Marcio Nucci.
Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil.
Correspondence to: Marcio
Nucci, M.D. Hospital Universitário Clementino Fraga Filho. Rua Prof.
Rodolpho Paulo Rocco 255 Sala 4A 12 – 21941-913 – Brazil. Tel:
+5521-39382463, E-mail:
mnucci@hucff.ufrj.br
Published: March 1, 2021
Received: December 28, 2020
Accepted: February 13, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021025 DOI
10.4084/MJHID.2021.025
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
management of febrile neutropenia is a backbone of treating patients
with hematologic malignancies and has evolved over the past decades.
This article reviews my approach to the evaluation and treatment of
febrile neutropenic patients. Key topics discussed include
antibacterial and antifungal prophylaxis, the initial workup for fever,
the choice of the empiric antibiotic regimen and its modifications, and
criteria for discontinuation. For each of these questions, I review the
literature and present my perspective.
|
Introduction
The
management of febrile neutropenia is a backbone of the treatment of
patients with hematologic malignancies. Since the introduction of the
concept of empiric antibiotic therapy upon the first fever in
neutropenic patients,[1] the management of febrile
neutropenia has evolved, reflecting changes in the epidemiology of
infection, the development of new diagnostic tools and antimicrobial
agents, and changes in the treatment of the underlying malignancies.
Over these years, guidelines for managing febrile neutropenia have been
published, and have helped hematologists and infectious diseases
clinicians to treat febrile neutropenic patients. These guidelines were
built based on the available literature, experts' opinions, and were
endorsed by regional and national medical societies.[2-13]
However, while these guidelines are of great usefulness, some
recommendations may not apply because of differences in infection
epidemiology in different regions. Therefore, the "blind" application
of international guideline recommendations not taking into account
local epidemiologic aspects may result in inappropriate use of
antimicrobial agents and compromise treatment success.
In this
review, I present my perspective of the management of febrile
neutropenia, based on my experience in a tertiary care
university-affiliated hospital. The purpose of this review is to
provide a practical approach to the management of neutropenic cancer
patients, taking into consideration current recommendations, local
epidemiologic aspects, and the experience in managing this complication
for over 30 years. A summary of my approach to the management of
febrile neutropenia is presented in Table 1.
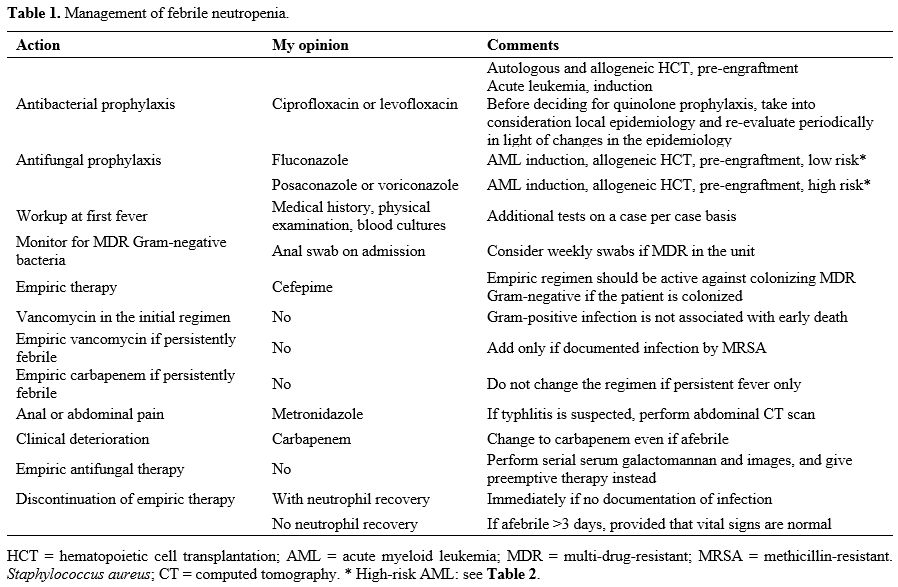 |
Table
1. Management of febrile neutropenia.
|
The "add-on" Strategy
Over
the past decades, significant advances in the management of infections
have occurred, including improvements in culture methods,[14] faster and more accurate identification of microorganisms and patterns of resistance,[15] the incorporation of biomarkers and new diagnostic tools,[16] new antimicrobial agents,[17]
and concepts of pharmacokinetics and pharmacodynamics guiding the
choice of appropriate doses and schedules for the administration of
antimicrobial agents.[18] These advances represent a
great challenge for hematologists because they are already overwhelmed
by the multitude of new information regarding the management of the
underlying hematologic malignancy, including the incorporation of new
molecular markers of disease, risk stratifications, and targeted
therapies. As a consequence, hematologists use the recommendations of
international guidelines to manage their febrile neutropenic patients,
usually taking the help of the "add-on" strategy: a beta-lactam is
started in the first fever, vancomycin is added after a few days of
persistent fever, the beta-lactam is changed after a few days if the
patient is still febrile, and finally, empiric antifungal therapy if
started in case of persistent fever. The add-on strategy is successful
in keeping the patient alive, but with the expense of overusing
antimicrobial agents, with its consequences: side effects, drug
interactions, selection of resistant organisms, and increased cost. In
addition, the overuse of antibiotics reduces the diversity of the
intestinal microbiota, increasing the risk of severe acute graft versus
host disease in allogeneic hematopoietic cell transplant (HCT)
recipients, with a potential increase in mortality.[19,20]
Therefore, the treatments' goal in febrile neutropenia is not just to
keep the patient alive but to do it with the least exposure to
antimicrobial agents possible. To do so, hematologists must abandon the
add-on strategy and develop a strategy that takes into consideration
the underlying disease and its status, recent chemotherapy with an
estimate of the predicted duration of neutropenia, local epidemiologic
features, a bedside risk assessment of infection, daily visits with
special attention to subtle clinical manifestations of infection, and
an aggressive attempt to diagnose infection with the help of a good
microbiology laboratory.
Should I Give Antibacterial Prophylaxis?
The
use of a quinolone (ciprofloxacin or levofloxacin) to afebrile
neutropenic patients has been associated with a reduction in fever and
bacterial infection frequency and a modest impact on mortality, as
shown by randomized trials and meta-analyses.[21-24]
However, with the emergence of infection by Gram-negative bacteria, the
possibility that the use of quinolones might increase resistance rates
has been a concern among experts. Recently the European Conference of
Infection in Leukemia (ECIL) revisited this topic, emphasizing the
impact of quinolone prophylaxis on antibiotic resistance.[25] The authors reviewed 18 studies, including one published by our group.[26]
Except for three observational studies (2 from the same institution),
the literature review failed to show an increase in resistance with the
use of quinolones, including two randomized trials and one
meta-analysis. More recently, alerts about quinolones' side effects
such as mental disturbances, fatal hypoglycemia, aortic dissection and
rupture of aortic aneurysm, disabling side effects on tendons muscles,
joints and nerves, brought new concerns about the use of quinolones (https://www.drugs.com/fda-alerts/672-0.html).
A reflection about the benefits and potential harms of quinolone
prophylaxis should be advanced, taking into consideration local
epidemiology. In addition, those who argue against the use of quinolone
prophylaxis highlight the lack of survival benefit. However, while
bacterial infections may increase febrile neutropenic patients'
mortality, the additional risk is not high enough to be apparent in a
randomized trial or meta-analysis of quinolone prophylaxis.
My opinion.
Unless there is an additional risk for potentially severe side effects,
I give ciprofloxacin 500 mg BID (or levofloxacin 500 mg/d) to
autologous and allogeneic HCT recipients, starting with the
conditioning regimen until engraftment or until the patient develops
fever requiring the initiation of empiric antibiotic therapy. I also
give ciprofloxacin to patients with acute myeloid leukemia (AML)
receiving consolidation chemotherapy with high-dose cytarabine. This is
particularly attractive because most patients are discharged after
chemotherapy and spend the period of neutropenia at home. In such
situations, quinolone prophylaxis may reduce the chance of readmission
to treat febrile neutropenia.
Most AML patients in induction
remission are already febrile on admission. For these patients, I start
empiric antibiotic therapy, even acknowledging that fever is most
likely caused by the underlying leukemia. However, if there is no
documentation of infection and fever resolves with chemotherapy, I
discontinue empiric therapy and start ciprofloxacin. The other
situation in which I consider giving quinolone prophylaxis is in
induction remission for acute lymphoid leukemia (ALL). The more
intensive induction remission I give, the more likely I prescribe
quinolone prophylaxis. Like AML, fever in newly diagnosed ALL patients
may be due to underlying leukemia,[27] and the same
strategy as described for AML applies. It is important to emphasize
that quinolone prophylaxis should be considered according to local
epidemiology and a periodic re-evaluation of its benefit in light of
potential changes in the epidemiology over time.
Should I Give Antifungal Prophylaxis?
The
frequency of invasive fungal disease (IDF) in hematologic patients
increased with improvements in the outcome of patients with acute
leukemia, and the expansion in the population of patients undergoing
HCT. Studies published in the 1980s reported high infection rates
caused by Candida species, and less frequently, Aspergillus and other molds.[28,29]
These epidemiologic features and fluconazole availability prompted
investigators to test this agent as prophylaxis in neutropenic cancer
patients. Compared with placebo, the best results favoring fluconazole
were reported in allogeneic HCT[30,31] and AML.[32]
Furthermore, a meta-analysis showed that a survival benefit was evident
among patients with prolonged neutropenia in addition to a reduction in
the incidence of invasive candidiasis.[33]
With
the widespread use of fluconazole as prophylaxis, the incidence of
invasive candidiasis dropped sharply, and invasive aspergillosis became
the most frequent IFD in neutropenic patients.[34,35] In addition, other filamentous fungi such as Fusarium species and the agents of mucormycosis emerged as important pathogens in neutropenic patients.[36,37]
As a consequence, primary prophylaxis with mold-active agents became an
attractive strategy and has been tested in randomized clinical trials.
The best evidence is for the use of posaconazole or caspofungin in AML.
A study comparing posaconazole with fluconazole or itraconazole oral
solution in adults showed that IFD and mortality incidence was
significantly lower in posaconazole recipients. [38]
In another study conducted in children and young adults, caspofungin
use resulted in a reduction in IFD overall and aspergillosis compared
with fluconazole.[39] In this trial, most children
received a protocol consisting of four cycles of intensive
chemotherapy, and the benefit of caspofungin was only apparent after
the second cycle. Considering that adults with AML are usually treated
with one or two cycles of intensive chemotherapy. Considering that,
adults with AML are usually treated with one or two cycles of intensive
chemotherapy, it is not clear if caspofungin will also benefit adults
with AML receiving induction remission.
A significant benefit of
anti-mold prophylaxis in the pre-engraftment period after allogeneic
HCT has not been observed since two randomized trials comparing
voriconazole with fluconazole or itraconazole failed to show a dramatic
advantage of voriconazole in terms of a reduction in the incidence of
mold infection.[40,41] Likewise, a benefit of micafungin in reducing the incidence of invasive aspergillosis was not demonstrated in three studies.[42-44]
Finally, in ALL, where azoles' use is restricted because of prohibitive
drug interactions with vincristine, a study comparing intravenous
liposomal amphotericin B (5 mg/kg twice weekly) with placebo showed
similar rates of IFD.[45]
The choice of which
antifungal prophylaxis to give in neutropenic patients influences the
strategies of diagnosis and monitoring for IFD during neutropenia.
Patients receiving fluconazole prophylaxis are at increased risk for
invasive aspergillosis. In these patients, active monitoring with
serial (2-3x/week) serum galactomannan should be strongly considered.[46]
On the other hand, if posaconazole is given as prophylaxis, the rates
of false-positive galactomannan increase because the pre-test
probability of invasive aspergillosis is much lower.[47]
In these circumstances, serum galactomannan testing is best performed
upon clinical suspicion of invasive aspergillosis rather serially.[48]
Another
consequence of the choice of antifungal prophylaxis is the selection of
non-prophylactic antifungal agents during neutropenia. If empiric or
preemptive antifungal therapy is considered in patients receiving
fluconazole prophylaxis, the options include an echinocandin,
voriconazole, and an amphotericin B's lipid formulation. However, if
the patient receives posaconazole prophylaxis, the most likely choice
is amphotericin B's lipid formulation.
Recently, new targeted
therapies for the treatment of AML have emerged, including midostaurin,
gilteritinib, enasidenib, ivosidenib, venetoclax, and others, with
significant improvements in the outcome.[49,50] Most of these agents are metabolized by CYP3A4 enzymes, which are strongly inhibited by both posaconazole and voriconazole.[51,52]
Incorporating these new compounds in the treatment of AML will
represent a challenge for the use of mold-active azoles as prophylaxis,
because the overexposure of target therapies may increase toxicity and
underexposure may reduce their efficacy.[53] An
alternative would be isavuconazole, a moderate CYP3A4 inhibitor,
although there are no solid data on its efficacy as prophylaxis.
My opinion.
I give antifungal prophylaxis to patients with AML receiving induction
remission chemotherapy and in the pre-engraftment period of allogeneic
HCT. In AML, my choice between fluconazole and posaconazole is based on
a bedside risk assessment of IFD that takes into account the
probability of achieving complete remission with one cycle of
chemotherapy (older age, high white blood cell count, relapsed AML, and
high cytogenetic and/or molecular risk),[54] co-morbidities and environmental exposure (Table 2).[55] I give posaconazole to patients with high-risk AML and fluconazole to patients with intermediate or low-risk AML.
In
the pre-engraftment period of allogeneic HCT, I use a risk
stratification strategy that takes into account the predicted duration
of neutropenia (stem cell source, conditioning regimen), T-cell
depletion, co-morbidities, and environmental factors (Table 2).
I give voriconazole or posaconazole to high-risk patients and
fluconazole to low or intermediate-risk patients. In patients receiving
any of the new drugs metabolized by CYP3A4, I prefer not to give a mold
active azole (voriconazole or posaconazole) and consider giving an
echinocandin as prophylaxis in patients at high risk for invasive
aspergillosis. I also give echinocandins to high-risk patients who
present increased liver enzymes during azole prophylaxis or who have
severe gastrointestinal mucositis.
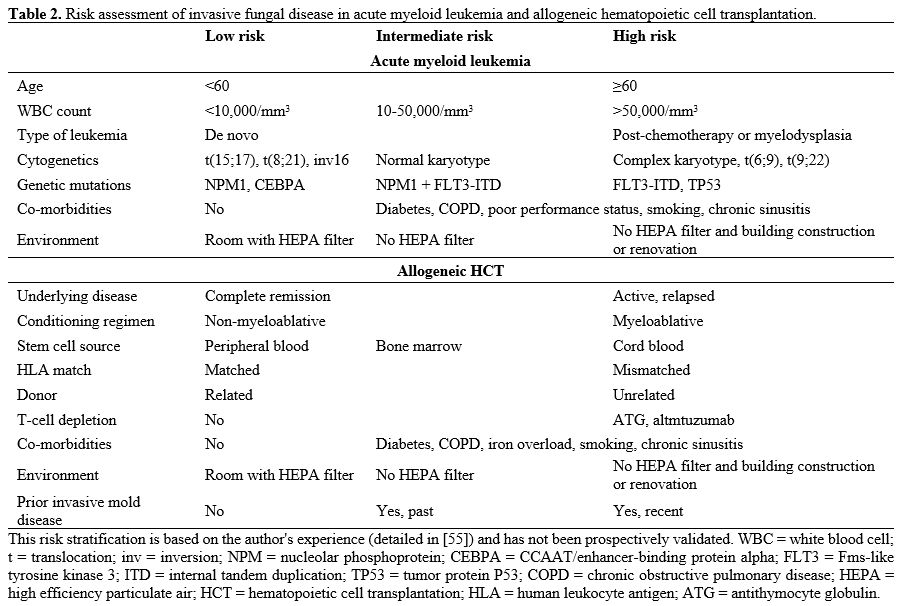 |
Table 2. Risk assessment of invasive fungal disease in acute myeloid leukemia and allogeneic hematopoietic cell transplantation.
|
In
both AML and allogeneic HCT, if the patient is receiving fluconazole
prophylaxis, I monitor for invasive aspergillosis with serial (3x/week)
serum galactomannan. In contrast, for patients receiving posaconazole,
I only perform serum galactomannan (3 consecutive days) if there is any
suspicion of aspergillosis or fusariosis (persistent or recurrent
fever, respiratory symptoms, images, skin lesions).
What is the Workup in the First Fever?
Because
the clinical presentation of infection in febrile neutropenic patients
is subtle, any sign or symptom must be seriously taken into account.[56]
Specifically, pain, fever, and erythema should prompt a thorough workup
for infection. The most common sites of infection are the skin, and the
respiratory and gastrointestinal tract. The workup for the first fever
comprises history, physical examination and blood cultures. The routine
performance of chest X-ray is not indicated.[57] On the other hand, reports of invasive aspergillosis occurring before the start of treatment in AML[58-60]
brought the discussion of obtaining a chest CT scan before induction
chemotherapy. Indeed, a web-based questionnaire, answered by 142
physicians from 43 countries, reported that 24% obtained baseline chest
CT scan routinely.[61]
My opinion. My working definition of fever is any axillary temperature ≥38oC.
Occasionally, the patient presents signs of infection (e.g., abdominal
pain in the context of gastrointestinal mucositis or cellulitis)
without fever. In these situations, I trigger the workup and the
initiation of empiric antibiotic therapy, regardless of body
temperature. My workup starts with a detailed medical history that
includes co-morbidities and prior infections (e.g., chronic lung
disease, sinusitis, diabetes, smoking habit, herpes virus infection,
varicella, tuberculosis), underlying disease and its status, past and
recent treatment for the underlying disease, prior episodes of febrile
neutropenia with information about the documentation of infection and
colonization by resistant organisms, concomitant medications, and
symptoms of infection. On the basis of the status of the underlying
disease and recent treatment (type and date), I estimate the probable
duration of neutropenia and anticipate potential non-infectious
complications that may mimic infection (e.g., engraftment syndrome
after HCT[62] and differentiation syndrome in AML patients receiving retinoic acid, ivosidenib or enasidenib).[63,64] This approach is essential for the correct interpretation of clinical signs of infection throughout neutropenia.
I
perform a physical examination with particular attention to the skin,
nails, and respiratory and digestive tracts. I obtain at least two sets
of blood cultures (aerobic, anaerobic, and fungal bottles), one from a
peripheral vein and another from a catheter. I only order additional
tests such as computed tomography (CT) scans or cultures from other
sites if clinically indicated. These tests include PCR panel for
respiratory viruses and PCR panel for diarrhea in patients with such
symptoms.
What is the Empiric Antibiotic Regimen for the First Fever?
Over
the past decades, various antibiotic regimens have been tested as
empiric therapy for febrile neutropenic patients. In early studies,
combinations of two or three antibiotics were usually given,[1,65]
but since the late 1990s, monotherapy with a beta-lactam has been
preferred, usually cefepime, piperacillin-tazobactam, or a carbapenem.[66,67]
The addition of vancomycin is not recommended routinely since a
meta-analysis of randomized trials comparing regimens with or without
vancomycin did not show any advantage of vancomycin in the initial
empiric regimen.[68,69] However, the use of
vancomycin in the initial empiric antibiotic regimen is recommended by
guidelines in certain circumstances such as suspected catheter-related
infection, skin and soft tissue infection, pneumonia, or hemodynamic
instability.[10,12] However, the level of evidence is weak, reflecting the lack of clinical data supporting these recommendations.
The
main objective of empiric antibiotic therapy in febrile neutropenic
patients is to prevent early death, an event that occurs mostly with
Gram-negative bacteremia.[70] We have recently analyzed 1,305 febrile neutropenia episodes looking at factors associated with early death and shock.[71]
None of the circumstances in which guidelines recommend the use of
vancomycin was associated with shock or early death, including
bacteremia due to Gram-positive organisms, catheter-related infection,
skin or soft tissue infection, or inadequate Gram-positive coverage,
suggesting that the empiric use of vancomycin in the first fever in
neutropenic patients is likely unnecessary in the overwhelming majority
of cases. Another study evaluated the impact of inappropriate
antibiotic coverage at first fever in 1,605 episodes of bloodstream
infections in neutropenic patients. While the mortality rate was
significantly higher in episodes of Gram-negative bacteremia with
inappropriate antibiotic coverage, there was no different in mortality
in Gram-positive bacteremia.[72] In other study, the
implementation of a rapid microbial identification via MALDI-TOF
(matrix-assisted laser desorption ionization time of flight) reduced
mortality in bacteremia caused by Gram-negative but not Gram-positive
bacteria, further indicating that Gram-positive infections do not
result in early death in febrile neutropenic patients.[73]
The
empiric antibiotic regimen must cover the most frequent Gram-negative
bacteria causing bloodstream infection in febrile neutropenic patients,
taking into account local epidemiology. The emergence of infection
caused by multi-drug resistant (MDR) Gram-negative bacteria has brought
a great challenge for the management of febrile neutropenic patients
because they are associated with high mortality rates.[74]
Strategies to overcome this problem include active screening with
weekly (or on admission) rectal swabs and the initiation of an empiric
antibiotic regimen active against the colonizing MDR Gram-negative
bacteria.[75,76] In addition, a de-escalation strategy is applied if the patient is stable and blood cultures are negative.[12]
A study tested the time to positive blood cultures to guide early
de-escalation and found that the median time to positivity of MDR
Gram-negative bacteria was 10.5 hours, and 100% of cultures turned
positive in less than 24 hours.[77]
My opinion.
All new patients admitted to my unit are put in contact precautions and
have an anal swab performed. I strongly consider repeating the swab
weekly if another patient in the unit is colonized by MDR Gram-negative
bacteria. Suppose the patient is colonized by MDR Gram-negative
bacteria, or had a documented infection caused by MDR Gram-negative
bacteria in a previous febrile neutropenia episode. In that case, I
choose an antibiotic regimen with activity against the colonizing (or
previously infecting) organism. On day 3 of febrile neutropenia, if
blood cultures are negative and the patient is stable, I change the
antibiotic regimen to cefepime, even is the patient is still febrile (Figure 1).
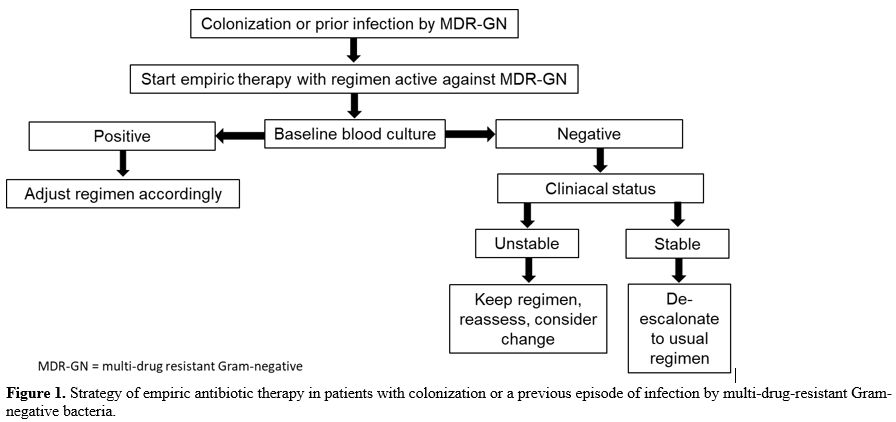 |
Figure 1. Strategy of
empiric antibiotic therapy in patients with colonization or a previous
episode of infection by multi-drug-resistant Gram-negative bacteria.
|
For
patients without colonization by MDG Gram-negative bacteria, I give
cefepime in extended infusion (3-4 hours), with the dose and schedule
adjusted for the creatinine clearance. If the patient presents signs of
typhlitis, I add metronidazole to cefepime. I do not give vancomycin or
any other anti-Gram positive antibiotic such as teicoplanin,
daptomycin, or linezolid. Instead, I wait for blood culture results and
add vancomycin if the patient presents with bacteremia due to
methicillin-resistant Staphylococcus aureus (MRSA).
When Should I Change the Empiric Antibiotic Regimen?
Persistent
fever after the start of empiric antibiotic therapy is frequent and may
have various causes, not necessarily indicating the need to change the
antibiotic regimen. In general, it is recommended that clinical and
microbiologic data should guide modifications, and persistent fever in
a stable patient rarely requires changes in the empiric regimen.[10] However, in practice, empiric changes in the initial regimen are very frequent, in general, without a reasonable reason. The
time to defervescence of a febrile neutropenic patient may vary
depending on the presence or absence of infection. For example, in our
febrile neutropenia database with over 2,500 episodes, the median time
to defervescence was three days in episodes without documented
infection and four days in those with clinical or microbiological
documentation. Among patients with bacteremia, the median time to
defervescence was four days in Gram-negative bacteremia and five days
in Gram-positive bacteremia (unpublished data). A randomized study
comparing cefepime with ceftazidime plus amikacin has shown that the
median time to defervescence of "responding" patients was three days.
However, less than 30% of patients were afebrile after three days of
antibiotics.[78] In another study comparing cefepime
with or without amikacin in febrile neutropenic patients, the
proportion of patients who became afebrile after 3, 7 and 10 days of
antibiotics was 39%, 70% and 83%, respectively.[79]
Taken these data, it is clear that the strategy of empiric change in
the antibiotic regimen after 3-4 days of a patient with persistent
fever and no new signs of infection is inappropriate and will likely
result in the overuse of antibiotics without improving the outcome. One
of the most common actions of clinicians treating febrile neutropenia
is to add an anti-Gram-positive antibiotic (usually vancomycin) in
persistently febrile patients. A study randomized 165 neutropenic
patients with a persistent fever after 2-3 days of
piperacillin-tazobactam to receive vancomycin or placebo. No
differences between the two groups were observed in time to
defervescence, the proportion of afebrile patients in different time
points, Gram-positive infections, or mortality.[80]Another
situation in which clinicians add vancomycin empirically is when there
are signs of a skin infection, such as cellulitis. A useful tool to
help decision making is to check the results of baseline nasal swabs
usually performed on admission to detect MRSA colonization. A study
analyzed the correlation between the results of 484 nasal swabs in 194
patients with AML and subsequent documentation of infection. A negative
MRSA nasal swab had a 99% negative predictive value for subsequent MRSA
infections.[81]Another
frequent empiric change in the antibiotic regimen in persistently
febrile neutropenic patients is to expand Gram-negative coverage,
usually switching from cefepime or piperacillin-tazobactam to
meropenem. Even considering the emergence of MDR bacterial infections
in neutropenic patients, this practice is not recommended for
persistently febrile patients this practice is not recommended for
persistently febrile patients who do not have signs of clinical
deterioration. Instead, a diagnostic workup for infection and other
causes of fever's persistence should be undertaken, including a
thorough physical examination, repeated blood cultures, serum
biomarkers of infection, and images.[12]My opinion.
I do not change the empiric regimen on the basis of just persistent
fever. I perform a careful review of symptoms and physical examination,
obtain additional blood cultures, and check for results of biomarkers
of infection, including serum C-reactive protein and galactomannan. On
the other hand, if there are new signs of infection, I change the
regimen as follows: add metronidazole if there is anal or abdominal
pain, and switch beta-lactam if there is any sign of clinical
deterioration, even if the patient is afebrile. In addition, I check
the results of baseline blood cultures and make appropriate adjustments
to the antibiotic regimen accordingly, including adjusting the dose of
cefepime, taking into consideration the minimal inhibitory
concentration of a Gram-negative bacteria grown in blood cultures. If
the patient presents signs of a skin infection, I only add vancomycin
if the patient is colonized by MRSA. I give linezolid or daptomycin to
patients with documented infection by vancomycin-resistant
Gram-positive bacteria, such as enterococci. I
do not give empiric antifungal therapy for persistently febrile
patients. Instead, I combine serum galactomannan results with images
(chest and sinuses CT scan), and start antifungal therapy in a
preemptive strategy. If a chest CT scan shows images suspicious of
invasive mold disease (macronodules, wedge-shaped images) and serum
galactomannan is negative, I perform bronchoalveolar lavage unless the
patient is hypoxemic. Additional tests that I perform frequently are
abdominal CT scan in patients with clinical manifestations suspicious
of typhlitis, stool tests for Clostridioides difficile in patients with diarrhea, and skin biopsy in any new skin nodular lesion. When Should I Discontinue Antibiotics in Febrile Neutropenia?
In
general, the parameters that guide the duration of antimicrobial
therapy in febrile neutropenia are documentation of infection and
neutrophil recovery. For patients with infection documentation, the
usual recommendation is to define the duration of treatment based on
the infection that was diagnosed, keeping the antibiotic regimen at
least until neutrophil recovery.[10] For patients
with no infection documentation, the recommendation had been to keep
the empiric regimen until neutrophil recovery. This practice was
supported by a study that randomized 33 neutropenic patients who were
afebrile on day 7 of antibiotics to keep (16 patients) or discontinue
(17 patients) the antibiotic regimen. None of the patients who
continued antibiotics until neutrophil recovery became febrile or had
documentation of infection. By contrast, 7 of the 17 patients who
discontinued the antibiotic regimen developed fever, with infection
documentation in 5 patients (2 deaths).[82] However,
more recently, a series of studies have explored the strategy of early
discontinuation of antibiotics in persistently neutropenic afebrile
patients,[83-85] including one randomized controlled
study. In this multicenter trial, patients with an expected duration of
neutropenia >7 days who had no documentation of infection, were
afebrile after three days of empiric antibiotics and had normal vital
signs (blood pressure, heart and respiratory rate, arterial oxygen
saturation, and daily diuresis) were randomized to continue antibiotics
until neutrophil recovery (control arm) or to discontinue the
antibiotic regimen (experimental arm). The number of empiric antibiotic
therapy-free days (primary endpoint) was significantly higher in the
experimental arm, with no differences in the total number of days with
fever or the fever recurrence rates, documentation of infection, or
death.[86]My opinion.
For patients who recover from neutropenia, I promptly discontinue the
antibiotic regimen if there was no documentation of infection,
regardless of the duration of empiric antibiotic treatment. For
patients who recover from neutropenia but had documentation of
infection, I adjust the antibiotic regimen to treat the documented
infection for as long as it is needed (based on the type of infection
that was diagnosed). For patients with are still neutropenic and have a
documented infection, I adjust the regimen to cover the pathogen
recovered in the documented infection but keep the beta-lactam until
neutrophil recovery. If there is no infection documentation, I
discontinue the empiric antibiotic regimen, provided that vital signs
are normal and the patient has no oral or gastrointestinal mucositis.
In some patients at high risk for infection (e.g., expected long
duration of neutropenia), I discontinue the empiric antibiotic regimen
and give a quinolone. In any case, once the empiric antibiotic regimen
is discontinued, I monitor the temperature very closely and reintroduce
empiric antibiotic therapy if fever recurs.
Conclusions
The
management of febrile neutropenia should be individualized, considering
the underlying hematologic disease, prior and recent chemotherapy, with
an estimate of the duration of neutropenia, local epidemiology, and
diagnostic resources in the center, and daily bedside assessment of
infection. Once the patient develops a fever, an antibiotic regimen
that is active against the most likely Gram-negative bacteria should be
promptly initiated and further adjusted based on clinical and
microbiologic parameters.
References
- Schimpff S, Satterlee W, Young VM, Serpick A.
Empiric therapy with carbenicillin and gentamicin for febrile patients
with cancer and granulocytopenia. N Engl J Med.
1971;284:1061-5.PM:4994878 https://doi.org/10.1056/NEJM197105132841904 PMid:4994878
- Hughes
WT, Armstrong D, Bodey GP, Brown AE, Edwards JE, Feld R, Pizzo P,
Rolston KV, Shenep JL, Young LS. 1997 guidelines for the use of
antimicrobial agents in neutropenic patients with unexplained fever.
Infectious Diseases Society of America. Clin Infect Dis.
1997;25:551-73.PM:9314442 https://doi.org/10.1086/513764 PMid:9314442
- Viscoli
C, Castagnola E, Caniggia M, De SL, Garaventa A, Giacchino M, Indolfi
P, Izzi GC, Manzoni P, Rossi MR, Santoro N, Zanazzo GA, Masera G.
Italian guidelines for the management of infectious complications in
pediatric oncology: empirical antimicrobial therapy of febrile
neutropenia. Oncology. 1998;55:489-500.PM:9732231 https://doi.org/10.1159/000011901 PMid:9732231
- NCCN
practice guidelines for fever and neutropenia. National Comprehensive
Cancer Network. Oncology (Williston Park). 1999;13:197-257.PM:10370929
- Wade JC, Rubenstein EB. NCCN: Fever and neutropenia. Cancer Control. 2001;8:16-21.PM:11760554
- Link
H, Bohme A, Cornely OA, Hoffken K, Kellner O, Kern WV, Mahlberg R,
Maschmeyer G, Nowrousian MR, Ostermann H, Ruhnke M, Sezer O, Schiel X,
Wilhelm M, Auner HW. Antimicrobial therapy of unexplained fever in
neutropenic patients--guidelines of the Infectious Diseases Working
Party (AGIHO) of the German Society of Hematology and Oncology (DGHO),
Study Group Interventional Therapy of Unexplained Fever,
Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the
Deutsche Krebsgesellschaft (DKG-German Cancer Society). Ann Hematol.
2003;82 Suppl 2:S105-S117.PM:13680173 https://doi.org/10.1007/s00277-003-0764-4 PMid:13680173
- Hughes
WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo
PA, Rolston KV, Shenep JL, Young LS. 2002 guidelines for the use of
antimicrobial agents in neutropenic patients with cancer. Clin Infect
Dis. 2002;34:730-51.PM:11850858 https://doi.org/10.1086/339215 PMid:11850858
- Guidelines
for the use of antimicrobial agents in patients with febrile
neutropenia in Taiwan. J Microbiol Immunol Infect.
2005;38:455-7.PM:16341349
- de NJ,
Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F. Management of
febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol.
2010;21 Suppl 5:v252-v256.PM:20555092 https://doi.org/10.1093/annonc/mdq196 PMid:20555092
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young JA, Wingard JR. Clinical practice guideline for the
use of antimicrobial agents in neutropenic patients with cancer: 2010
update by the infectious diseases society of america. Clin Infect Dis.
2011;52:e56-e93.PM:21258094 https://doi.org/10.1093/cid/cir073 PMid:21258094
- Flowers
CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, Kuderer NM,
Langston AA, Marr KA, Rolston KV, Ramsey SD. Antimicrobial prophylaxis
and outpatient management of fever and neutropenia in adults treated
for malignancy: American Society of Clinical Oncology clinical practice
guideline. J Clin Oncol. 2013;31:794-810.PM:23319691 https://doi.org/10.1200/JCO.2012.45.8661 PMid:23319691
- Averbuch
D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, Gyssens
IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M. European
guidelines for empirical antibacterial therapy for febrile neutropenic
patients in the era of growing resistance: summary of the 2011 4th
European Conference on Infections in Leukemia. Haematologica.
2013;98:1826-35.PM:24323983 https://doi.org/10.3324/haematol.2013.091025 PMid:24323983 PMCid:PMC3856957
- Lehrnbecher
T, Robinson P, Fisher B, Alexander S, Ammann RA, Beauchemin M, Carlesse
F, Groll AH, Haeusler GM, Santolaya M, Steinbach WJ, Castagnola E,
Davis BL, Dupuis LL, Gaur AH, Tissing WJE, Zaoutis T, Phillips R, Sung
L. Guideline for the Management of Fever and Neutropenia in Children
With Cancer and Hematopoietic Stem-Cell Transplantation Recipients:
2017 Update. J Clin Oncol. 2017;35:2082-94.PM:28459614 https://doi.org/10.1200/JCO.2016.71.7017 PMid:28459614
- Opota
O, Croxatto A, Prod'hom G, Greub G. Blood culture-based diagnosis of
bacteraemia: state of the art. Clin Microbiol Infect.
2015;21:313-22.PM:25753137 https://doi.org/10.1016/j.cmi.2015.01.003 PMid:25753137
- Luethy
PM, Johnson JK. The Use of Matrix-Assisted Laser Desorption/Ionization
Time-of-Flight Mass Spectrometry (MALDI-TOF MS) for the Identification
of Pathogens Causing Sepsis. J Appl Lab Med. 2019;3:675-85.PM:31639735 https://doi.org/10.1373/jalm.2018.027318 PMid:31639735
- Maertens
J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E,
Wilmer A, Verhaegen J, Boogaerts M, Van EJ. Galactomannan and computed
tomography-based preemptive antifungal therapy in neutropenic patients
at high risk for invasive fungal infection: a prospective feasibility
study. Clin Infect Dis. 2005;41:1242-50.PM:16206097 https://doi.org/10.1086/496927 PMid:16206097
- van
DD, Bonomo RA. Ceftazidime/Avibactam and Ceftolozane/Tazobactam:
Second-generation beta-Lactam/beta-Lactamase Inhibitor Combinations.
Clin Infect Dis. 2016;63:234-41.PM:27098166 https://doi.org/10.1093/cid/ciw243 PMid:27098166 PMCid:PMC4928383
- Goulenok
T, Fantin B. Antimicrobial treatment of febrile neutropenia:
pharmacokinetic-pharmacodynamic considerations. Clin Pharmacokinet.
2013;52:869-83.PM:23807657 https://doi.org/10.1007/s40262-013-0086-1 PMid:23807657
- Golob
JL, Pergam SA, Srinivasan S, Fiedler TL, Liu C, Garcia K, Mielcarek M,
Ko D, Aker S, Marquis S, Loeffelholz T, Plantinga A, Wu MC, Celustka K,
Morrison A, Woodfield M, Fredricks DN. Stool Microbiota at Neutrophil
Recovery Is Predictive for Severe Acute Graft vs Host Disease After
Hematopoietic Cell Transplantation. Clin Infect Dis.
2017;65:1984-91.PM:29020185 https://doi.org/10.1093/cid/cix699 PMid:29020185 PMCid:PMC5850019
- Holler
E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, Zhu W,
Sporrer D, Hehlgans T, Kreutz M, Holler B, Wolff D, Edinger M,
Andreesen R, Levine JE, Ferrara JL, Gessner A, Spang R, Oefner PJ.
Metagenomic analysis of the stool microbiome in patients receiving
allogeneic stem cell transplantation: loss of diversity is associated
with use of systemic antibiotics and more pronounced in
gastrointestinal graft-versus-host disease. Biol Blood Marrow
Transplant. 2014;20:640-5.PM:24492144 https://doi.org/10.1016/j.bbmt.2014.01.030 PMid:24492144 PMCid:PMC4973578
- Bucaneve
G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G,
Allione B, D'Antonio D, Buelli M, Nosari AM, Cilloni D, Zuffa E,
Cantaffa R, Specchia G, Amadori S, Fabbiano F, Deliliers GL, Lauria F,
Foa R, Del FA. Levofloxacin to prevent bacterial infection in patients
with cancer and neutropenia. N Engl J Med. 2005;353:977-87.PM:16148283 https://doi.org/10.1056/NEJMoa044097 PMid:16148283
- Cullen
M, Steven N, Billingham L, Gaunt C, Hastings M, Simmonds P, Stuart N,
Rea D, Bower M, Fernando I, Huddart R, Gollins S, Stanley A.
Antibacterial prophylaxis after chemotherapy for solid tumors and
lymphomas. N Engl J Med. 2005;353:988-98.PM:16148284 https://doi.org/10.1056/NEJMoa050078 PMid:16148284
- Gafter-Gvili
A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis
reduces mortality in neutropenic patients. Ann Intern Med.
2005;142:979-95.PM:15968013 https://doi.org/10.7326/0003-4819-142-12_Part_1-200506210-00008 PMid:15968013
- Leibovici
L, Paul M, Cullen M, Bucaneve G, Gafter-Gvili A, Fraser A, Kern WV.
Antibiotic prophylaxis in neutropenic patients: new evidence, practical
decisions. Cancer. 2006;107:1743-51.PM:16977651 https://doi.org/10.1002/cncr.22205 PMid:16977651
- Mikulska
M, Averbuch D, Tissot F, Cordonnier C, Akova M, Calandra T, Ceppi M,
Bruzzi P, Viscoli C. Fluoroquinolone prophylaxis in haematological
cancer patients with neutropenia: ECIL critical appraisal of previous
guidelines. J Infect. 2018;76:20-37.PM:29079323 https://doi.org/10.1016/j.jinf.2017.10.009 PMid:29079323
- Garnica
M, Nouer SA, Pellegrino FL, Moreira BM, Maiolino A, Nucci M.
Ciprofloxacin prophylaxis in high risk neutropenic patients: effects on
outcomes, antimicrobial therapy and resistance. BMC Infect Dis.
2013;13:356.PM:23899356 https://doi.org/10.1186/1471-2334-13-356 PMid:23899356 PMCid:PMC3729823
- Khurana
M, Lee B, Feusner JH. Fever at Diagnosis of Pediatric Acute
Lymphoblastic Leukemia: Are Antibiotics Really Necessary? J Pediatr
Hematol Oncol. 2015;37:498-501.PM:26376233 https://doi.org/10.1097/MPH.0000000000000417 PMid:26376233
- DeGregorio
MW, Lee WM, Linker CA, Jacobs RA, Ries CA. Fungal infections in
patients with acute leukemia. Am J Med. 1982;73:543-8.PM:7124778 https://doi.org/10.1016/0002-9343(82)90334-5
- Bodey
G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, Mall G, Martino
P, Meunier F, Milliken S, . Fungal infections in cancer patients: an
international autopsy survey. Eur J Clin Microbiol Infect Dis.
1992;11:99-109.PM:1396746 https://doi.org/10.1007/BF01967060 PMid:1396746
- Slavin
MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, Meyers
JD, Bowden RA. Efficacy and safety of fluconazole prophylaxis for
fungal infections after marrow transplantation--a prospective,
randomized, double-blind study. J Infect Dis.
1995;171:1545-52.PM:7769290 https://doi.org/10.1093/infdis/171.6.1545 PMid:7769290
- Goodman
JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H,
Shadduck RK, Shea TC, Stiff P, Friedman DJ, . A controlled trial of
fluconazole to prevent fungal infections in patients undergoing bone
marrow transplantation. N Engl J Med. 1992;326:845-51.PM:1542320 https://doi.org/10.1056/NEJM199203263261301 PMid:1542320
- Winston
DJ, Chandrasekar PH, Lazarus HM, Goodman JL, Silber JL, Horowitz H,
Shadduck RK, Rosenfeld CS, Ho WG, Islam MZ, Buell DN. Fluconazole
prophylaxis of fungal infections in patients with acute leukemia.
Results of a randomized placebo-controlled, double-blind, multicenter
trial. Ann Intern Med. 1993;118:495-503.PM:8442620 https://doi.org/10.7326/0003-4819-118-7-199304010-00003 PMid:8442620
- Bow
EJ, Laverdiere M, Lussier N, Rotstein C, Cheang MS, Ioannou S.
Antifungal prophylaxis for severely neutropenic chemotherapy
recipients: a meta analysis of randomized-controlled clinical trials.
Cancer. 2002;94:3230-46.PM:12115356 https://doi.org/10.1002/cncr.10610 PMid:12115356
- Pagano
L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D,
Picardi M, Bonini A, Chierichini A, Fanci R, Caramatti C, Invernizzi R,
Mattei D, Mitra ME, Melillo L, Aversa F, Van Lint MT, Falcucci P,
Valentini CG, Girmenia C, Nosari A. The epidemiology of fungal
infections in patients with hematologic malignancies: the SEIFEM-2004
study. Haematologica. 2006;91:1068-75.PM:16885047
- Pagano
L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, Aloisi T,
Irrera G, Bonini A, Picardi M, Caramatti C, Invernizzi R, Mattei D,
Melillo L, de WC, Reddiconto G, Fianchi L, Valentini CG, Girmenia C,
Leone G, Aversa F. Fungal infections in recipients of hematopoietic
stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza
Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect
Dis. 2007;45:1161-70.PM:17918077 https://doi.org/10.1086/522189 PMid:17918077
- Nucci
M, Garnica M, Gloria AB, Lehugeur DS, Dias VC, Palma LC, Cappellano P,
Fertrin KY, Carlesse F, Simoes B, Bergamasco MD, Cunha CA, Seber A,
Ribeiro MP, Queiroz-Telles F, Lee ML, Chauffaille ML, Silla L, De Souza
CA, Colombo AL. Invasive fungal diseases in haematopoietic cell
transplant recipients and in patients with acute myeloid leukaemia or
myelodysplasia in Brazil. Clin Microbiol Infect.
2013;19:745-51.PM:23009319 https://doi.org/10.1111/1469-0691.12002 PMid:23009319
- Pagano
L, Offidani M, Fianchi L, Nosari A, Candoni A, Piccardi M, Corvatta L,
D'Antonio D, Girmenia C, Martino P, Del FA. Mucormycosis in hematologic
patients. Haematologica. 2004;89:207-14.PM:15003897
- Cornely
OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott
D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R,
Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole
prophylaxis in patients with neutropenia. N Engl J Med.
2007;356:348-59.PM:17251531 https://doi.org/10.1056/NEJMoa061094 PMid:17251531
- Fisher
BT, Zaoutis T, Dvorak CC, Nieder M, Zerr D, Wingard JR, Callahan C,
Villaluna D, Chen L, Dang H, Esbenshade AJ, Alexander S, Wiley JM, Sung
L. Effect of Caspofungin vs Fluconazole Prophylaxis on Invasive Fungal
Disease Among Children and Young Adults With Acute Myeloid Leukemia: A
Randomized Clinical Trial. JAMA. 2019;322:1673-81.PM:31688884 https://doi.org/10.1001/jama.2019.15702 PMid:31688884 PMCid:PMC6865545
- Wingard
JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, Gersten ID,
Mendizabal AM, Leather HL, Confer DL, Maziarz RT, Stadtmauer EA,
Bolanos-Meade J, Brown J, DiPersio JF, Boeckh M, Marr KA. Randomized,
double-blind trial of fluconazole versus voriconazole for prevention of
invasive fungal infection after allogeneic hematopoietic cell
transplantation. Blood. 2010;116:5111-8.PM:20826719 https://doi.org/10.1182/blood-2010-02-268151 PMid:20826719 PMCid:PMC3012532
- Marks
DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel CP, Kantecki M,
Miller PJ, Ribaud P, Schlamm HT, Solano C, Cook G. Voriconazole versus
itraconazole for antifungal prophylaxis following allogeneic
haematopoietic stem-cell transplantation. Br J Haematol.
2011;155:318-27.PM:21880032 https://doi.org/10.1111/j.1365-2141.2011.08838.x PMid:21880032 PMCid:PMC3253339
- Park
S, Kim K, Jang JH, Kim SJ, Kim WS, Chung DR, Kang CI, Peck KR, Jung CW.
Randomized trial of micafungin versus fluconazole as prophylaxis
against invasive fungal infections in hematopoietic stem cell
transplant recipients. J Infect. 2016;73:496-505.PM:27394404 https://doi.org/10.1016/j.jinf.2016.06.011 PMid:27394404
- Huang
X, Chen H, Han M, Zou P, Wu D, Lai Y, Huang H, Chen X, Liu T, Zhu H,
Wang J, Hu J. Multicenter, randomized, open-label study comparing the
efficacy and safety of micafungin versus itraconazole for prophylaxis
of invasive fungal infections in patients undergoing hematopoietic stem
cell transplant. Biol Blood Marrow Transplant.
2012;18:1509-16.PM:22469884 https://doi.org/10.1016/j.bbmt.2012.03.014 PMid:22469884
- van
Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole
DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ. Micafungin
versus fluconazole for prophylaxis against invasive fungal infections
during neutropenia in patients undergoing hematopoietic stem cell
transplantation. Clin Infect Dis. 2004;39:1407-16.PM:15546073 https://doi.org/10.1086/422312 PMid:15546073
- Cornely
OA, Leguay T, Maertens J, Vehreschild MJGT, Anagnostopoulos A,
Castagnola C, Verga L, Rieger C, Kondakci M, Harter G, Duarte RF,
Allione B, Cordonnier C, Heussel CP, Morrissey CO, Agrawal SG, Donnelly
JP, Bresnik M, Hawkins MJ, Garner W, Gokbuget N. Randomized comparison
of liposomal amphotericin B versus placebo to prevent invasive mycoses
in acute lymphoblastic leukaemia. J Antimicrob Chemother.
2017;72:2359-67.PM:28575414 https://doi.org/10.1093/jac/dkx133 PMid:28575414 PMCid:PMC5890735
- Maertens
J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E,
Wilmer A, Verhaegen J, Boogaerts M, Van EJ. Galactomannan and computed
tomography-based preemptive antifungal therapy in neutropenic patients
at high risk for invasive fungal infection: a prospective feasibility
study. Clin Infect Dis. 2005;41:1242-50.PM:16206097 https://doi.org/10.1086/496927 PMid:16206097
- Duarte
RF, Sanchez-Ortega I, Cuesta I, Arnan M, Patino B, Fernandez de SA,
Gudiol C, Ayats J, Cuenca-Estrella M. Serum galactomannan-based early
detection of invasive aspergillosis in hematology patients receiving
effective antimold prophylaxis. Clin Infect Dis.
2014;59:1696-702.PM:25165088 https://doi.org/10.1093/cid/ciu673 PMid:25165088
- Girmenia
C, Micozzi A, Gentile G, Santilli S, Arleo E, Cardarelli L, Capria S,
Minotti C, Cartoni C, Brocchieri S, Guerrisi V, Meloni G, Foa R,
Martino P. Clinically driven diagnostic antifungal approach in
neutropenic patients: a prospective feasibility study. J Clin Oncol.
2010;28:667-74.PM:19841328 https://doi.org/10.1200/JCO.2009.21.8032 PMid:19841328
- Bewersdorf
JP, Stahl M, Zeidan AM. Are we witnessing the start of a therapeutic
revolution in acute myeloid leukemia? Leuk Lymphoma.
2019;60:1354-69.PM:30652518 https://doi.org/10.1080/10428194.2018.1546854 PMid:30652518
- Kucukyurt S, Eskazan AE. New drugs approved for acute myeloid leukemia (AML) in 2018. Br J Clin Pharmacol. 2019.PM:31469910 https://doi.org/10.1111/bcp.14105 PMid:31469910 PMCid:PMC6955409
- Agarwal
SK, DiNardo CD, Potluri J, Dunbar M, Kantarjian HM, Humerickhouse RA,
Wong SL, Menon RM, Konopleva MY, Salem AH. Management of
Venetoclax-Posaconazole Interaction in Acute Myeloid Leukemia Patients:
Evaluation of Dose Adjustments. Clin Ther. 2017;39:359-67.PM:28161120 https://doi.org/10.1016/j.clinthera.2017.01.003 PMid:28161120
- Dutreix
C, Munarini F, Lorenzo S, Roesel J, Wang Y. Investigation into
CYP3A4-mediated drug-drug interactions on midostaurin in healthy
volunteers. Cancer Chemother Pharmacol. 2013;72:1223-34.PM:24085261 https://doi.org/10.1007/s00280-013-2287-6 PMid:24085261 PMCid:PMC3834177
- Agarwal
S, Gopalakrishnan S, Mensing S, Potluri J, Hayslip J, Kirschbrown W,
Friedel A, Menon R, Salem AH. Optimizing venetoclax dose in combination
with low intensive therapies in elderly patients with newly diagnosed
acute myeloid leukemia: An exposure-response analysis. Hematol Oncol.
2019;37:464-73.PM:31251400 https://doi.org/10.1002/hon.2646 PMid:31251400
- Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93:1267-91.PM:30328165 https://doi.org/10.1002/ajh.25214 PMid:30328165
- Nucci
M, Anaissie E. How we treat invasive fungal diseases in patients with
acute leukemia: the importance of an individualized approach. Blood.
2014;124:3858-69.PM:25339358 https://doi.org/10.1182/blood-2014-04-516211 PMid:25339358
- Bodey
GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships
between circulating leukocytes and infection in patients with acute
leukemia. Ann Intern Med. 1966;64:328-40.PM:5216294 https://doi.org/10.7326/0003-4819-64-2-328 PMid:5216294
- Estacio
O, Loh Z, Baker A, Chong G, Grigg A, Churilov L, Hawkes EA. Limited
utility of routine chest X-ray in initial evaluation of neutropenic
fever in patients with haematological diseases undergoing chemotherapy.
Intern Med J. 2018;48:556-60.PM:29227565 https://doi.org/10.1111/imj.13712 PMid:29227565
- Bitterman R, Hardak E, Guralnik L, Paul M, Oren I. Reply to Nucci. Clin Infect Dis. 2020;70:347-9.PM:31075162 https://doi.org/10.1093/cid/ciz382 PMid:31075162
- Bitterman
R, Hardak E, Raines M, Stern A, Zuckerman T, Ofran Y, Lavi N, Guralnik
L, Frisch A, Nudelman O, Paul M, Oren I. Baseline Chest Computed
Tomography for Early Diagnosis of Invasive Pulmonary Aspergillosis in
Hemato-oncological Patients: A Prospective Cohort Study. Clin Infect
Dis. 2019;69:1805-8.PM:30855077 https://doi.org/10.1093/cid/ciz194 PMid:30855077
- Nucci M. Is Early Invasive Pulmonary Aspergillosis Coming of Age? Clin Infect Dis. 2020;70:347.PM:31075161 https://doi.org/10.1093/cid/ciz381 PMid:31075161
- Stemler
J, Bruns C, Mellinghoff SC, Alakel N, Akan H, Ananda-Rajah M, Auberger
J, Bojko P, Chandrasekar PH, Chayakulkeeree M, Cozzi JA, de Kort EA,
Groll AH, Heath CH, Henze L, Hernandez JM, Kanj SS, Khanna N, Koldehoff
M, Lee DG, Mager A, Marchesi F, Martino-Bufarull R, Nucci M, Oksi J,
Pagano L, Phillips B, Prattes J, Pyrpasopoulou A, Rabitsch W, Schalk E,
Schmidt-Hieber M, Sidharthan N, Soler-Palacin P, Stern A, Weinbergerova
B, El ZA, Cornely OA, Koehler P. Baseline Chest Computed Tomography as
Standard of Care in High-Risk Hematology Patients. J Fungi (Basel).
2020;6.PM:32183235 https://doi.org/10.3390/jof6010036 PMid:32183235 PMCid:PMC7151030
- Maiolino
A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M. Engraftment
syndrome following autologous hematopoietic stem cell transplantation:
definition of diagnostic criteria. Bone Marrow Transplant.
2003;31:393-7.PM:12634731 https://doi.org/10.1038/sj.bmt.1703855 PMid:12634731
- Montesinos
P, Bergua JM, Vellenga E, Rayon C, Parody R, De la Serna J, Leon A,
Esteve J, Milone G, Deben G, Rivas C, Gonzalez M, Tormo M,
Diaz-Mediavilla J, Gonzalez JD, Negri S, Amutio E, Brunet S, Lowenberg
B, Sanz MA. Differentiation syndrome in patients with acute
promyelocytic leukemia treated with all-trans retinoic acid and
anthracycline chemotherapy: characteristics, outcome, and prognostic
factors. Blood. 2009;113:775-83.PM:18945964 https://doi.org/10.1182/blood-2008-07-168617 PMid:18945964
- Norsworthy
KJ, Mulkey F, Scott EC, Ward AF, Przepiorka D, Charlab R, Dorff SE,
Deisseroth A, Kazandjian D, Sridhara R, Beaver JA, Farrell AT, de Claro
RA, Pazdur R. Differentiation Syndrome with Ivosidenib and Enasidenib
Treatment in Patients with Relapsed or Refractory IDH-Mutated AML: A
U.S. Food and Drug Administration Systematic Analysis. Clin Cancer Res.
2020;26:4280-8.PM:32393603 https://doi.org/10.1158/1078-0432.CCR-20-0834 PMid:32393603
- Calandra
T, Klastersky J, Gaya H, Glauser MP, Meunier F, Zinner SH. Ceftazidime
combined with a short or long course of amikacin for empirical therapy
of gram-negative bacteremia in cancer patients with granulocytopenia. N
Engl J Med. 1987;317:1692-8.PM:2892130 https://doi.org/10.1056/NEJM198712313172703 PMid:2892130
- Paul
M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K,
Leibovici L. Beta-lactam versus beta-lactam-aminoglycoside combination
therapy in cancer patients with neutropenia. Cochrane Database Syst
Rev. 2013;CD003038.PM:23813455 https://doi.org/10.1002/14651858.CD003038.pub2 PMid:23813455 PMCid:PMC6457814
- Pizzo
PA, Hathorn JW, Hiemenz J, Browne M, Commers J, Cotton D, Gress J,
Longo D, Marshall D, McKnight J, . A randomized trial comparing
ceftazidime alone with combination antibiotic therapy in cancer
patients with fever and neutropenia. N Engl J Med.
1986;315:552-8.PM:3526155 https://doi.org/10.1056/NEJM198608283150905 PMid:3526155
- Paul
M, Borok S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics
against Gram-positive infections for febrile neutropenia: systematic
review and meta-analysis of randomized controlled trials. J Antimicrob
Chemother. 2005;55:436-44.PM:15722392 https://doi.org/10.1093/jac/dki028 PMid:15722392
- Beyar-Katz
O, Dickstein Y, Borok S, Vidal L, Leibovici L, Paul M. Empirical
antibiotics targeting gram-positive bacteria for the treatment of
febrile neutropenic patients with cancer. Cochrane Database Syst Rev.
2017;6:CD003914.PM:28577308 https://doi.org/10.1002/14651858.CD003914.pub4 PMid:28577308 PMCid:PMC6481386
- Gustinetti
G, Mikulska M. Bloodstream infections in neutropenic cancer patients: A
practical update. Virulence. 2016;7:280-97.PM:27002635 https://doi.org/10.1080/21505594.2016.1156821 PMid:27002635 PMCid:PMC4871679
- Guarana
M, Nucci M, Nouer SA. Shock and Early Death in Hematologic Patients
with Febrile Neutropenia. Antimicrob Agents Chemother. 2019.PM:31405857
https://doi.org/10.1128/AAC.01250-19 PMid:31405857 PMCid:PMC6811434
- Martinez-Nadal
G, Puerta-Alcalde P, Gudiol C, Cardozo C, Albasanz-Puig A, Marco F,
Laporte-Amargos J, Moreno-Garcia E, Domingo-Domenech E, Chumbita M,
Martinez JA, Soriano A, Carratala J, Garcia-Vidal C. Inappropriate
Empirical Antibiotic Treatment in High-risk Neutropenic Patients With
Bacteremia in the Era of Multidrug Resistance. Clin Infect Dis.
2020;70:1068-74.PM:31321410 https://doi.org/10.1093/cid/ciz319 PMid:31321410
- Zadka
H, Raykhshtat E, Uralev B, Bishouty N, Weiss-Meilik A, Adler A. The
implementation of rapid microbial identification via MALDI-ToF reduces
mortality in gram-negative but not gram-positive bacteremia. Eur J Clin
Microbiol Infect Dis. 2019;38:2053-9.PM:31359256 https://doi.org/10.1007/s10096-019-03640-w PMid:31359256
- Righi
E, Peri AM, Harris PN, Wailan AM, Liborio M, Lane SW, Paterson DL.
Global prevalence of carbapenem resistance in neutropenic patients and
association with mortality and carbapenem use: systematic review and
meta-analysis. J Antimicrob Chemother. 2017;72:668-77.PM:27999023 https://doi.org/10.1093/jac/dkw459 PMid:27999023
- Forcina
A, Baldan R, Marasco V, Cichero P, Bondanza A, Noviello M, Piemontese
S, Soliman C, Greco R, Lorentino F, Giglio F, Messina C, Carrabba M,
Bernardi M, Peccatori J, Moro M, Biancardi A, Nizzero P, Scarpellini P,
Cirillo DM, Mancini N, Corti C, Clementi M, Ciceri F. Control of
infectious mortality due to carbapenemase-producing Klebsiella
pneumoniae in hematopoietic stem cell transplantation. Bone Marrow
Transplant. 2017;52:114-9.PM:27668762 https://doi.org/10.1038/bmt.2016.234 PMid:27668762
- Nouer
SA, Nucci M, Anaissie E. Tackling antibiotic resistance in febrile
neutropenia: current challenges with and recommendations for managing
infections with resistant Gram-negative organisms. Expert Rev Hematol.
2015;8:647-58.PM:26115679 https://doi.org/10.1586/17474086.2015.1060576 PMid:26115679
- Puerta-Alcalde
P, Cardozo C, Suarez-Lledo M, Rodriguez-Nunez O, Morata L, Feher C,
Marco F, Del RA, Martinez JA, Mensa J, Rovira M, Esteve J, Soriano A,
Garcia-Vidal C. Current time-to-positivity of blood cultures in febrile
neutropenia: a tool to be used in stewardship de-escalation strategies.
Clin Microbiol Infect. 2019;25:447-53.PM:30096417 https://doi.org/10.1016/j.cmi.2018.07.026 PMid:30096417
- Erman
M, Akova M, Akan H, Korten V, Ferhanoglu B, Koksal I, Cetinkaya Y, Uzun
O, Unal S. Comparison of cefepime and ceftazidime in combination with
amikacin in the empirical treatment of high-risk patients with febrile
neutropenia: a prospective, randomized, multicenter study. Scand J
Infect Dis. 2001;33:827-31.PM:11760163 https://doi.org/10.1080/00365540110076679 PMid:11760163
- Tamura
K, Imajo K, Akiyama N, Suzuki K, Urabe A, Ohyashiki K, Tanimoto M,
Masaoka T. Randomized trial of cefepime monotherapy or cefepime in
combination with amikacin as empirical therapy for febrile neutropenia.
Clin Infect Dis. 2004;39 Suppl 1:S15-S24.PM:15250016 https://doi.org/10.1086/383046 PMid:15250016
- Cometta
A, Kern WV, de BR, Paesmans M, Vandenbergh M, Crokaert F, Engelhard D,
Marchetti O, Akan H, Skoutelis A, Korten V, Vandercam M, Gaya H, Padmos
A, Klastersky J, Zinner S, Glauser MP, Calandra T, Viscoli C.
Vancomycin versus placebo for treating persistent fever in patients
with neutropenic cancer receiving piperacillin-tazobactam monotherapy.
Clin Infect Dis. 2003;37:382-9.PM:12884163 https://doi.org/10.1086/376637 PMid:12884163
- Perreault
SK, Binks B, McManus DS, Topal JE. Evaluation of the negative
predictive value of methicillin-resistant Staphylococcus aureus nasal
swab screening in patients with acute myeloid leukemia. Infect Control
Hosp Epidemiol. 2020;1-4.PM:33228818 https://doi.org/10.1017/ice.2020.1299 PMid:33228818
- Pizzo
PA, Robichaud KJ, Gill FA, Witebsky FG, Levine AS, Deisseroth AB,
Glaubiger DL, Maclowry JD, Magrath IT, Poplack DG, Simon RM. Duration
of empiric antibiotic therapy in granulocytopenic patients with cancer.
Am J Med. 1979;67:194-200.PM:380336 https://doi.org/10.1016/0002-9343(79)90390-5
- Le
CL, Talarmin JP, Couturier MA, Ianotto JC, Nicol C, Le CR, Dos SS,
Hutin P, Tande D, Cogulet V, Berthou C, Guillerm G. Early
discontinuation of empirical antibacterial therapy in febrile
neutropenia: the ANTIBIOSTOP study. Infect Dis (Lond).
2018;50:539-49.PM:29451055 https://doi.org/10.1080/23744235.2018.1438649 PMid:29451055
- Van
de Wyngaert Z, Berthon C, Debarri H, Bories C, Bonnet S, Nudel M,
Carpentier B, Legrand C, Barbieux S, Chauvet P, Simonnet A, Willaume A,
Bossard JB, Renaud L, Wattebled KJ, Escure G, Branche N, Arib I,
Titecat M, Quesnel B, Alfandari S. Discontinuation of antimicrobial
therapy in adult neutropenic haematology patients: A prospective
cohort. Int J Antimicrob Agents. 2019;53:781-8.PM:30831232 https://doi.org/10.1016/j.ijantimicag.2019.02.020 PMid:30831232
- Niessen
FA, van Mourik MSM, Bruns AHW, Raijmakers RAP, de Groot MCH, van der
Bruggen T. Early discontinuation of empirical antibiotic treatment in
neutropenic patients with acute myeloid leukaemia and high-risk
myelodysplastic syndrome. Antimicrob Resist Infect Control.
2020;9:74.PM:32460887 https://doi.org/10.1186/s13756-020-00729-2 PMid:32460887 PMCid:PMC7251665
- Aguilar-Guisado
M, Espigado I, Martin-Pena A, Gudiol C, Royo-Cebrecos C, Falantes J,
Vazquez-Lopez L, Montero MI, Rosso-Fernandez C, de la Luz MM, Parody R,
Gonzalez-Campos J, Garzon-Lopez S, Calderon-Cabrera C, Barba P,
Rodriguez N, Rovira M, Montero-Mateos E, Carratala J, Perez-Simon JA,
Cisneros JM. Optimisation of empirical antimicrobial therapy in
patients with haematological malignancies and febrile neutropenia (How
Long study): an open-label, randomised, controlled phase 4 trial.
Lancet Haematol. 2017;4:e573-e583.PM:29153975 https://doi.org/10.1016/S2352-3026(17)30211-9
[TOP]


