Jassada Buaboonnam, Chayamon
Takpradit, Vip Viprakasit, Nattee Narkbunnam, Nassawee Vathana, Kamon
Phuakpet, Kleebsabai Sanpakit and Bunchoo Pongtanakul.
Division of Hematology
and Oncology, Department of Pediatrics, Faculty of Medicine Siriraj
Hospital, Mahidol University, Bangkok, Thailand.
Correspondence to:
Bunchoo
Pongtanakul, MD. Associate Professor of Pediatrics, Division of
Hematology and Oncology, Department of Pediatrics, Faculty of Medicine
Siriraj Hospital, Mahidol University 2 Wanglang Road, Bangkok Noi,
Bangkok 10700, Thailand. Tel: +66 2 419 5960; Fax: +66 2 411 3010.
E-mail:
pongtanakul@yahoo.com
Published: November 1, 2021
Received: August 1, 2021
Accepted: October 16, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021065 DOI
10.4084/MJHID.2021.065
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Patients with transfusion-dependent thalassemia (TDT) risk iron
overload and require iron chelation therapy. Second-line therapy is
warranted for patients demonstrating poor chelation responses.
Patients and methods:
We retrospectively studied the serum-ferritin (SF), and
liver-iron-concentration (LIC) outcomes of patients with TDT treated
with twice-daily dosing of deferasirox (TDD-DFX) > 24 months, after
failing to respond to once-daily deferasirox (OD-DFX).
Results:
We enrolled 22 OD-DFX nonresponders (14 males and eight females; median
age, 9.2 [3–15.5] years). The median blood transfusion was 216
(206–277) ml/kg/year. The median TDD-DFX treatment period was 30
(24–35) months. Before initiating TDD-DFX, the median SF level was
2,486 (1,562–8,183) ng/ml, while the median LIC was 6.6 (3.2–19) mg/g
dry wt. There were 18 TDD-DFX responders (81.8%) and 4 TDD-DFX
nonresponders. The median SF-level change was -724 (-4,916 to 1,490)
ng/mL. The median LIC change was -2.14 (-13.7 to 6.8) mg/g dry wt. The
1-year and 2-year SF levels and LICs were statistically significant
(SF, P = 0.006/0.005; and LIC, 0.006/0.005, respectively). There were
no treatment interruptions secondary to adverse events. In the
follow-up of the TDD-DFX responder group, 11 of the 18 had a reduced
dose, whereas the remaining seven continued with the same dose.
Conclusions:
TDD-DFX appears to be an alternative treatment approach for patients
refractory to OD-DFX, with a favorable long-term safety profile.
Further studies with larger groups and pharmacogenetic analyses of
OD-DFX responders are warranted to determine the efficacy and safety
profile of TDD-DFX.
|
Introduction
Thalassemia is one of the most common hereditary hemolytic anemias found in Southeast Asia, including in Thailand.[1]
The severity ranges from asymptomatic to transfusion-dependent
thalassemia (TDT). Patients with TDT requiring regular blood
transfusion may succumb to iron overload. This condition ultimately
results in organ damage, especially cardiomyopathy and liver cirrhosis,
secondary to cardiac and liver siderosis, respectively. Once-daily
deferasirox (OD-DFX) dispersible tablets can be administered as an oral
iron chelator to patients with transfusional iron overload, depending
on factors such as the iron burden, rate of transfusion, and adequacy
of the dosage.[2] Although the recommended dosage of
OD-DFX is 30 mg/kg/day, about 30% of patients with TDT cannot maintain
a negative iron balance with OD-DFX; they require a dosage greater than
30 mg/kg/day.[3] The dosage can be increased to 40 mg/kg/day while maintaining an acceptable safety profile.[4]
Nevertheless, even when treated with a higher dosage, some patients
still need other treatments to achieve adequate iron chelation. Our
earlier research found that twice-daily dosing of DFX (TDD-DFX) showed
improved clinical efficacy in patients with TDT who were OD-DFX
nonresponders.[5] However, the long-term
effectiveness, safety, and tolerability of TDD-DFX in children with TDT
who were unresponsive to OD-DFX have not yet been studied. The primary
objective of this study was to determine the clinical efficacy of
TDD-DFX, and the secondary objective was to evaluate the safety and
tolerability of TDD-DFX in patients with TDT who failed to respond
OD-DFX during their long-term treatment.
Patients and Methods
Patients
with TDT who were treated with OD-DFX at Siriraj Hospital, Mahidol
University, Bangkok, Thailand, between January 2013 and December 2020
were retrospectively analyzed. The red blood cell (PRC) transfusion was
given every 3–4 weeks at a dosage of 12–15 mL/kg, depending on the
pretransfusion hemoglobin level, to maintain the pretransfusion
hemoglobin level at greater than 9 g/dL. On two consecutive tests, iron
chelation was initiated in patients with TDT if their serum ferritin
(SF) level was greater than 1,000 ng/mL. Monitoring of the treatment
regimen was based on the results of the patients' trimonthly SF level
assessments. Cardiac T2* magnetic resonance imaging (MRI) and liver
iron concentration (LIC) monitoring were performed every 6 to 12 months
at our institute.[6] The dose of DFX was initiated at
15-20 mg/kg/day and adjusted according to their SF-level and MRI
results. Patients unable to undergo an MRI study were monitored solely
by SF concentration. Complete blood count, liver function test, renal
function test, and urine analysis were monitored every 6 to 8 weeks,
depending on the transfusion schedules. In addition, an ophthalmic
examination and audiometry were performed annually during treatment
with DFX. OD-DFX nonresponders were defined using modified criteria by
Chirnomas et al. as follows: (1) their average DFX-OD dosage exceeded
35 mg/kg/day for six months and (2) their SF level tended to increase,
and/or there was less than a 30% reduction in their SF level relative
to baseline for three consecutive months with more than 2 SF
measurements exceeding 1,500 ng/mL during the time that dosage exceeded
35 mg/kg/day.[7] The OD-DFX nonresponders were
switched to TDD-DFX, using the same dose as the previous OD-DFX
regimen. Treatment responses after TDD-DFX were reviewed one year after
initiation of the chelators, with evaluations based on the patients' SF
levels and MRI studies. TDD-DFX responders were defined based on our
institutional criteria as follows: (1) their 1-year SF level declined
by more than 15% of the baseline, or (2) their LIC decreased from the
baseline value. Patients did not respond to TDD-DFX were classified as
TDD-DFX nonresponder. Tolerability to DFX and treatment compliance were
evaluated by history taking and reviewing the drug dosages prescribed
during the study period. Patients with grade I proteinuria were advised
to have adequate hydration without dose adjustment. Those who
experienced > grade I proteinuria were tested for urine
protein/creatinine ratio. If the ratio was greater than 0.5 for two
consecutive times, the medication was temporarily omitted. The 50%
reduction dose was restarted if the ratio was less than 0.5. Treatment
toxicity was graded according to the US National Cancer Institute's
Common Terminology Criteria for Adverse Events (version 4.03). Before
the commencement of this research, its protocol was approved by the
Siriraj Institutional Review Board, Faculty of Medicine, Siriraj
Hospital, Mahidol University, Bangkok, Thailand (COA 175/2564, IRB4).
Statistical Analysis.
Statistical analyses were performed using STATA IC (release 16;
StataCorp LLC., College Station, TX, USA). Continuous variables were
analyzed and reported using descriptive statistics (medians and
ranges). In addition, a Wilcoxon matched-pairs signed-rank test
compared SF levels, LICs, and other parameters at the baseline, 1-year,
and 2-year time points. Statistically significant differences were
defined as P values of < 0.05.
Results
Of
the 22 OD-DFX nonresponders in this study, 21 had hemoglobin E/β
thalassemia, while 1 had β thalassemia major. The baseline
characteristics of the patients are summarized in Table 1.
No patient had cardiac T2* MRI < 20 ms. The median TDD-DFX treatment
time was 30 (24–35) months. The median dose of OD-DFX was 37.5 (35-40)
mg/kg/day.
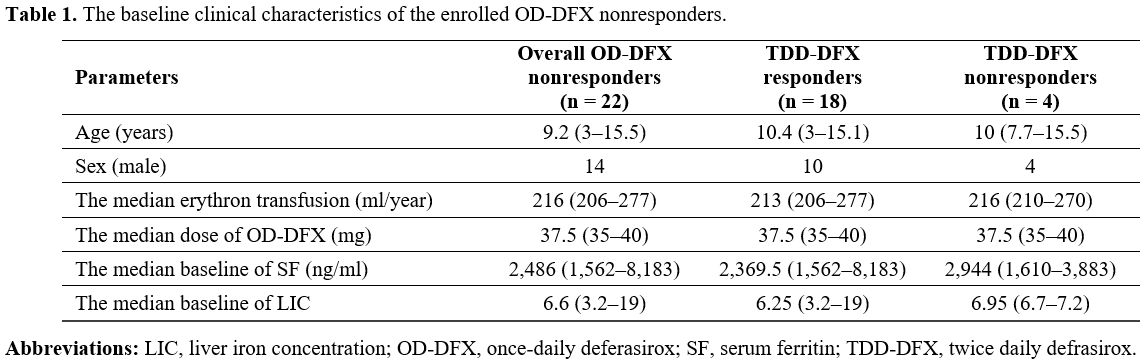 |
Table 1. The baseline clinical characteristics of the enrolled OD-DFX nonresponders. |
With
17 of the OD-DFX nonresponders, their responses were based on their
SF-level and MRI results, whereas with the other 5 OD-DFX
nonresponders, the responses were determined by SF level only. In all,
there were 18 TDD-DFX responders (81.8%) and 4 TDD-DFX nonresponders.
Fifteen of the 18 TDD-DFX responders had evidence of declined LICs,
while the remaining 3 TDD-DFX responders only exhibited decreased SF
levels. The four patients, classified as TDD-DFX nonresponders one year
after commencement of the TDD-DFX regimen, were switched to a
combination of DFX and deferoxamine. The median changes in the SF
levels and LICs at the 1-year-post-treatment and 2-year-post-treatment
time points for the TDD-DFX therapy are detailed in Table 2.
Of 3 TDD-DFX responders who only exhibited decreased SF levels, the
median reduction of SF at a 1-year time point was 31% (range
23-62%). Figure 1 illustrates
the changes in the SF levels and LICs of the TDD-DFX responders. As to
the TDD-DFX nonresponder group, 2 of the four patients experienced
increased SF levels and LICs, while the other two only saw a rise in
their SF levels. A disparity between the SF level and the LIC was
observed in 2 patients: both were TDD-DFX responders with a decline in
the LIC but an elevation in the level of SF. In the follow-up study of
the 18 TDD-DFX responders, 11 could be treated with a reduced TDD-DFX
dose (median, 17.04 mg/kg/day), but the other seven patients continued
with the same TDD-DFX dose (median, 37.4 mg/kg/day). There were no
reported incidents of gastrointestinal intolerance or skin reaction.
However, three patients developed grade I proteinuria, which was
resolved without interruption or reducing the TDD-DFX therapy. Neither
severe transaminitis (alanine aminotransferase > 3 times of the
upper limit of normal) nor abnormal renal function test results were
reported for all 18 patients (Table 2).
Although the blood-urea-nitrogen and creatinine levels were
significantly increased at the 1-year-post-treatment and
2-year-post-treatment timepoints relative to baseline, they were still
within the normal ranges for age. The ophthalmic examinations and
audiometry results were normal throughout the treatment with TDD-DFX.
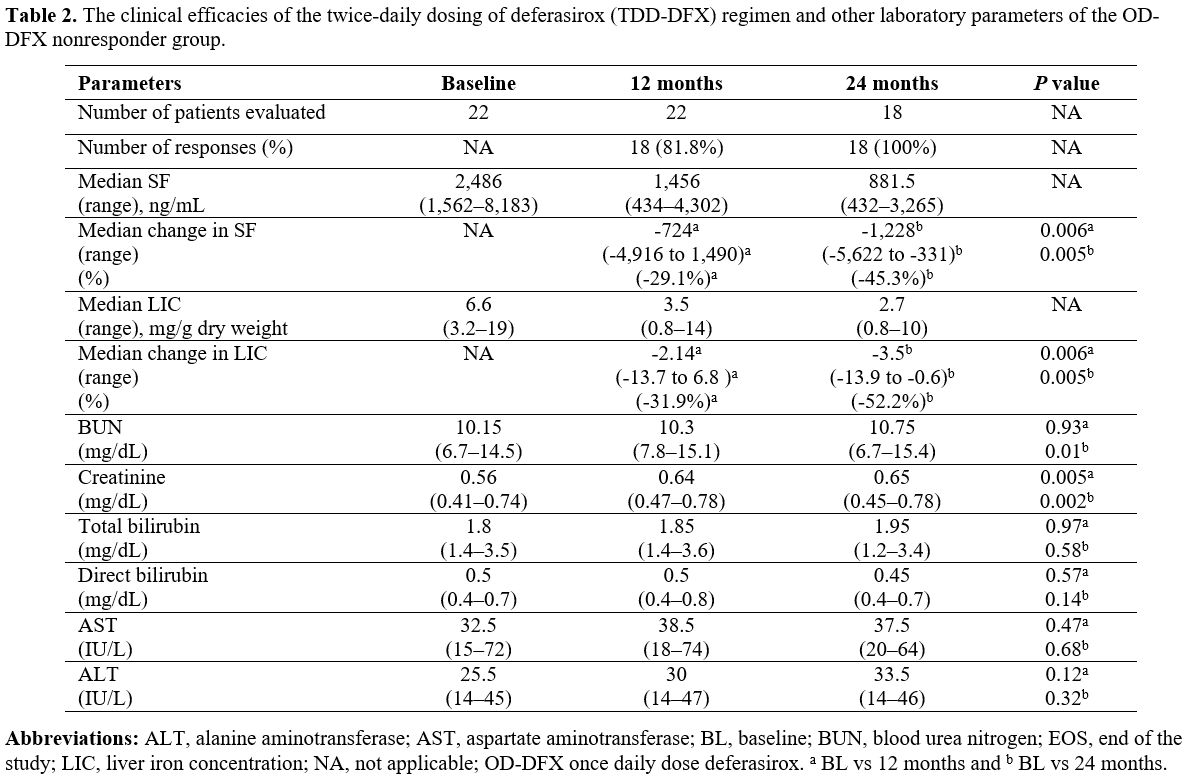 |
Table 2. The clinical
efficacies of the twice-daily dosing of deferasirox (TDD-DFX) regimen
and other laboratory parameters of the OD-DFX nonresponder group. |
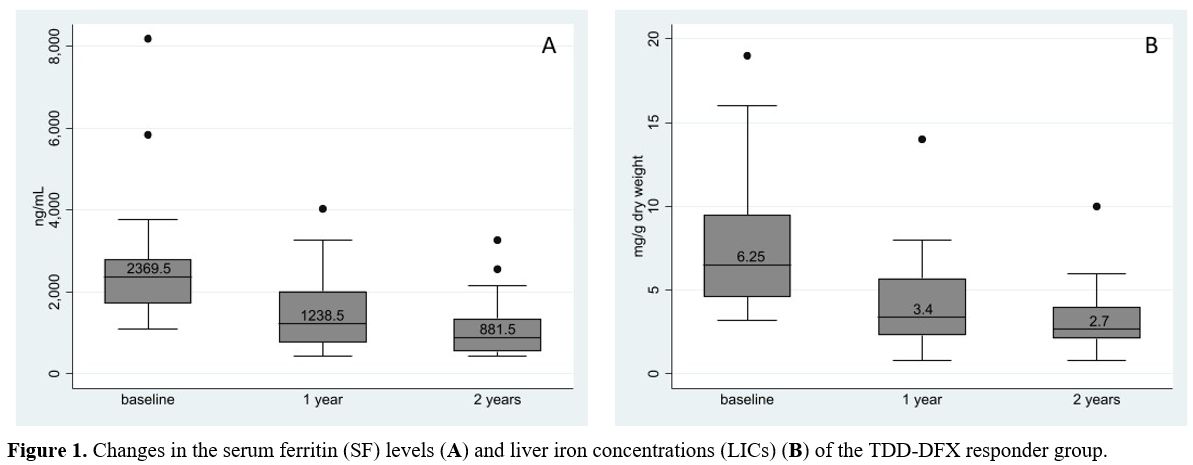 |
Figure 1. Changes in
the serum ferritin (SF) levels (A) and liver iron concentrations (LICs)
(B) of the TDD-DFX responder group. |
Discussion
Although
the survival of patients with TDT has improved dramatically with the
use of effective iron chelators, research showed that 40% of patients
had poor responses to standard monotherapy.[7,8] For example, in our previous study, 44% of patients with TDT were OD-DFX nonresponder.[5]
Fortunately, the alternative approach of using TDD-DFX can modify the
response in the majority of such recalcitrant cases (81.8%). In the
current investigation, 18% (4 of 22) of the TDD-DFX nonresponders
required other chelations (Figure 2). As we previously reported, the combination of DFX and another chelator should be considered for such patients.[9]
Hepatic siderosis was common in our study. All those with analyzable
LICs experienced liver siderosis, and 40% of such patients were
classified as having moderate to severe disease.
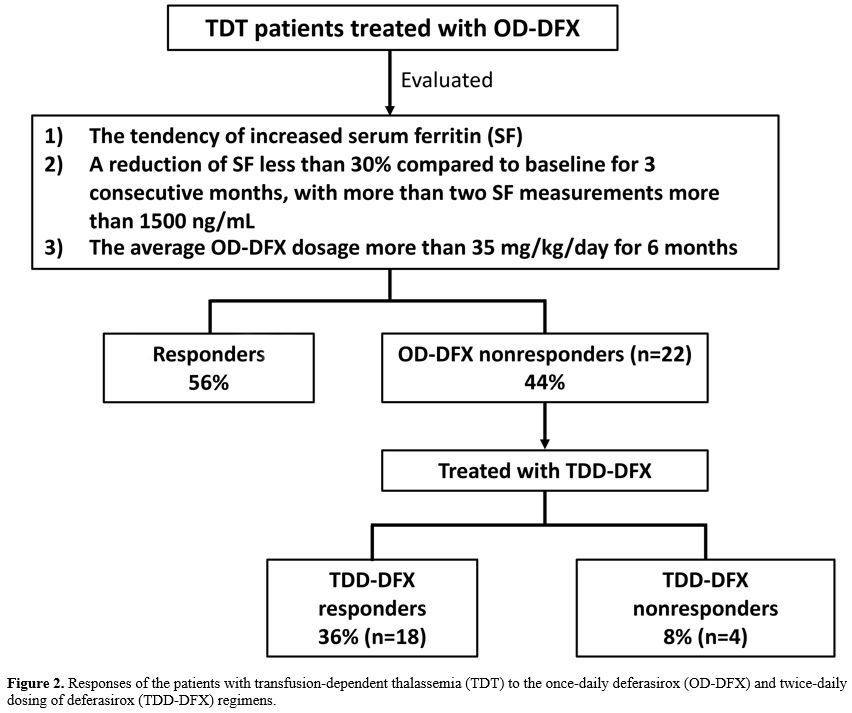 |
Figure 2. Responses of the
patients with transfusion-dependent thalassemia (TDT) to the once-daily
deferasirox (OD-DFX) and twice-daily dosing of deferasirox (TDD-DFX)
regimens. |
Nevertheless,
those with moderate to severe hepatic siderosis still responded to the
TDD-DFX regimen. Likewise, other clinical factors of the TDD-DFX
responder and TDD-DFX nonresponder groups were comparable, for example,
SF level, age, and sex. Thus, this response may highlight the value of
TDD-DFX therapy even for patients with a high iron accumulation.
A
study of TDD-DFX in an animal model revealed that it might yield a
broader suppression of non-transferrin-bound iron and a lower peak
concentration than OD-DFX, resulting in improved efficacy and decreased
toxicity, respectively.[10] Similarly, a
pharmacokinetic study in humans demonstrated that TDD-DFX tended to
produce higher Cmax and Ctrough values than OD-DFX, especially at a
dosage exceeding 30 mg/kg/day. Moreover, the difference between the
Cmax and Ctrough values for TDD-DFX was less than that for OD-DFX.
Hence, a more sustainable drug level with TDD-DFX may account for the
improved chelation results in patients who failed to respond to OD-DFX.
A clinical study conducted by Chang and associates demonstrated that
the efficacy of TDD-DFX was equivalent to that of OD-DFX during a
6-month treatment period, with a favorable tolerability for patients
experiencing gastrointestinal intolerance from OD-DFX.[3]
In a longer study of 1 year, Pongtanakul and coauthors reported the
clinical efficacy of TDD-DFX for children with TDT who had responded
poorly to the standard OD-DFX therapy. They found that TDD-DFX improved
the children's chelation response and had a favorable safety profile.[5]
Other studies also demonstrated the feasibility of TDD-DFX in terms of
reduced iron deposition and improved tolerability. However, the
evaluations of most of those investigations were based solely on the SF
levels at the 6-month treatment timepoint.[11,12] An
exception was the work of Karimi and colleagues, which used a
combination of the SF level and LIC as well as a 1-year follow-up.[13]
In the present study, the response to TDD-DFX was sustained for more
than one year when evaluated with SF and LIC. In terms of tolerability,
our study revealed a statistically significant increase in the serum
creatinine level from the baseline value to the 1-year follow-up.
Nevertheless, the rise seemed to be mild and nonprogressive since the
median serum creatinine level at the 2-year time point was static and
within the normal range for age. Furthermore, all patients could
continue the TDD-DFX therapy without any treatment interruption caused
by gastrointestinal disturbance or other adverse events. As the TDD-DFX
appears to have favorable safety profiles, it should be considered for
those experiencing side effects of OD-DFX before deciding to change to
other chelations.
This study has some limitations. Firstly,
consistent with the nature of retrospective studies, some data were
missing. More specifically, the LICs could not be evaluated for some
patients because of the generally limited ability of young children to
cooperate; the evaluation in some patients was solely based on SF.
Hence, the LICs that were obtained may not truly reflect the overall
outcomes of the study cohort. Moreover, due to the small number of
patients, the efficacy and toxicity of the study may not be salient.
Furthermore, factors that could be used to predict the response to
OD-DFX therapy could not be determined by this study. Lastly, the study
could not evaluate the effect of TDD-DFX on cardiac siderosis since all
22 patients had normal MRI T2* values.
Conclusions
To
summarize, TDD-DFX may be regarded as a second-line therapy for those
unresponsive to OD-DFX. Further studies with a larger cohort, a longer
follow-up, and pharmacogenetic analyses of inadequate-response patients
are warranted to elucidate the efficacy and safety profile of TDD-DFX.
References
- Fucharoen S, Winichagoon P. Thalassemia in
SouthEast Asia: problems and strategy for prevention and control.
Southeast Asian J Trop Med Public Health. 1992;23:647-55
- Cappellini
MD, Bejaoui M, Agaoglu L, et al. Iron chelation with deferasirox in
adult and pediatric patients with thalassemia major: efficacy and
safety during 5 years' follow-up. Blood. 2011;118:884-93. https://doi.org/10.1182/blood-2010-11-316646 PMid:21628399
- Chang
HH, Lu MY, Liao YM, et al. Improved efficacy and tolerability of oral
deferasirox by twice-daily dosing for patients with
transfusion-dependent beta-thalassemia. Pediatr Blood Cancer.
2011;56:420-4. https://doi.org/10.1002/pbc.22826 PMid:21072825
- Pennell
DJ, Porter JB, Cappellini MD, et al. Deferasirox for up to 3 years
leads to continued improvement of myocardial T2* in patients with
β-thalassemia major. Haematologica. 2012;97:842-8. https://doi.org/10.3324/haematol.2011.049957 PMid:22271905 PMCid:PMC3366648
- Pongtanakul
B, Viprakasit V. Twice daily deferasirox significantly improves
clinical efficacy in transfusion dependent thalassaemias who were
inadequate responders to standard once daily dose. Blood Cells Mol Dis.
2013;51:96-7. https://doi.org/10.1016/j.bcmd.2013.03.004 PMid:23566753
- Saiviroonporn
P, Viprakasit V, Maneesai A, et al. Inter-site validations of the
Pixel-Wise method for cardiac T2* analysis in transfusion-dependent
Thai thalassemia patients. Journal of the Medical Association of
Thailand = Chotmaihet thangphaet. 2012;95 Suppl 2:S165-72 https://doi.org/10.5144/1658-3876.2012.91 PMid:22828372
- Chirnomas
D, Smith AL, Braunstein J, et al. Deferasirox pharmacokinetics in
patients with adequate versus inadequate response. Blood.
2009;114:4009-13. https://doi.org/10.1182/blood-2009-05-222729 PMid:19724055 PMCid:PMC2774541
- Viprakasit
V, Nuchprayoon I, Chuansumrit A, et al. Deferiprone (GPO-L-ONE®)
monotherapy reduces iron overload in transfusion-dependent
thalassemias: 1-year results from a multicenter prospective, single
arm, open label, dose escalating phase III pediatric study (GPO-L-ONE;
A001) from Thailand. American Journal of Hematology. 2013;88:251-60. https://doi.org/10.1002/ajh.23386 PMid:23460233
- Takpradit C, Viprakasit V. Using of deferasirox and deferoxamine in refractory iron overload thalassemia. 2021;63:404-9. https://doi.org/10.1111/ped.14444 PMid:32856363
- Otto-Duessel
M, Aguilar M, Nick H, Moats R, Wood JC. Comparison of twice-daily vs
once-daily deferasirox dosing in a gerbil model of iron cardiomyopathy.
Experimental hematology. 2007;35:1069-73. https://doi.org/10.1016/j.exphem.2007.04.001 PMid:17588475 PMCid:PMC2892931
- Salehifar
E, Karami H, Kosaryan M, et al. Efficacy of Oral Deferasirox by
Twice-daily Dosing in Patients with Transfusion-dependent Beta
Thalassemia. Journal of Mazandaran University of Medical Sciences.
2015;25:1-8
- Gumruk F, Unal S, Bayhan T,
Hazirolan T, Tuncer AM, Cetin M. Twice Daily Use of Deferasirox Is More
Effective in Decreasing Serum Ferritin. Blood. 2014;124:2675-. https://doi.org/10.1182/blood.V124.21.2675.2675
- Karimi
M, Haghpanah S, Bahoush G, et al. Evaluation of Efficacy, Safety, and
Satisfaction Taking Deferasirox Twice Daily Versus Once Daily in
Patients With Transfusion-Dependent Thalassemia. J Pediatr Hematol
Oncol. 2020;42:23-6. https://doi.org/10.1097/MPH.0000000000001596 PMid:31568183
[TOP]



