Daniela Taurino1, Marco Frigeni1, Anna Grassi1, Gianluca Cavallaro1, Silvia Salmoiraghi1, Orietta Spinelli1, Alessandro Rambaldi1,2 and Federico Lussana1.
1Hematology and Bone Marrow Transplant Unit, ASST Papa Giovanni XXIII, Bergamo, Italy.
2 Department Oncology and Hematology, Università degli Studi di Milano, Milano, Italy.
Correspondence to:
Daniela Taurino, Hematology and Bone Marrow Transplant Unit, ASST Papa
Giovanni XXIII, Piazza OMS, 1, 24127, Bergamo, Italy. Tel.
+390352673684 - E-mail:
dtaurino@asst-pg23.it
Published: September 1, 2021
Received: June 30, 2021
Accepted: August 14, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021057 DOI
10.4084/MJHID.2021.057
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
SARS-COV2 pandemic has caused profound challenges
in health care systems worldwide. Patients affected by hematological neoplasms
appear to be particularly at risk of developing COVID-19 complications, with
unfavorable outcomes.
Here, we present the case of a 57-years-old woman
diagnosed with
severe COVID-19 pneumonia and concurrent acute myeloid leukemia (AML). At the
time of diagnosis, it was decided to postpone leukemia therapy to enable
adequate COVID-19 pneumonia treatment. When her conditions related to pneumonia
improved, the combination of Azacitidine-Venetoclax was used as first-line
treatment instead of conventional intensive chemotherapy. At the end of the
first two cycles, the patient showed complete remission, and a post-remission
consolidation with allogeneic hematopoietic stem cell transplantation has been
planned.
This case suggests that Azacytidine-Venetoclax
induction may represent a valid and safe alternative to intensive chemotherapy
in the challenging setting of patients with a concomitant diagnosis of AML and
severe COVID-19 infection.
|
Introduction
Since
SARS-COV2 infection was declared a pandemic, it has profoundly impacted
the health system worldwide, challenging established algorithms to
manage many diseases, including hematological malignancies. Older and
immunocompromised populations appear to be at a higher risk for severe
complications related to COVID-19 than the general population, with a
more significant number of patients admitted to intensive care units
requiring invasive ventilation or death.[1] As showed by Chinese
nationwide analysis, cancer patients are 3.5 times more likely to
develop severe SARS-COV2 pneumonia than the cancer-free population.[2]
Therefore, the clinical management of cancer patients is complicated by
considerable uncertainty about the risks and benefits of treatment
while the infection is ongoing.
Acute myeloid leukemia (AML)
patients with less than 70 years and without comorbidities are usually
candidates to receive induction chemotherapy and often allogeneic
transplantation (allo-HSCT) as optimal consolidation therapy.[3] This
type of therapy often leads to prolonged cytopenia, making patients
more susceptible to infectious complications.[4]
In the coming
months, a sizeable number of AML patients may be expected to experience
SARS-COV2 infection either at diagnosis or during the disease course,
with a potentially substantial impact on the possibility of receiving
the optimal standard chemotherapy. Given the lack of evidence-based
algorithms to guide clinicians in choosing the best therapeutic regimen
and timing for treatment initiation for newly diagnosed patients with
AML and concomitant symptomatic SARSCOV-2 infection, here we report an
emblematic case.
Case Report
In
November 2020, a 57-years-old woman with no significant medical history
was referred to the emergency room for cough and dyspnea, prompting a
SARS-COV-2 real-time polymerase chain reaction (RT‐PCR) test, which
resulted in positive results. Chest CT evidenced bilateral patchy
ground-glass opacities, consistent with COVID-19 pneumonia (Figure 1-A). Blood tests showed severe neutropenia (neutrophils 0.55 x 109/L), mild thrombocytopenia (platelets 115 x 109/L), and anemia (Hb 50 g/L) (Figure 2).
The patient was admitted to the intensive care unit (ICU), where she
was immediately treated with piperacillin/tazobactam 4.5 g QID,
posaconazole 300 mg QD, and dexamethasone per institutional guidelines
for the treatment of COVID-19 pneumonia. Ventilatory support with
continuous positive airway pressure (CPAP) and pronation cycles was
also started without the need for intubation. Antibiotic therapy was
continued for 41 days.
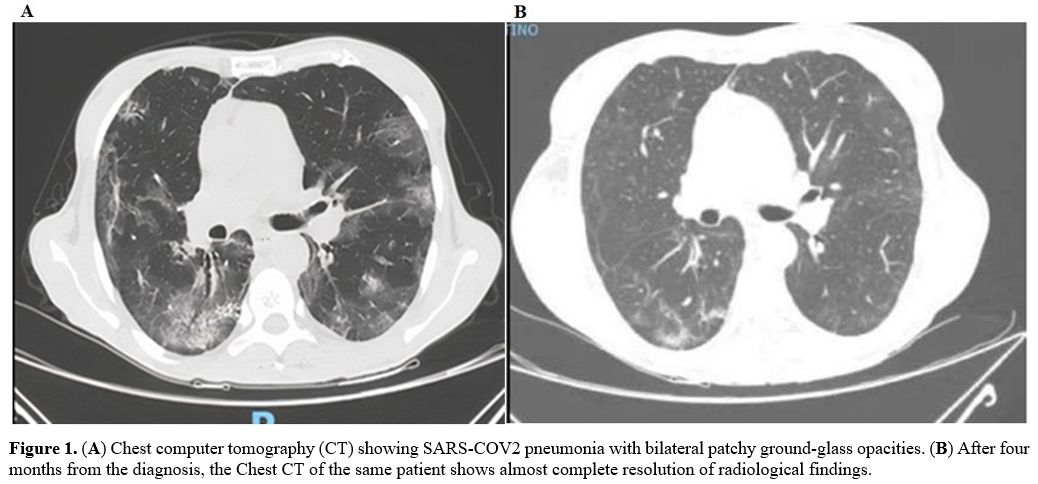 |
Figure
1. (A) Chest computer tomography (CT) showing SARS-COV2 pneumonia with bilateral patchy ground-glass opacities. (B)
After four months from the diagnosis, the Chest CT of the same patient
shows almost complete resolution of radiological findings. |
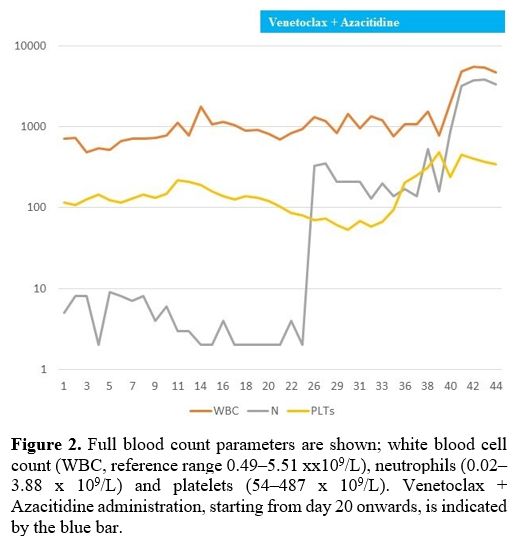 |
Figure 2. Full blood count parameters are shown; white blood cell count (WBC, reference range 0.49–5.51 xx109/L), neutrophils (0.02–3.88 x 109/L) and platelets (54–487 x 109/L). Venetoclax + Azacitidine administration, starting from day 20 onwards, is indicated by the blue bar. |
A
bone marrow aspirate was performed to investigate the abnormal blood
results, which were diagnostic for AML, with 69% blasts. The
immunophenotype was positive for CD34, CD117, human leukocyte antigen
DR isotype (HLADR), CD33, and weakly positive for CD13. Cytogenetic
revealed a 46, XX normal karyotype and molecular characterization by
NGS an isocitrate dehydrogenase 2 (IDH2) mutation with FLT3-ITD, NPM1, CEBPA
wild-type, therefore classifying the disease as AML
not-otherwise-specified according to the WHO 2016 classification,[5]
with an intermediate risk based on ELN 2017 recommendations.[3]
A
contrast chest CT was performed due to a marked D-dimer increase on day
two after ICU admission, showing bilateral pulmonary emboli and
confirmed the bilateral ground-glass opacities already highlighted in
the previous CT. Anticoagulant therapy with low-molecular-weight
heparin (LMWH) was therefore added.
Upon multidisciplinary
review, it was decided to postpone leukemia therapy to enable adequate
COVID-19 pneumonia treatment. The patient experienced progressive
clinical improvement and requiring gradually decreased oxygen
consumption. Oxygen weaning was obtained after 15 days after ICU
admission. Despite the clinical improvement, the persistent positivity
of the nasopharyngeal swab for RT-PCR for SARS-CoV2 was observed.
Therefore, we decided to infuse two cycles of convalescent plasma
collected from patients who had recovered from SARS-COV2 disease,[6-7]
unfortunately without achieving the clearance of the virus. A plausible
explanation for the benefit of hyperimmune plasma is to provide
immunity by giving patients virus-specific neutralizing antibodies,
which might result in immediate clearance of SARS-CoV-2.[8]
Due
to the persistent swab positivity for SARS-CoV-2 and the recent severe
pneumonia and pulmonary embolism, the risk of a standard intensive
chemotherapy induction was deemed unacceptable for this patient. Thus,
based on the current data showing that IDH1/2
mutations are responsive to venetoclax-based therapy,[9-11] a treatment
with Azacytidine-Venetoclax was preferred and started 32 days after
diagnosis. 5-Azacitidine was administered subcutaneously at 75 mg/m2
once daily from day 1 to day 7. Venetoclax was administered orally, 100
mg day 1, 200 mg day 2, 300 mg day 3, 400 mg day 4 to be continued till
day 28. Due to the well-known pharmacological interference of
Venetoclax with azoles, posaconazole was replaced with micafungin at a
dose of 50 mg QD. The patient tolerated the treatment exceptionally
well, without hemorrhagic or infectious events. Complete recovery of
blood counts was observed 46 days after treatment initiation (Figure 2), and antibiotic and antifungal therapy was suspended.
Notably,
on day 18 of the cycle (the seventh week since the onset of COVID-19
infection), the nasal swab became finally negative, and the patient was
discharged to complete the 28-day course of venetoclax at home. At the
end of the cycle, a bone marrow evaluation showed marrow hypoplasia,
with residual 6% myeloid blasts detected by immunophenotype.
After
19 days from the end of the first cycle, the patient received a second
cycle of Azacytidine-Venetoclax. After seven days from the end of the
second cycle, a bone marrow evaluation showed complete remission, and a
post-remission consolidation with alloHSCT was planned. A new chest CT
scan repeated four months later showed extensive resolution of lung
infiltrates (Figure 1-B). An
unrelated donor was identified, but the patient refused to undergo
allogeneic transplantation. We, therefore, planned to continue
Azacitidine-Venetoclax therapy until relapse or development of
unacceptable toxicity. After the second cycle of treatment, a bone
marrow evaluation was also performed, confirming the complete remission
of the disease.
Discussion
Recent
systematic reviews and pooled analysis showed that patients with cancer
and COVID-19 have an increased risk of severe disease and
mortality.[12,13] Desai et al.[13] found that increasing age, male sex,
hematologic malignancy, and current anticancer therapy contributed to
the increased mortality. Several studies have shown that T immunity
plays a fundamental role in viral clearance.[14] In this regard,
defective immunity due to both hematological disorders and chemotherapy
may cause a worse prognosis with an increased risk of mortality due to
COVID-19.[15]
In the pandemic era, the general recommendation in
patients newly diagnosed with AML and concomitant SARS-COV2 infection
is to postpone all treatments that do not require urgent initiation,
limiting cytoreductive therapies if necessary.[16] Although AML has
always been considered a medical emergency needing prompt treatment
intervention; nowadays, this dogma is changing according to the results
of a real-world study showing no significant difference in the rate of
complete remissions and overall survival after delaying treatment up to
15 days.[17]
Intensive chemotherapy with initial induction «7+3»
regimen represents the backbone of upfront AML treatment in young
patients intending to eradicate the disease. However, it is noteworthy
that treating patients with concomitant COVID-19 requires an individual
refinement of the standard therapeutic approach based on the clinical
conditions. In this regard, the use of recently approved drugs in AML,
such as the combination of hypomethylating agents and venetoclax,[18]
should be considered due to their promising results in the incidence of
complete remission and favorable safety profile.
Here, we
reported the clinical management with venetoclax and azacytidine of a
patient with a newly AML diagnosis with an IDH2 mutation and severe
COVID19 pneumonia. In this unique scenario where chemotherapy-induced
pancytopenia could adversely affect outcomes, this case confirmed the
high efficacy and safety profile of the venetoclax combination therapy.
Furthermore, this case report highlights the usefulness of an accurate
molecular characterization, especially in complex cases, and the
emerging interest for different AML molecular patterns associated with
favorable outcomes with venetoclax-based regimens. In this regard,
although promising results were obtained in the front-line use of
chemotherapy and IDH1/2 inhibitors,[19] the Azacytidine-Venetoclax
combination has been confirmed as a powerful treatment option for this
subset of AML.
Of interest, our experience is entirely in
keeping with a recent report of a patient with de novo
FLT3-ITD-positive AML and severe COVID-19, safely treated with
single-agent gilteritinib obtaining a complete remission.[20]
Conclusions
Treatment
of AML in patients with concurrent COVID-19 infection remains
challenging and calls a refinement of the standard therapeutic approach
into question. Therefore, the use of novel drugs approved in AML should
be considered a valid alternative to standard chemotherapy for these
frail patients.
References
- Wu Z, McGoogan JM. Characteristics of and important
lessons from the coronavirus disease 2019 (COVID-19) outbreak in china:
summary of a report of 72 314 cases from the Chinese center for disease
control and prevention. JAMA 2020; 323: 1239.
https://doi.org/10.1001/jama.2020.2648
- Liang
W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a
nationwide analysis in China. The Lancet Oncology 2020; 21: 335-337.
https://doi.org/10.1016/S1470-2045(20)30096-6
- Döhner
H, Estey E, Grimwade D, et al. Diagnosis and management of AML in
adults: 2017 ELN recommendations from an international expert panel.
Blood 2017; 129: 424-447. https://doi.org/10.1182/blood-2016-08-733196
- Ferrara
F, Schiffer CA. Acute myeloid leukemia in adults. The Lancet 2013; 381:
484-495. https://doi.org/10.1016/S0140-6736(12)61727-9
- Arber
DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health
Organization classification of myeloid neoplasms and acute leukemia.
Blood 2016; 127: 2391-2405.
https://doi.org/10.1182/blood-2016-03-643544
- Mair-Jenkins
J, Saavedra-Campos M, Baillie JK, et al. The Effectiveness of
Convalescent Plasma and Hyperimmune Immunoglobulin for the Treatment of
Severe Acute Respiratory Infections of Viral Etiology: A Systematic
Review and Exploratory Meta-analysis. J Infect Dis 2015; 211: 80-90.
https://doi.org/10.1093/infdis/jiu396
- Chen
L, Xiong J, Bao L, et al. Convalescent plasma as a potential therapy
for COVID-19. The Lancet Infectious Diseases 2020; 20: 398-400.
https://doi.org/10.1016/S1473-3099(20)30141-9
- Ferrari
S, Caprioli C, Weber A, et al. Convalescent hyperimmune plasma for
chemo-immunotherapy induced immunodeficiency in COVID-19 patients with
hematological malignancies. Leukemia & Lymphoma 2021; 62:
1490-1496. https://doi.org/10.1080/10428194.2021.1872070
- DiNardo
CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of
venetoclax with decitabine or azacitidine in elderly patients with
previously untreated acute myeloid leukemia: a non-randomized,
open-label, phase 1b study. The Lancet Oncology 2018; 19: 216-228.
https://doi.org/10.1016/S1470-2045(18)30010-X
- Chan
SM, Thomas D, Corces-Zimmerman MR, et al. Isocitrate dehydrogenase 1
and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat
Med 2015; 21: 178-184. https://doi.org/10.1038/nm.3788
- DiNardo
CD, Tiong IS, Quaglieri A et al. Molecular patterns of response and
treatment failure after front-line venetoclax combinations in older
patients with AML. Blood 2020; 135: 791-803.
https://doi.org/10.1182/blood.2019003988
- Saini
KS, Tagliamento M, Lambertini M, et al. mortality in patients with
cancer and coronavirus disease 2019: A systematic review and pooled
analysis of 52 studies. European Journal of Cancer 2020; 139: 43-50.
https://doi.org/10.1016/j.ejca.2020.08.011
- Desai
A, Gupta R, Advani S, et al. mortality in hospitalized patients with
cancer and coronavirus disease 2019: A systematic review and
meta‐analysis of cohort studies. Cancer 2021; 127: 1459-1468.
https://doi.org/10.1002/cncr.33386
- Chen
Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev
Immunol 2020; 20: 529-536. https://doi.org/10.1038/s41577-020-0402-6
- Aries
JA, Davies JK, Auer RL, et al. Clinical outcome of coronavirus disease
2019 in haemato‐oncology patients. Br J Haematol; 190. Epub ahead of
print July 2020. DOI: 10.1111/bjh.16852.
https://doi.org/10.1111/bjh.16852
- Zeidan
AM, Boddu PC, Patnaik MM, et al. Special considerations in the
management of adult patients with acute leukemias and myeloid neoplasms
in the COVID-19 era: recommendations from a panel of international
experts. The Lancet Haematology 2020; 7: e601-e612.
https://doi.org/10.1016/S2352-3026(20)30205-2
- Röllig
C, Kramer M, Schliemann C, et al. Does time from diagnosis to treatment
affect the prognosis of patients with newly diagnosed acute myeloid
leukemia? Blood 2020; 136: 823-830.
- DiNardo
CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in
Previously Untreated Acute Myeloid Leukemia. N Engl J Med 2020; 383:
617-629. https://doi.org/10.1056/NEJMoa2012971
- Stein
EM, DiNardo CD, Fathi AT, et al. Ivosidenib or enasidenib combined with
intensive chemotherapy in patients with newly diagnosed AML: a phase 1
study. Blood 2021; 137: 1792-1803.
https://doi.org/10.1182/blood.2020007233
- Wilson
AJ, Troy‐Barnes E, Subhan M, et al. Successful remission induction
therapy with gilteritinib in a patient with de novo FLT3 ‐mutated acute
myeloid leukemia and severe COVID‐19. Br J Haematol; 190. Epub ahead of
print August 2020. https://doi.org/10.1111/bjh.16962
[TOP]