Case #1. A 52-year-old male patient with no past medical history presented to the emergency room with gum bleeding and diffuse cutaneous purpura three weeks after administering the first dose of the AstraZeneca COVID-19 vaccine. He experienced gum bleeding as soon as three days after vaccination. On admission, complete blood count (CBC) showed isolated severe thrombocytopenia (platelet count, PLT=1.000/mm3), with normal white blood cell count and hemoglobin value. Coagulation tests were within normal limits, as well as renal and hepatic function tests. Hemolysis markers were negative. SARS-CoV-2 reverse-transcriptase–polymerase-chain-reaction assay of a nasopharyngeal swab was negative.
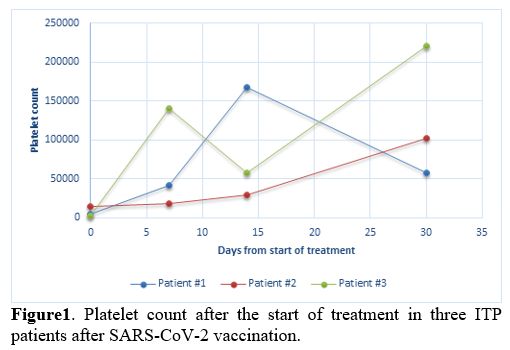
Furthermore, the autoimmune- including antinuclear antibodies (ANA), extractable nuclear antigens (ENA), antineutrophil cytoplasmic antibody (ANCA) and rheumatoid factor (RF)- and infectious screening for Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human immunodeficiency virus (HIV), Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) resulted all negative. Anti-platelet antibodies were not detected according to indirect immunofluorescence antibody assay (Euroimmun). He was hospitalized for seven days and treated with standard-dose steroid therapy (intravenous methylprednisolone 1 mg/kg). The patient achieved a complete remission of disease (defined as a platelet count greater than 100.000/mm3 and absence of bleeding) with a PLT count of 168.000/mm3 after 14 days of therapy. Nonetheless, after one month of 1 mg/kg steroid therapy, the platelet count dropped to 58.000/mm3 (Figure 1). The patient is currently in the course of steroid tapering.
 |
Figure
1. Platelet count after the start of treatment in three ITP patients
after SARS-CoV-2 vaccination. |
Case #2. A 24-year-old male came to our attention for thrombocytopenia following the Pfizer-BioNTech COVID-19 vaccination. He underwent heart transplantation at the age of 2 years due to congenital heart disease and, since then, he is on immunosuppressive treatment with tacrolimus and everolimus. Furthermore, he was diagnosed with Hodgkin lymphoma when he was fourteen years old and treated with chemotherapy achieving a complete remission of the disease. Four days after receiving the second dose of vaccine, he performed routine analyses, and CBC showed severe thrombocytopenia (PLT=15.000/mm3), with normal hemoglobin value and white blood cell count. The patient was asymptomatic and in a previous CBC performed two weeks before the administration of the first dose, he had a normal platelet count (PLT=150.000/mm3). Coagulation tests, renal and hepatic function tests were all within normal limits, and hemolysis markers were negative. The autoimmune (ANA, ANCA, ENA, RF) and infectious screening for HBV, HCV, HIV, CMV, and EBV resulted negative. Due to previous hematological malignancy and chemotherapy treatment, a diagnostic bone marrow aspirate smear was done. Microscopic examination of the smear revealed the presence of normal megakaryocytes and the absence of blast cells, compatible with the diagnosis of ITP. He was started on standard-dose oral steroids (prednisone 1 mg/kg), obtaining a complete remission of disease with a PLT count of 102.000/mm3 after a month of therapy. The patient is currently in the course of steroid tapering.
Case #3. A 73-year-old male patient with a medical history of hypertension, diabetes mellitus on insulin therapy, hyperlipidemia, coronary artery bypass grafting, and iron deficiency anemia received the first dose of Pfizer-BioNTech COVID-19 vaccination on 21st, 2021. Eighteen days after vaccination, ecchymosis appeared on the injection site of insulin. He received the second dose of the SARS-CoV-2 vaccine on April 12th, 2021. Two days after administering the second dose, he presented to the emergency department with tongue and oral mucosa petechiae along with subcutaneous ecchymosis on forearms and abdomen. Complete blood count showed an extremely low platelet count of 2.000/mm3, with normal hemoglobin and white blood cell count. A previous CBC report performed a few days before the second dose administration revealed a platelet count of 8.000/mm3. Coagulation tests, renal and hepatic function tests were all normal, excluding consumption coagulopathy and thrombotic microangiopathy in the absence of both schistocytes and blasts in the peripheral blood smear. Hemolysis markers were negative. Viral hepatitis panel, HIV, CMV, EBV, rheumatological markers, and anti-platelet antibodies resulted negative. ANA, ANCA, ENA, RF were absent. A previous CBC performed two weeks before the vaccination showed a platelet count of 256,000/mm3. He was hospitalized for five days and received standard-dose of methylprednisolone (1 mg/kg) and intravenous immunoglobulin (IVIG 400 mg/kg/die) with a quick improvement of the platelet count. After three days of therapy, the platelet count increased to 96.000/mm3, and at the time of discharge, the platelet count was 140.000/mm3. The patient continued oral steroid therapy per os (prednisone 1 mg/kg) for a total of 4 weeks, and at last, follow-up steroids were gradually tapered, maintaining platelet response (Figure 1).
Primary immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by isolated thrombocytopenia (peripheral blood platelet count<100.000/mm3) and, depending upon the degree of thrombocytopenia, increased risk of bleeding in the absence of other causes or disorders that may be associated with thrombocytopenia. Secondary ITP, on the other hand, develops in the context of other disorders, including autoimmune diseases, chronic infections or lymphoproliferative disorders. Secondary ITP has also been associated with different types of vaccinations4. Although the pathogenesis remains unclear, a plausible hypothesis is that vaccines-related ITP could be caused by molecular mimicry.[4] Furthermore, a new syndrome called “Autoimmune/inflammatory syndrome induced by adjuvants” (ASIA) was described and includes a spectrum of reactions due to vaccine adjuvant stimulation.[4]
The incidence of ITP is 6 per 100,000 adults/year. To date, few other cases of ITP following the administration of mRNA-1273 (Moderna), Pfizer- BioNTech,[5–7] and AstraZeneca COVID-19 vaccines[8] have been reported. The majority of cases described followed the administration of mRNA vaccines. Among the reported cases, 22 patients received an mRNA vaccine (13 patients received Moderna and nine patients received Pfizer vaccine), and only one patient received an adenovirus-based vaccine (AstraZeneca). Furthermore, in a recent study, the FDA evaluated the incidence of thrombocytopenia, including immune thrombocytopenia after mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS).[9] The reporting rates of thrombocytopenia were equal to 0.80 per million doses for both mRNA vaccines, suggesting a possible coincidental onset not attributable to the mRNA vaccine. As the authors also highlight, it should be said that VAERS is a passive safety surveillance system that is operator-dependent and that can underestimate the entity of thrombocytopenic episodes.
In Sicily, according to the last vaccination update of the ISTAT (Italian National Institute of Statistics) database, nearly half of the population received at least one COVID-19 dose at this time, while 16% received both scheduled doses. Also, according to the Italian government vaccinating campaign program, the majority of the vaccinated population is older than 60 years. In our case series, the median age at diagnosis was 49 years, and they were all male. In all three clinical cases, secondary causes (autoimmune, infectious, and lymphoproliferative disorders) were excluded, and the short-latency period following the administration of the vaccine suggests a possible temporal connection.
Patient #1 experienced hemorrhagic manifestations as soon as three days after administering the first dose, while patient #3 showed ecchymosis on the injection site of insulin eighteen days after the first dose. On the other hand, patient #2 did not have any hemorrhagic manifestation and accidentally discovered a low platelet count by performing routine blood analyses four days after receiving the second shot of the vaccine. The patients had no clinical signs and laboratoristic features of consumption coagulopathy, thrombotic microangiopathy, or thrombosis (Table 1), and the diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) was therefore excluded.[10] All three patients had a favorable response to “ITP directed” therapies, including corticosteroids and IVIG. Furthermore, episodes of VITT following SARS-CoV-2 vaccines showed that most of the patients were female younger than 50 years.[10]
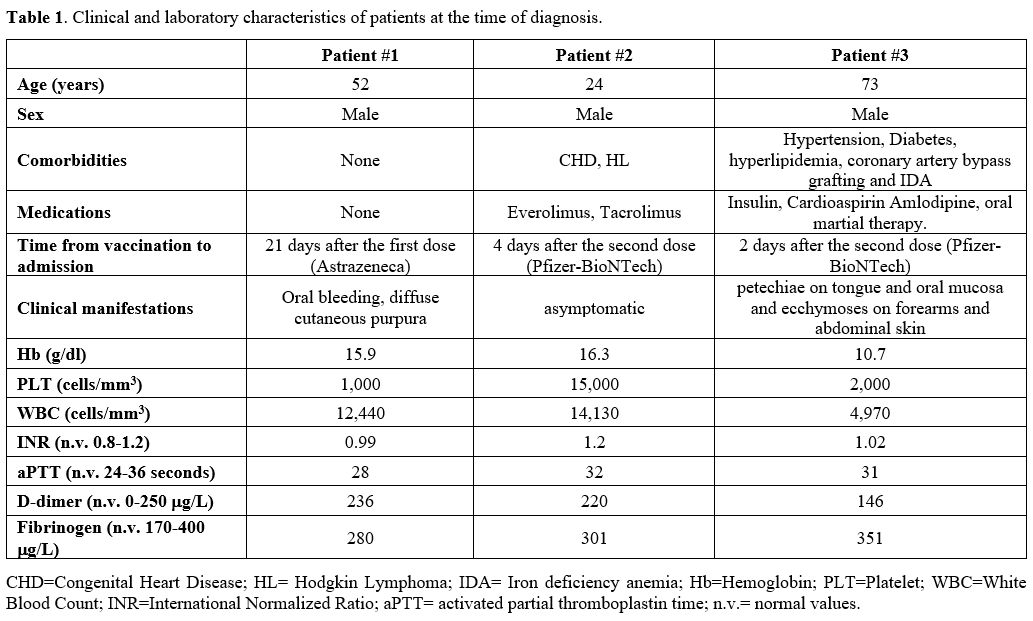 |
Table
1. Clinical and laboratory characteristics of patients at the time of
diagnosis. |
On the other hand, in all three case reports regarding ITP patients after the SARS-CoV-2 vaccine, the patient was male,[5-6,8] like our case series. However, the largest case series consisting of 20 patients vaccinated with mRNA vaccines (Pfizer-BioNtech or Moderna)[7] showed a slight female predominance (11 females and eight males, sex unknown in one patient). Of 20 patients, three patients had pre-existing thrombocytopenia, and one patient had thrombocytopenia found after a hemorrhagic stroke that was resolved with platelet transfusions alone. In the rest of the study population, seven patients were male and eight females, thus failing to confirm the male predominance of ITP cases following SARS-CoV-2 vaccines.
We cannot know exactly the time of insurgence of thrombocytopenia, how many days after the first dose, the platelet count started to fall, or, in the case of patient #2, whether it happened after the first or the second administration. Patients #2 and #3 performed a CBC two weeks before vaccination revealing normal platelet count. Patient #1 did not perform any blood tests before receiving the vaccination; therefore, we cannot exclude a pre-existing ITP, although he did not evidence hemorrhagic manifestations before vaccination. Unfortunately, measurement of SARS-Cov-2 antibodies was not done, although patients #1 and #3 did not have important comorbidities. Therefore, the vaccine-induced antibody response was expected. Also, patient #2 was on immunosuppressive therapy without steroids before vaccination with normal lymphocyte count; immune response with the production of SARS-Cov-2 antibodies is possible. Finally, the timing of thrombocytopenic onset is compatible with the production of the virus antibodies, from 3 to 5 weeks from the first vaccine dose.
Nonetheless, it remains difficult to distinguish incidental from post-vaccination ITP, and additional monitoring is fundamental to better characterize the incidence of immune thrombocytopenic purpura following COVID19 vaccination. Given the extremely limited number of cases, further information is needed in order to evaluate male predominance. Also, the heterogeneity in age at disease onset is present in the literature ranging from 22 until 74 years, although the older population is prevalent. In Italy, the vaccination campaign was first initiated in the elderly population; therefore, it is difficult to have concrete conclusions until all age groups are equally vaccinated.