Details about patients, laboratory characteristics of the disease, and therapeutic approaches are summarized in Table 1. We did not observe patients with myeloid sarcoma without bone marrow involvement during the study period.
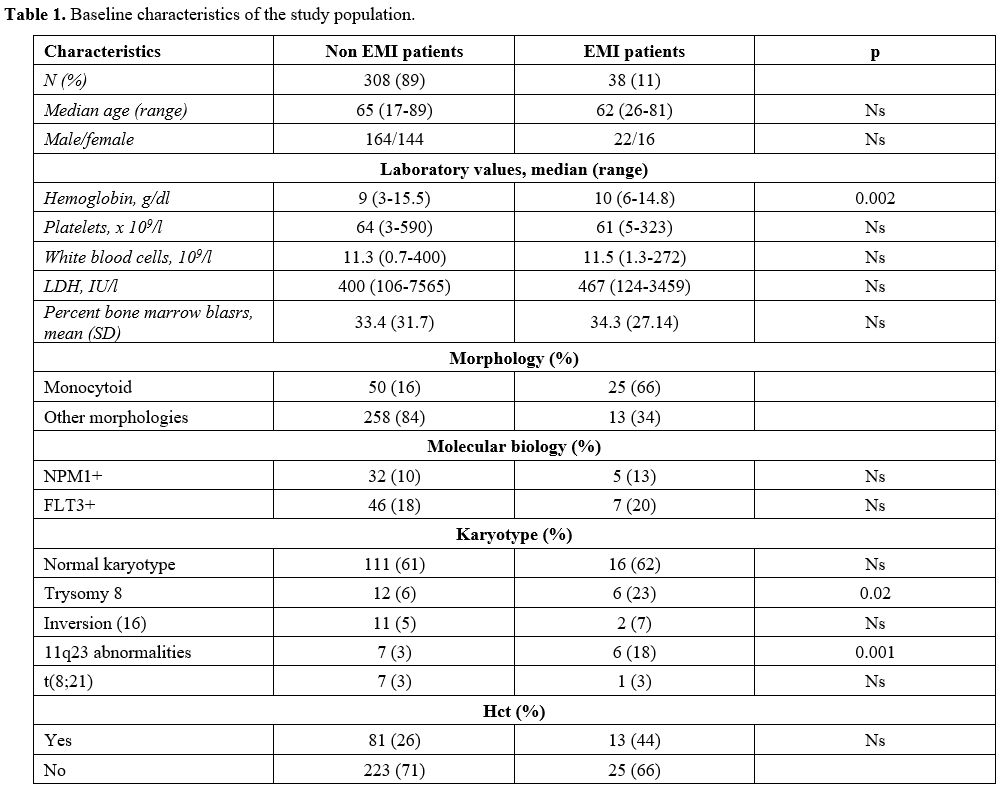 |
Table
1. Baseline characteristics of the study population. |
In the subgroup of patients with EMI, 24/38 were diagnosed with extramedullary involvement at diagnosis (63%), 10/38 had an extramedullary relapse (26%), while 4/38 patients had extramedullary disease both at diagnosis and at relapse (11%). Sites of EMI were: skin (22 patients, 58%), CNS (6 patients, 16%), lymph nodes (1 patient, 3%), pleura (2 patients, 5%), spleen (1 patient, 3%), bone (1 patient, 3%), peritoneum (1 patient, 3%). Four patients had multiple localizations (11%) (skin, CNS, bones, pancreas, breast, pleural). (Figure 1) All patients with CNS involvement presented signs and symptoms related to localization represented by facial nerve paresis in 5 cases and diplopy in 1 case.
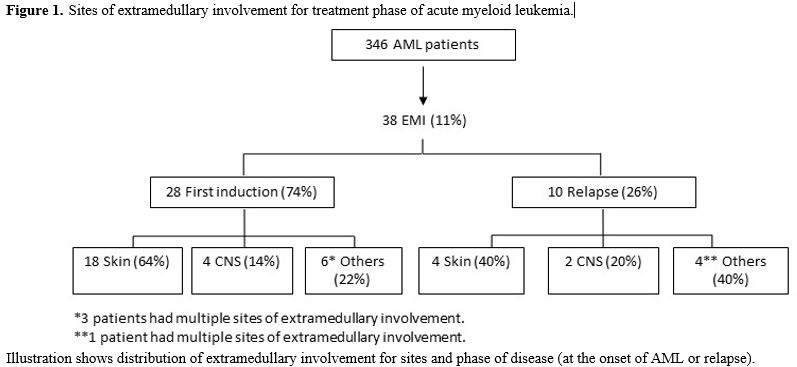 |
Figure 1. Sites of extramedullary involvement for treatment phase of acute myeloid leukemia. |
Median age was 65 years (18-89) for AML patients without and 62 (26-81) for patients with EMI (p-value = ns). The median value of hemoglobin for AML patients without EMI was 9 g/dl (range 3-15.5), while for EMI patients resulted 10 g/dl (range 6-14.8) [p- value 0.002]; the median WBC count was 11x109/L for AML patients without EMI (0.7-400) and 11.5x109/L (1.3-272) for EMI ones (p-value: ns). The mean percent bone marrow blasts were 33% for patients with AML (+/- 31%) and 34% (+/- 27%) for EMI patients (p-value: ns) There were no differences in the average percentage of bone marrow blasts among EMI-negative and EMI-positive patients.
The morphology was monocytoid in 16% of AML patients without EMI (50 patients), and in 66% of EMI patients (25 patients) (p-value = 0.0001).
At flow cytometry analysis, blasts of patients diagnosed with EMI often lacked the expression of CD117: 15/38 patients with EMI (43%) were CD117 negative, versus 37/308 (19%) AML patients without EMI (p-value = 0.0035). 24 AML patients without EMI (12%) vs 9 EMI patients (26%) were CD56 positive (p = 0.061); 28 AML patients (14%) vs 10 (29%) EMI patient were both CD56 negative and CD117 negative (p = 0.046); 9 AML patients (5%) vs 5 (14%) EMI patients were at the same time CD56 positive and CD117 negative (p = 0.42) (Table 2).
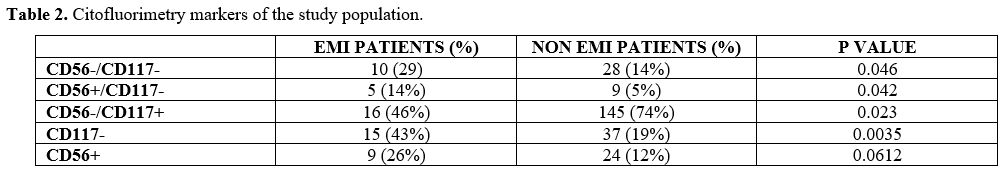 |
Table 2. Citofluorimetry markers of the study population. |
The most frequent cytogenetic anomaly in EMI patients was trisomy 8 (23% of EMI patients vs 6% AML; p = 0.02), while t(8;21) and inv(16) were not significantly associated with EMI in our series (5% vs 7% for inv16; 3% vs 3% for t(8;21)).
NPM1 mutation was not more frequent in AML patients without EMI than EMI ones (10% vs 13%); similarly, 46/308 (18%) AML patients had FLT3 mutation vs 7/38 (20%) EMI patients (p-value: 0.78).
Thirteen patients among 346 AML patients (3.8%) had MLL rearrangements; 6/13 (46%) has been diagnosed with EMI (p = 0.001): 4 had leukemia cutis, one had a cutaneous and meningeal disease, and one had CNS localization only.
Further analysis regarding treatment, OS, and DFS was performed only on the 28 patients who experienced EMI at the onset of their disease. One EMI elderly patient, judged unfit for chemotherapy or hypomethylating agents due to age and comorbidities, received best supportive care only and was consequently excluded from OS analysis. Among the other EMI patients, 21 (55%) were treated with conventional chemotherapy, 5 with hypomethylating agents (13%), and 1 with low doses of cytarabine (3%).
Only eight patients (28.5%) were considered eligible, by age and absence of significant comorbidities, for a consolidation therapy with allogeneic stem cell transplantation (allo-HSCT) from a matched sibling (6 cases) or unrelated donor (2 cases): 5 patients received a myeloablative conditioning regimen, and three patients received a reduced-intensity conditioning regimen (RIC). Among the 27 patients with EMI included in the analysis, only 6 (22%) achieved CR versus an overall response rate of 123/163 AML patients without EMI (75 %) receiving similar therapeutic approaches (p-value < 0.0085).
No differences in DFS between EMI patients (7.4 months) and non-EMI AML patients (14.7 months) (p-value = 0.45) were observed.
The median OS of the 27 EMI patients was 11.6 months (2-79) (Figure 2).
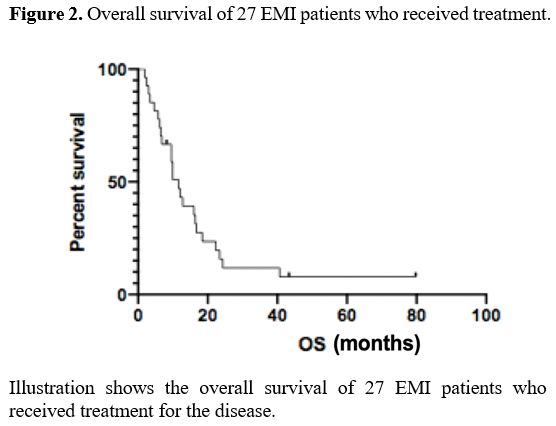 |
Figure 2. Overall survival of 27 EMI patients who received treatment. |
Focusing on OS of AML patients treated with standard chemotherapy, not significant differences emerged between OS of 21 EMI patients (12 months) versus OS of 163 AML patients without EMI treated with a similar approach (15.8 months) (p-value = 0.09).
On the other hand, the OS of EMI patients who undergone allo-HSCT (16.7 months) resulted significantly different from OS of EMI patients who did not receive allo-HSCT (8.2 months) (p-value was 0.02). (Figure 3)
Both univariate and multivariate analyses showed that the achievement of CR and allo-HSCT were the main prognostic factors for survival in the EMI population (p < 0.0001, IC 0.39-0.63; p < 0.0001, IC 0.25-0.48 respectively).
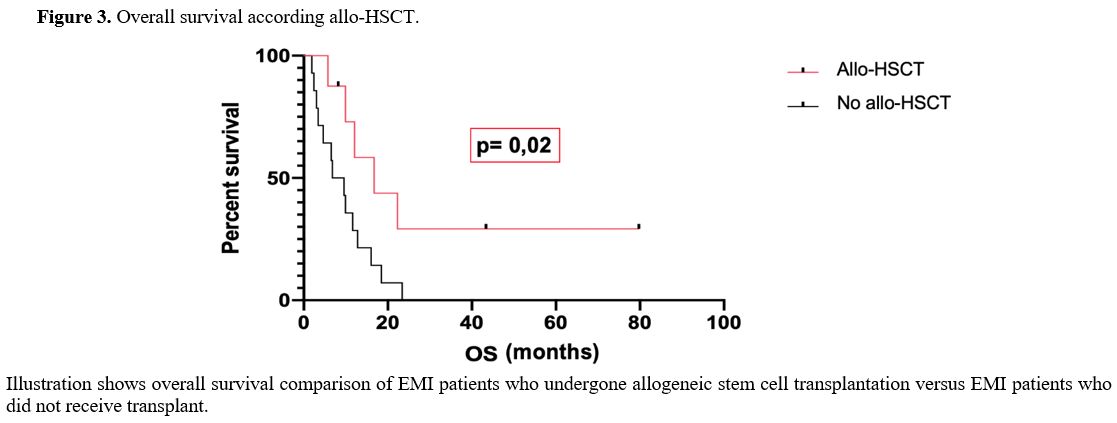 |
Figure 3. Overall survival according allo-HSCT. |