Since
December 2020, humankind, in an attempt to control the Covid-19
pandemic, experienced the biggest vaccine rollout and immunization
process ever remembered. As of May 1, 2021, more than 1 billion doses
of a Covid-19 vaccine have been administered worldwide. There are
several Covid-19 vaccines available, but only four of them have
received emergency use approval by EMA and/or FDA.[1]
Ongoing studies are still analyzing their efficacy against the new
emerging mutant SARS-CoV2 variants and their safety profile.[2]
We
present a case of vaccine-induced prothrombotic immune thrombocytopenic
(VIPIT) disorder following ChAdOx1 nCoV-19 vaccine in a 29 years old,
previously healthy, Caucasian female, which manifested with superior
ophthalmic vein (SOV) thrombosis and thrombocytopenia.[3,4]
The patient was referred to our Clinic on March 30, 2021, complaining
of severe headache, left orbital swelling, and blurred left eye vision.
Initial findings showed thrombocytopenia of 18 × 109
per L in association with high D-dimers levels of 35712 μg/L. At
physical examination, the patient showed left eyeball swelling with
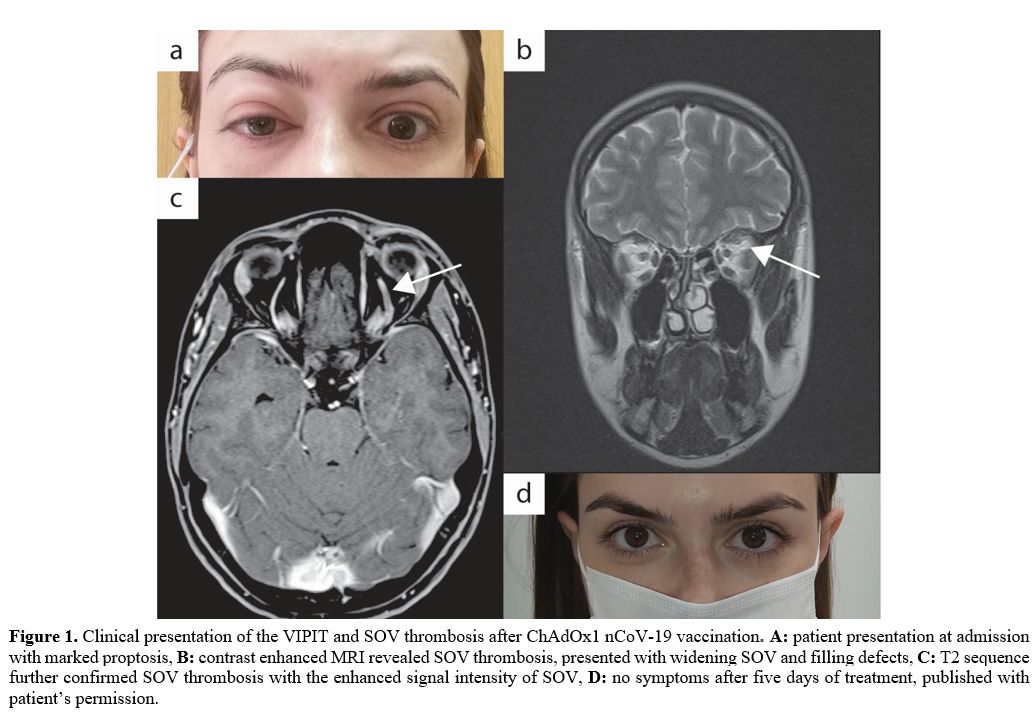
proptosis, limited ocular motility, and diplopia at the vertical gaze (Figure 1a).
The
symptoms dated one day before admission, starting with flu-like
symptoms and fever. She has received the first dose of vaccine against
SARS-CoV-2, ChAdOx1 nCoV-19, ten days before admission. Her medical and
pharmacological history were unremarkable negative. She also reported
one day of fever after the vaccination. The CT scan of the head was
normal. Diagnostic blood examinations showed a normal blood smear,
apart from the thrombocytopenia, elevated CRP (142 mg/L), and normal
prothrombin, activated partial thromboplastin, and thrombin time and
fibrinogen level (2.5 g/L). The nasopharyngeal swab for SARS-CoV2
nucleic acid testing was negative, and serology revealed the presence
of SARS-CoV-RBD IgG 59.46 and IgM 1.5 AU/ml, although the patient had
no history of infection since the pandemic started.
Contrast-enhanced MRI demonstrated central filling defects (Figure 1b) and hyper T2 signal (Figure 1c) in the left superior ophthalmic vein (SOV), revealing thrombosis.[5]
Screening
for antibodies for Heparin/Platelet Factor 4 (PF4) complex using a
particle gel immune assay (ID-PaGIA Heparin/PF4 Antibody test) revealed
a very high level of antibodies agents the PF4 complex.[6]
Testing
for hereditary thrombophilia (Factor V Leiden R506Q mutation,
Prothrombin G20210A gene mutation) and other triggers for thrombosis
and thrombocytopenia, including lupus anticoagulant, protein C and S
activity, cardiolipin IgG and IgM antibodies, hepatitis B and C virus,
HIV, cytomegalovirus, and Helicobacter pylori infection, were all
negative.
The presence of elevated D-dimers in association with
immune thrombocytopenia and MRI-confirmed thrombosis make the diagnosis
of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)
probable.[3] Treatment for this condition was
initiated following the recommendation of the Drugs & Biologics
Clinical Practice Guidelines Working Group and the Ontario COVID-19
Science Advisory Table.[4] Intravenous immunoglobulin
(IVIG) 1 g/kg of body weight daily for two days, broad-spectrum
antibiotics and direct oral anti-Xa inhibitor, Rivaroxaban, 15 mg twice
daily for 21 days were administrated. Afterward, immunosuppression was
continued with oral prednisolone 1 mg/kg bw for seven days, with
tapering afterward. The patient recovered remarkably rapidly, all the
symptoms resolved within 4 to 5 days (Figure 1d),
platelets raised to normal level after a week of treatment, and the
D-dimer levels went to normal after two weeks of treatment. The patient
was discharged home after six days. She is still on prednisolone and
Rivaroxaban 20 mg daily and will continue for another two months.
Since
April 9, 2021, the EMA has been listed the condition of unusual blood
clots with low blood platelets occurring in the first two weeks after
the ChAdOx1 nCoV-19 vaccine as a very rare side effect of this vaccine.
A few weeks later, the FDA associated the same side effect with the
Janssen ad26.cov2.s (COVID-19) vaccine. Thus, both regulatory agencies
underline that the benefits of both vaccines are far greater than their
risk.[7,8]
Most of the reported VIPT patients
presented with cerebral venous sinus and splanchnic vein thrombosis,
but other rare thrombotic complications are possible, and our case of
SOV thrombosis is the second-ever described.[5]