 hours in neonates with EONS, the high specificity and
PPV of these parameters coupled with small-sample bias may have led to
the high PLR estimate.
Conclusions
Our
study has evaluated the diagnostic performance of a clinical tool,
'STOPS', for identifying neonates with sepsis for early discontinuation
of antibiotics in those with no sepsis. Our study has shown that
abnormal 'STOPS' parameters are superior to PCT alone in EONS and as
good as PCT in LONS. A simple bedside clinical tool incorporated in the
NICU monitoring charts allows early identification of neonatal sepsis
and optimizes antibiotic use.
Acknowledgments
The
authors gratefully acknowledge parents of the enrolled neonates,
nursing staff, and neonatal fellows for their wholehearted
participation in the study.
References
- Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER,
Lavelle J, Chilutti M, Grundmeier R, Nadkarni VM, Thomas NJ. Delayed
antimicrobial therapy increases mortality and organ dysfunction
duration in pediatric sepsis. Crit Care Med 2014; 42:2409-17. https://doi.org/10.1097/CCM.0000000000000509 PMid:25148597 PMCid:PMC4213742
- Schmatz
M, Srinivasan L, Grundmeier RW, Elci OU, Weiss SL, Masino AJ, Tremoglie
M, Ostapenko S, Harris MC. Surviving sepsis in a referral neonatal
intensive care unit: association between time to antibiotic
administration and in-hospital outcomes. J Pediatr 2020;217:59-65.e1. https://doi.org/10.1016/j.jpeds.2019.08.023 PMid:31604632
- The
Young Infants Clinical Signs Study Group. Clinical signs that predict
severe illness in children under age 2 months: a multicentre study.
Lancet 2008; 371: 135-42. https://doi.org/10.1016/S0140-6736(08)60106-3
- Cotten
CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, Ambalavanan
N, Benjamin DK Jr; NICHD Neonatal Research Network. Prolonged duration
of initial empirical antibiotic treatment is associated with increased
rates of necrotizing enterocolitis and death for extremely low birth
weight infants. Pediatrics 2009; 123:58-66. https://doi.org/10.1542/peds.2007-3423 PMid:19117861 PMCid:PMC2760222
- Schulfer A, Blaser MJ. Risks of antibiotic exposures early in life on the developing microbiome. PLoS Pathog 2015; 11:e1004903. https://doi.org/10.1371/journal.ppat.1004903 PMid:26135581 PMCid:PMC4489621
- Connell
TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative
blood culture result? Volume of blood submitted for culture in routine
practice in a children's hospital. Pediatrics 2007; 119:891-6. https://doi.org/10.1542/peds.2006-0440 PMid:17473088
- Buttery
JP. Blood cultures in newborn and children: Optimizing an everyday
test. Arch Dis Child Fetal Neonatal Ed 2002; 87:F25-F28. https://doi.org/10.1136/fn.87.1.F25 PMid:12091285 PMCid:PMC1721431
- Yu
Z, Liu J, Sun Q, Qiu Y, Han S, Guo X. The accuracy of the procalcitonin
test for the diagnosis of neonatal sepsis: A Meta-analysis. Scand
Infect Dis J 2010; 42:723-733. https://doi.org/10.3109/00365548.2010.489906 PMid:20840003
- Stocker
M, van Herk W, El Helou S, Dutta S, Fontana MS, Schuerman FABA, van den
Tooren-de Groot RK, Wieringa JW, Janota J, van der Meer-Kappelle LH,
Moonen R, Sie SD, de Vries E, Donker AE, Zimmerman U, Schlapbach LJ, de
Mol AC, Hoffman-Haringsma A, Roy M, Tomaske M, Kornelisse RF, van
Gijsel J, Visser EG, Willemsen SP, van Rossum AMC; NeoPInS Study Group.
Procalcitonin-guided decision making for duration of antibiotic therapy
in neonates with suspected early-onset sepsis: a multicentre randomised
controlled trial (NeoPIns). Lancet 2017; 390:871-81. https://doi.org/10.1016/S0140-6736(17)31444-7
- The
WHO Young Infants Study Group. Clinical prediction of serious bacterial
infections in young infants in developing countries. Pediatr Infect Dis
J 1999; 18 (10 suppl): S23-31. https://doi.org/10.1097/00006454-199910001-00005 PMid:10530570
- Puopolo
KM, Benitz WE, Zaoutis TE; Committee on fetus and newborn; committee on
infectious diseases. Management of Neonates Born at ≤34 6/7 Weeks'
Gestation With Suspected or Proven Early-Onset Bacterial Sepsis.
Pediatrics 2018;142: e20182896. https://doi.org/10.1542/peds.2018-2896 PMid:30455344
- Verstraete
EH, Blot K, Mahieu L, Vogelaers D, Blot S. Prediction models for
neonatal health care-associated sepsis: a meta-analysis. Pediatrics
2015;135: e1002-14. https://doi.org/10.1542/peds.2014-3226 PMid:25755236
- Lopez
Sastre JB, Solis DP, Serradilla VR, Colomer BF, Cotallo GD; Grupo de
Hospitales Castrillo. Evaluation of procalcitonin for diagnosis of
vertical transmission. BMC Pediatrics 2007; 7:9. https://doi.org/10.1186/1471-2431-7-9 PMid:17324267 PMCid:PMC1828911
- Rossi
P, Botgross R (eds). Report on the expert meeting on neonatal and
pediatric sepsis in the Pediatric committee of the European Medicines
Agency. EMA London.2010. EMA/477725/2010.
- McGovern
M, Giannoni E, Kuester H, Turner MA, van den Hoogen A, Bliss JM, Koenig
JM, Keij FM, Mazela J, Finnegan R, Degtyareva M, Simons SHP, de Boode
WP, Strunk T, Reiss IKM, Wynn JL, Molloy EJ; Infection, Inflammation,
Immunology and Immunisation (I4) section of the ESPR. Challenges in
developing a consensus definition of neonatal sepsis. Pediatr Res
2020;88:14-26. https://doi.org/10.1038/s41390-020-0785-x PMid:32126571
- Tuzun
F, Ozkan H, Cetinkaya M, Yucesoy E, Kurum O, Cebeci B, Cakmak E,
Ozkutuk A, Keskinoglu P, Baysal B, Kumral A, Duman Net al. Is European
Medicines Agency (EMA) sepsis criteria accurate for neonatal sepsis
diagnosis or do we need new criteria? PLoS One 2019;14:e0218002. https://doi.org/10.1371/journal.pone.0218002 PMid:31170237 PMCid:PMC6553766
- National
Collaborating Centre for Women's and Children's Health (UK).
Antibiotics for Early-Onset Neonatal Infection: Antibiotics for the
Prevention and Treatment of Early-Onset Neonatal Infection. London:
RCOG Press; 2012.
- Tewari VV, Jain N.
Monotherapy with Amikacin or Piperacillin-Tazobactum Empirically in
Neonates at Risk for Early-onset Sepsis: A Randomized Controlled Trial.
J Trop Pediatr 2014; 60:297-302 https://doi.org/10.1093/tropej/fmu017 PMid:24699298
- Carley
S, Dosman S, Jones SR, Harrison M. Simple nomogram to calculate sample
size in diagnostic studies. Emerg Med J 2005; 22:180-81. https://doi.org/10.1136/emj.2003.011148 PMid:15735264 PMCid:PMC1726700
- Modi
N, Dore CJ, Saraswatula A, Richards M, Bamford KB, Coello R, Holmes A.
A case definition for national and international neonatal blood stream
infection surveillance. Arch Dis Fetal Neonatal Ed 2009; 94:F8-F12. https://doi.org/10.1136/adc.2007.126458 PMid:18499771
- Tollner U. Early diagnosis of septicemia in the newborn, Clinical studies and sepsis score. Eur J Pediatr 1982; 138:331-37. https://doi.org/10.1007/BF00442511 PMid:7128642
- Bang
AT, Bang RA, Reddy MH, Baitule SB, Deshmukh MD, Paul VK, de C Marshal
TF. Simple clinical criteria to identify sepsis or pneumonia in
neonates in the community needing treatment or referral. Pediatr Infect
Dis J 2005; 24:335-41. https://doi.org/10.1097/01.inf.0000157094.43609.17 PMid:15818294
- Ohlin
A, Bjorkqvist M, Montgomery SM, Schollin J. Clinical signs and CRP
values associated with blood culture results in neonates evaluated for
suspected sepsis. Acta Pediatrica 2010; 99:1635-40. https://doi.org/10.1111/j.1651-2227.2010.01913.x PMid:20560896
- Achten
NB, Klingenberg C, Benitz WE, Stocker M, Schlapbach LJ, Giannoni E,
Bokelaar R, Driessen GJA, Brodin P, Uthaya S, van Rossum AMC, Plötz FB.
Association of Use of the Neonatal Early-Onset Sepsis Calculator With
Reduction in Antibiotic Therapy and Safety: A Systematic Review and
Meta-analysis. JAMA Pediatr 2019. https://doi.org/10.1001/jamapediatrics.2019.2825 PMid:31479103 PMCid:PMC6724419
- Singh
SA, Dutta S, Narang A. Predictive Clinical Scores for Diagnosis of Late
Onset Neonatal Septicemia. J Trop Pediatr 2003; 49:235-39. https://doi.org/10.1093/tropej/49.4.235 PMid:12929886
- Husada
D, Chanthavanich P, Chotigeat U, Sunttarattiwong P, Sirivichayakul C,
Pengsaa K, Chokejindachai W, Kaewkungwal J. Predictive model for
bacterial late-onset neonatal sepsis in a tertiary care hospital in
Thailand. BMC Infectious Diseases 2020; 20:15. https://doi.org/10.1186/s12879-020-4875-5 PMid:32070296 PMCid:PMC7029566
- Arayici
S., Kadioglu Simsek G., Canpolat F.E., Oncel M.Y., Uraş N., Oguz S.S.
Can base excess be used for prediction to early diagnosis of neonatal
sepsis in preterm newborns? Mediterr J Hematol Infect Dis 2019, 11(1):
e2019014, https://doi.org/10.4084/mjhid.2019.014 PMid:30858952 PMCid:PMC6402550
- Beardsall
K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van
Weissenbruch M, Midgley P, Thompson M, Thio M, Cornette L, Ossuetta I,
Iglesias I, Theyskens C, de Jong M, Ahluwalia JS, de Zegher F, Dunger
DB. Early Insulin Therapy in Very-Low-Birth-Weight Infants. New Engl J
Med 2008; 359:1873-84. https://doi.org/10.1056/NEJMoa0803725 PMid:18971490
- Islam
MS, Mia MAH, Akhter KR, Haque M, Malik MA. Glycemic Status and its
Effect in Neonatal Sepsis in a Tertiary Care Hospital. Bangladesh J
Child Health 2016; 40:21-5. https://doi.org/10.3329/bjch.v40i1.31551
[TOP]







