Nawfal R Hussein1, Brisik H Rashad1, Lokman A Almizori2, Shawkat Sabri Yousif2, Ahmed Tayar Sadeeq2, Yaseen Rashad Abdulkareem2, Alan Mobarek Mahmood2 and Zhiyan K Salih2.
1 Department of Biomolecular Sciences, College of Medicine, University of Zakho, Kurdistan Region of Iraq.
2 Azadi teaching hospital, Duhok city, Kurdistan Region of Iraq.
Correspondence to: Nawfal
R Hussein. Department of Biomolecular Sciences, College of Medicine,
University of Zakho, Kurdistan Region of Iraq. E-mail:
Nawfal.hussein@yahoo.com
Published: May 1, 2021
Received: February 6, 2021
Accepted: April 04, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021035 DOI
10.4084/MJHID.2021.035
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor.
Novel
coronavirus disease (COVID-19) or SARS-CoV-2 was discovered in December
2019 in Wuhan City, China. The infection became a global pandemic over
few months.[1] Duhok is a big city in the Kurdistan
Region of Iraq with a population of 1.3 million. The city experienced a
large epidemic with one of the highest COVID-19 infection and case
fatality rates in Iraq.[2] The centralized COVID-19
database registration system and the intensity of infection spread in
the city provided an excellent opportunity to evaluate the risk of
reinfection in the city. It is worth mentioning that reinfection may be
possible after recent reports showing recovered patients testing
positive after a symptom-free period.[3-5] This study aimed to evaluate the risk of reinfection in a cohort of 445,660 COVID-19 RT-PCR-tested cases.
Methods
The
database for COVID-19 testing, management, and clinical outcomes at
Duhok city, Kurdistan Region of Iraq, was analyzed. This database
covers all COVID-19 confirmed cases in the city and shows RT-PCR
testing results between March 1, 2020, and January 24, 2021. Besides,
the database contains the number of tests performed, number of
suspected cases, cure rate, case fatality rates, and clinical outcomes.
We analyzed the centralized and standardized national SARS-CoV-2
testing and hospitalization. In addition, data from all COVID-19
centers and clinics were matched and analyzed. All COVID-19 confirmed
cases, with at least one RT-PCR positive result after ≥45 days after
the first positive swab, were considered suspected cases. Previous
studies showed that a cutoff of 45 days could be used as a mark for the
end of prolonged PCR positivity.[6] Suspected cases of
reinfection were classified as showing either strong or weak evidence
of reinfection. Strong evidence of reinfection included positive RT-PCR
cases, the appearance of signs and symptoms, resolution of signs and
symptoms, negative RT-PCR confirming cure (two negative RT-PCR results
on sequential samples taken at least 24 hours apart), the re-appearance
of symptoms, and positive RT-PCR after ≥45 days. Any case that missed
one of these conditions was considered reinfection with weak evidence
and was excluded from further analysis. The risk of reinfection
was calculated by quantifying the proportion of cases with strong
evidence for reinfection out of all confirmed COVID-19 cases. Apart
from the patient with cancer, our patients were immunocompetent, and
they had no disorders that may facilitate reinfection.
Confirmed
cases were defined as patients with laboratory confirmation of
COVID-19. Mild cases were defined as confirmed cases of COVID-19
without evidence of viral pneumonia or hypoxia, whereas moderate cases
were defined as patients with confirmed COVID-19 with radiological
findings of pneumonia, SpO2 ≥ 90% on room air but no signs of severe
pneumonia.[7] Severe cases were defined as patients
with confirmed COVID-19 with radiological findings of pneumonia plus
one of the following: respiratory rate > 30 breaths/min; severe
respiratory distress; or SpO2 < 90% on room air.[7]
RT-PCR testing.
Each RT-PCR testing was composed of two reactions using two different
kits. First, LightMix Modular SARS Wuhan CoV E-gene was used to target
a 76 bp long fragment from a conserved region in the E gene. Second,
LightMix Modular Wuhan CoV RdRP-gene was used to target a 100 bp long
fragment from a conserved region of the RNA-dependent RNA polymerase
(RdRP) gene. If the results of both reactions were positive, then the
test was considered positive. The test was considered negative when the
results of both reactions were negative. If one reaction was positive
and the other is negative, the test result was considered indeterminate.
Results
For
the period between March 1, 2020, and January 24, 2021, the database
showed that the RT-PCR test was performed on 445,660 cases. Among
those, 35272 tests were positive. Amongst RT-PCR-positive cases, 30486
(86.4%; 95% CI: 86.1-86.8%) were cured and 703 (1.99%; 95% CI:
1.84-2.14%) died, while the rest were under medical observation.
Out of 35272 confirmed COVID-19 cases, 29663 (84%; 95% CI: 83.7-84.5%)
had only one positive RT-PCR result and therefore were excluded from
the study. Of the remaining 5609 cases with more than one positive
swab, only 34 (0.6%; 95% CI: 0.42-0.84%) cases had at least one
subsequent positive swab that was ≥45 days from the first positive swab
and thus qualified for inclusion in analysis. Detailed history taking
with individual investigations of the 34 cases yielded 11 cases with
strong evidence for reinfection. This gave reinfection risk rate of
0.031% (95% CI:0.012-0.049%) (Figure 1).
Among our cases, 6/11 (54.54%; 95% CI:23-83%) were males. All patients
were not related, apart from patients 8 and 9, who were twins (Table 1).
The average age was 39.5±14.6 years (range: 26-79), and the average
time between the first swab and the reinfection swab was 79.9±38.3 days
(range: 49-174). The infection in 4/11 (36.36%; 95% CI:10-69%) patients
was more severe than the first round of infection. The infection was
severe in both rounds in one patient.
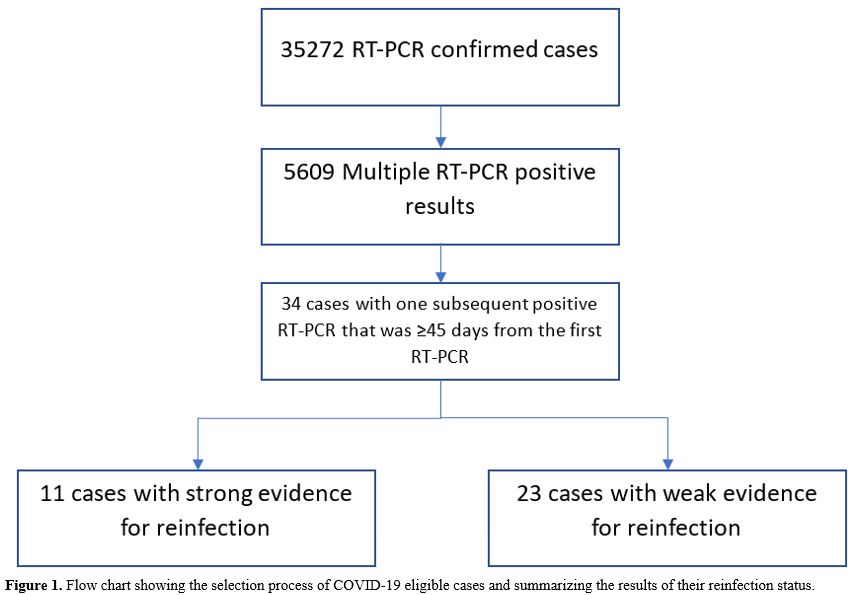 |
Figure 1. Flow chart showing the selection
process of COVID-19 eligible cases and summarizing the results of their
reinfection status. |
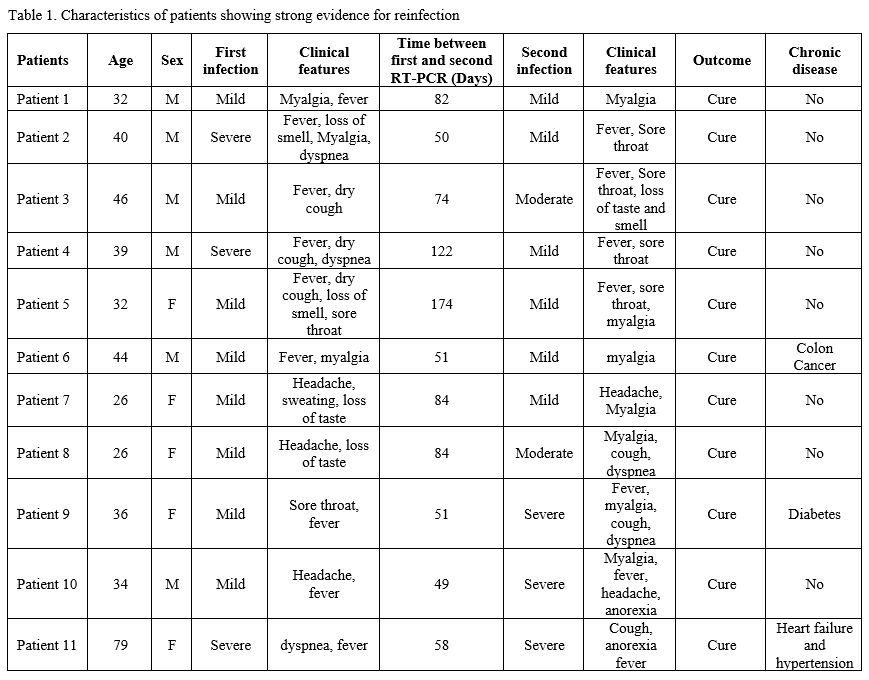 |
Table 1. Characteristics of patients showing strong evidence for reinfection |
Discussion
In
the Duhok City, in the Kurdistan Region of Iraq, strict measures were
put to combat COVID-19 infection spread. Such measures started March 1,
2020, and continued until May 21, 2020.[8,9] Then,
political necessity and mounting economic pressure on the local
government demanded the start of a reopening process at a progressively
rapid pace.[8] After reopening, the number of COVID-19
cases increased sharply, with a concurrent increase in the number of
symptomatic patients and a two-fold increase in the case-fatality rate.[2]
In this study, we found that our cases' cure and case fatality rates
were 86.4% and 1.99%, respectively. In a study conducted in China, the
cure and case fatality rates were estimated to be 85.97% and 14.03%,
respectively.[10] In a study conducted in Iran and
Turkey, neighboring countries to Iraq, the case fatality rates were
6.35% and 2.6%, respectively.[11] The variation in
cure and case fatality rates among different geographical regions can
be explained in part by the variation in preventive measures, the
health system's efficiency, the genomic makeup of the virus, and host
genetics. International cooperation and more studies are needed to
explore this.
Additionally, the risk of reinfection was 0.031% in
the city. The risk of reinfection in our study is comparable to what
was found in Qatar as they found risk of reinfection at 0.04%.[6]
However, it seemed that reinfection is a rare phenomenon and may not
pose a considerable threat. Previous case reports showed different
severity in reinfection. While a case report from the USA showed
increased symptoms in the reinfection that required giving antiviral
treatment,[12] reports from Hong Kong, Belgium and
Netherlands demonstrated that the severity of reinfection cases was the
same in both rounds of infection.[4,5] In this study,
four patients showed more severe symptoms that required giving
antiviral treatment, and that can be explained by a very high dose of
virus that led to a high viral load. Other possibility is that the
reinfection might be caused by mutated version of the virus. Viral load
studies and genetic comparison, both of which not available in Iraq,
were needed to confirm this. The majority of the patients had mild or
minimal symptoms and none of reinfection cases were critical or fatal.
This may suggest that the vast majority of the patients developed
immunity against the infection that may last for months and if
reinfection occurs, it might be mild and none fatal. Long-term follow
up study is suggested to determine the waning time of immunity. Our
study has limitations. Firstly, this study provided epidemiological
evidence for reinfections that needs confirmation by sequencing
analysis of the paired viral samples. Secondly, we may have missed some
cases that received treatment in non-governmental clinics. However, we
are confident that the vast majority of patients were covered in this
study. To conclude, COVID-19 reinfection appeared to be possible but
rare; most patients showed mild infection, and further research is
needed to investigate the reasons behind that.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao
X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao
GF, Tan W. A Novel Coronavirus from Patients with Pneumonia in China,
2019. The New England journal of medicine 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017 PMid:31978945 PMCid:PMC7092803
- Hussein
NR, Naqid IA, Saleem ZSM, Almizori LA, Musa DH, Ibrahim N. A sharp
increase in the number of COVID-19 cases and case fatality rates after
lifting the lockdown in Kurdistan region of Iraq. Annals of medicine
and surgery 2020;57:140-142. https://doi.org/10.1016/j.amsu.2020.07.030 PMid:32754314 PMCid:PMC7377994
- Tillett
RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A,
Laverdure C, Verma SC, Rossetto CC, Jackson D, Farrell MJ, Van Hooser
S, Pandori M. Genomic evidence for reinfection with SARS-CoV-2: a case
study. Lancet Infect Dis 2020; 21: 52–58 https://doi.org/10.1016/S1473-3099(20)30764-7
- Wu
Z, McGoogan JM. Characteristics of and Important Lessons From the
Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a
Report of 72 314 Cases From the Chinese Center for Disease Control and
Prevention. JAMA 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648 PMid:32091533
- Mao
R, Qiu Y, He J-S, Tan J-Y, Li X-H, Liang J, Shen J, Zhu L-R, Chen Y,
Iacucci M, Ng SC, Ghosh S, Chen M-H. Manifestations and prognosis of
gastrointestinal and liver involvement in patients with COVID-19: a
systematic review and meta-analysis. The Lancet Gastroenterology &
Hepatology 2020;5(7):667-678. https://doi.org/10.1016/S2468-1253(20)30126-6
- Abu-Raddad
LJ, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S,
Ayoub HH, Al Kanaani Z, Al Khal A, Al Kuwari E, Butt AA, Coyle P,
Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Rahim HFA, Yassine HM,
Al Kuwari MG, Al Romaihi HE, Al-Thani MH, Bertollini R. Assessment of
the risk of SARS-CoV-2 reinfection in an intense re-exposure setting.
Clinical infectious diseases : an official publication of the
Infectious Diseases Society of America 2020. https://doi.org/10.1101/2020.08.24.20179457
- IMAI
District Clinician Manual. Hospital care for adolescents and adults.
Geneva: World Health Organization. 2020 [updated 2020; cited
05/01/2021]; Available from: https://apps.who.int/iris/bitstream/handle/10665/77751/9789241548290_Vol2_eng.pdf?sequence=3.
- Hussein
NR. The Role of Self-Responsible Response Versus Lockdown Approach in
Controlling COVID-19 Pandemic in Kurdistan Region of Iraq.
International Journal of Infection 2020;7(4). https://doi.org/10.5812/iji.107092
- Hussein
NR, Naqid IA, Saleem ZSM. A retrospective descriptive study
characterizing coronavirus disease epidemiology among people in the
Kurdistan Region, Iraq. Mediterranean Journal of Hematology and
Infectious Diseases 2020;12(1). https://doi.org/10.4084/mjhid.2020.061 PMid:32952972 PMCid:PMC7485477
- Diao
Y, Liu X, Wang T, Zeng X, Dong C, Zhang Y, Zhou C, She X, Liu D, Hu Z.
Estimating the cure rate and case fatality rate of the ongoing epidemic
COVID-19. medRxiv 2020:2020.2002.2018.20024513. https://doi.org/10.1101/2020.02.18.20024513
- Oke
J, Heneghan C. Global Covid-19 Case Fatality Rates - CEBM. The Centre
for Evidence-Based Medicine; 2020 [updated 2020; cited 28/01/2021];
Available from: https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/.
- Esme
M, Koca M, Dikmeer A, Balci C, Ata N, Dogu BB, Cankurtaran M, Yilmaz M,
Celik O, Unal GG, Ulgu MM, Birinci S. Older Adults With Coronavirus
Disease 2019; A Nationwide Study in Turkey. The journals of gerontology
Series A, Biological sciences and medical sciences 2020. https://doi.org/10.1093/gerona/glaa219 PMid:32871002 PMCid:PMC7499528



