Benedetta Fumarola1,2, Stefano Calza3, Stefano Renzetti3, Issa El Hamad1, Maria C. Pezzoli1, Ilaria Izzo1, Melania Degli Antoni1,2, Annacarla Chiesa1,2, Maria De Francesco4, Eugenia Quiros-Roldan1,2, Arnaldo Caruso4, Francesco Castelli1,2 and Emanuele Focà1,2.
1Division of Infectious and Tropical Diseases, ASST Spedali Civili, University of Brescia, Brescia, Italy.
2 Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
3
Unit of Biostatistics and Bioinformatics, Department of Molecular and
Translational Medicine, University of Brescia, Brescia, Italy.
4
Institute of Microbiology, Department of Molecular and Translational
Medicine, University of Brescia – ASST Spedali Civili, Brescia, Italy.
Published: March 1, 2022
Received: September 15, 2021
Accepted: February 6, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022016 DOI
10.4084/MJHID.2022.016
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Human
Immunodeficiency Virus type 2 (HIV-2) affects a minority of patients in
Italy; nevertheless, the increasing migratory flow from higher
prevalence areas led to the spread of this virus into our Country. We
evaluate clinical, viro-immunological, and therapeutic characteristics
of patients with HIV-2 infection and HIV-1/HIV-2 dual-infection and the
early treatment impact on overall survival and incidence of AIDS
events.
Methods: We
retrospectively analyzed all HIV-2, and HIV-1/HIV-2 positive patients
followed in a large Italian clinic from January 1987 to December 2020.
We recorded demographic, viro-immunological, clinical, and therapeutic
data. We performed a descriptive analysis followed by a longitudinal
analysis to explore the factors associated with the CD4+ lymphocyte
trend; lastly, we studied the possible predictors of death and AIDS in
our cohort in a multivariable model.
Results: 32
subjects were enrolled, 17 (53%) HIV-2 infected and 15 (46.8%)
HIV-1/HIV-2 dual-infected; 12 patients were lost to follow up, while 3
died. We found a lack of HIV-2 viremia in 12/32 subjects (37.5%). Most
of the patients at baseline had a good viro-immunological profile with
HIV-2 RNA <200 copies/ml and CD4+ lymphocyte >200 cell/mcl. We
found a CD4+ lymphocyte improvement over time, both in the absolute
number (𝛽 472.61, 95%CI 383.05-562.18, p<0.001) and in percentage
(𝛽 25.28, 95%CI 21.91 – 28.66, p<0.001). Nevertheless, subjects
taking cART had CD4+ lymphocyte percentage increase over time, and this
trend appeared significantly better than those who did not receive
therapy. Lastly, in the multivariable model CD4+, T-cell count increase
was negatively associated with AIDS (HR 0.34 95%CI 0.13-0.91, p=0.032).
Conclusion: We
found a higher prevalence of HIV-1/2 dual infection than in previous
observations. Subjects with HIV-2 infection showed a favorable
immunological condition at diagnosis, and the benefits of cART in those
who received treatment are undiscussed. Moreover, our data suggest a
different disease course based on age at diagnosis, as in HIV-1
infections. We encourage starting cART at diagnosis in HIV-2 patients,
regardless of CD4+ lymphocyte, because even in the new cART era, CD4+
lymphocyte decrease remains the strongest predictor of death and AIDS
also in this population.
|
Introduction
In 2019 the World Health Organisation (WHO) estimated that HIV infection affected approximately 38 million people worldwide.[1]
Most of the statistics are built based on data of HIV-1 infection;
however, HIV-2 is very peculiar for its epidemiological, virological,
and clinical characteristics. It started as a zoonosis from the SIV of
the sooty mangabey.[2,3]
To date, at least nine
groups of HIV-2 have been described (termed A-I), with groups A and B
being the most prevalent and the only two groups that have continued to
transmit from person to person.[4,5]
The two
viruses have similar transmission routes and cellular targets, but
HIV-2 remains much less transmissible than HIV-1 and shows differences
in the natural history of infection.[6,7]
Because
of the lower prevalence and the less virulence of HIV-2, accurate
estimates on the number of cases are lacking. By crude estimates, in
the 1990s, one or two million patients were infected with HIV-2.[8,9]
In the United States of America, to enumerate and describe HIV-2 cases,
a working case definition was developed, and, during 1988 - June 2010,
a total of 242 HIV-2 cases were reported to CDC.[10] In Italy there is a lack of systematic informations about the epidemiology of this infection.[11] HIV-2 prevalence has declined in several West African countries during the last two decades, but the reasons are unclear.[12]
Dual
HIV-1 and HIV-2 mixed infections have been reported mostly in West
Africa, where the two viruses are endemic (HIV-2) and epidemic (HIV-1)
and where it is estimated that approximately 5-10 percent of HIV-1
infected individuals are coinfected with HIV-2.[13]
Limited
data exist on the natural history of HIV-1/ HIV-2 mixed infections.
However, a meta-analysis performed in 2014 showed no evidence that
HIV-2 delays progression to death in HIV-1/HIV-2 coinfected patients,[14] and initial infection with HIV-2 does not appear to protect against a subsequent HIV-1 acquisition.[15]
On the other hand, one study suggests that people with both viruses
have a slower progression of the disease and a delayed death when
compared with people who have HIV-1 alone, with the greatest benefits
when HIV-2 infection occurs earlier than HIV-1.[16]
Several
studies showed that HIV-2 is generally less pathogenic than HIV-1, with
a longer asymptomatic stage of infection, a slower decline of CD4
T-cell count, and a lower level of plasma viremia.[17]
There
are no antiretroviral medications approved by the US Food and Drug
Administration for HIV-2, and the selection of combination
antiretroviral therapy (cART) for these patients appears to be
complicated because of the lack of randomized clinical trial data
giving indications on when to start and on the choice of initial or
subsequent cART regimens. In fact, all the antiretroviral therapies
have been developed against HIV-1, and many are inactive against HIV-2.
HIV-2 is intrinsically resistant to the non-nucleoside reverse
transcriptase inhibitors (NNRTIs) and the fusion inhibitor,
enfuvirtide. Moreover, the only protease inhibitors active against
HIV-2 are lopinavir and darunavir, both boosted by ritonavir or
cobicistat. International guidelines[18] suggest
starting the antiretroviral treatment in HIV-2 infected patients with
an integrase inhibitor (INI) plus 2 NRTIs or, as a second-line regimen,
to choose boosted protease inhibitors (PIs) active against HIV-2 plus 2
NRTIs.
The existing data suggest starting antiretroviral therapy
at or soon as the diagnosis of HIV-2 infection is made to prevent
disease progression and transmission of HIV-2 to others.[18]
Physicians
need to face some problems when making decisions about patients living
with HIV-2 infection. The first one is the lack of standardized
guidelines for HIV-2 infection treatment; the decision to start therapy
is not methodical because of the lower virulence, and the minor damage
HIV-2 can cause to the host. In addition, plasmatic viremia is often
not detectable or very low, even without any treatment. The lack of
cohort of patients with HIV-2 infection is another issue; the available
literature is set up on study with a few patients, and data are
fragmented and often set up as case reports, not as real observational
studies.
The aim of this study is to evaluate clinical,
viro-immunological, and therapeutic characteristics of a cohort of
patients with HIV-2 infection and HIV-1/HIV-2 co-infection and to
assess the treatment impact on the viro-immunological conditions and
incidence of AIDS events.
Methods
The
present was a retrospective, observational single-center study. We
analyzed all ART-naïve, and ART-experienced HIV-2 infected, and
HIV-1/HIV-2 coinfected adult patients followed at the outpatient Clinic
of Infectious and Tropical Disease Department, University of Brescia
and ASST Spedali Civili Hospital of Brescia, Italy, from January 1987,
when patients were included in our cohort, to December 2020.
Data
were retrieved from medical records and included gender, age, medical
history, risk factors for HIV acquisition, viro-immunological
characteristics including CD4+ T-cell, CD8+ T-cell, CD4+/CD8+ ratio,
plasma HIV-1 and HIV-2 viral load, presence of single HIV-2 infection
or HIV-1/HIV-2 co-infection, antiretroviral regimen prescribed, therapy
starting year and presence of AIDS event. After initiation into care,
patients were typically followed every six months. Patients' outcomes
included death, loss to follow-up, or still in care.
HIV-2 was
diagnosed through WB and/or immunoblotting in case screening test was
suspected of HIV-2 infection. HIV PCR was required, if available, in
case of HIV-2 positive WB or in patients coming from endemic areas with
characteristics compatible with HIV-2 infection.
HIV 1 and 2 viral
loads were detected with biomolecular methods that changed during the
observational time. HIV-1 RNA was detected with Versant HIV-1 RNA 1.5
Assay (kPCR) from 1997 until the end of the data collection. For HIV-2
RNA, the first commercial test used by our laboratory was HIV-2
Real-Time RT-PCR Kit, followed from 2014 by Human Immunodeficiency
Virus type 2 genesig Standard Kit. We divided our sample in two groups:
patients with HIV-2 infection (Group 1) and patients with HIV-1 and HIV-2 mixed infection (Group 2).
We compared the two groups and assessed whether there was any
significant difference between viro-immunological conditions,
demographic characteristics, number of AIDS events, and antiretroviral
treatment received. Regarding HAART, we compared whether there was any
difference in the viro-immunological conditions between subjects who
started therapy and those who did not.
A favorable viro-immunological profile
(FP) was defined as the simultaneous presence of a CD4+ T-cell count
> 200 cells/µL and an HIV2 RNA < 200 cp/ml, while any other
combination defined a non-favorable viro-immunological profile (nFP). Early treatment start was defined as the initiation of cART within one month from the diagnosis of HIV-2 infection.
Statistical analysis.
Continuous and categorical variables were summarized by the median and
interquartile range and by count and percentages. A Kruskal-Wallis test
was applied for continuous variables, while a chi-square test was
performed for categorical variables to look at differences between
Group 1 and Group 2. A linear mixed effect model was then applied to
test for the trend over time of CD4+ lymphocyte (absolute and
percentage) between age and therapy groups. We finally fitted a Cox
proportional hazard regression model to test for the effect of CD4+
lymphocyte, age, and therapy on time to death and AIDS events.
Ethics.
This study was approved by the Ethical Board of Brescia Provence and
conducted according to the Declaration of Helsinki and to principles of
Good Clinical Practice (GCP). As this study had a retrospective design
and was based on routinely collected data, patients' informed consent
was not required according to the Italian law (Italian Guidelines for
classification and conduction of observational studies, established by
the Italian Drug Agency, "Agenzia Italiana del Farmaco – AIFA" on March
20, 2008). Moreover, for this study, we used the general authorization
of the Italian Guarantor for the use of retrospective demographical and
clinical data, which have been anonymized and treated according to
current Italian laws.
Results
In
our study, we enrolled 32 patients (47% female), 17 (53%) with HIV-2,
and 15 (46.8%) with an HIV1/HIV2. During the observational period, 12
patients were lost to follow-up after a median of 8 years, while 3
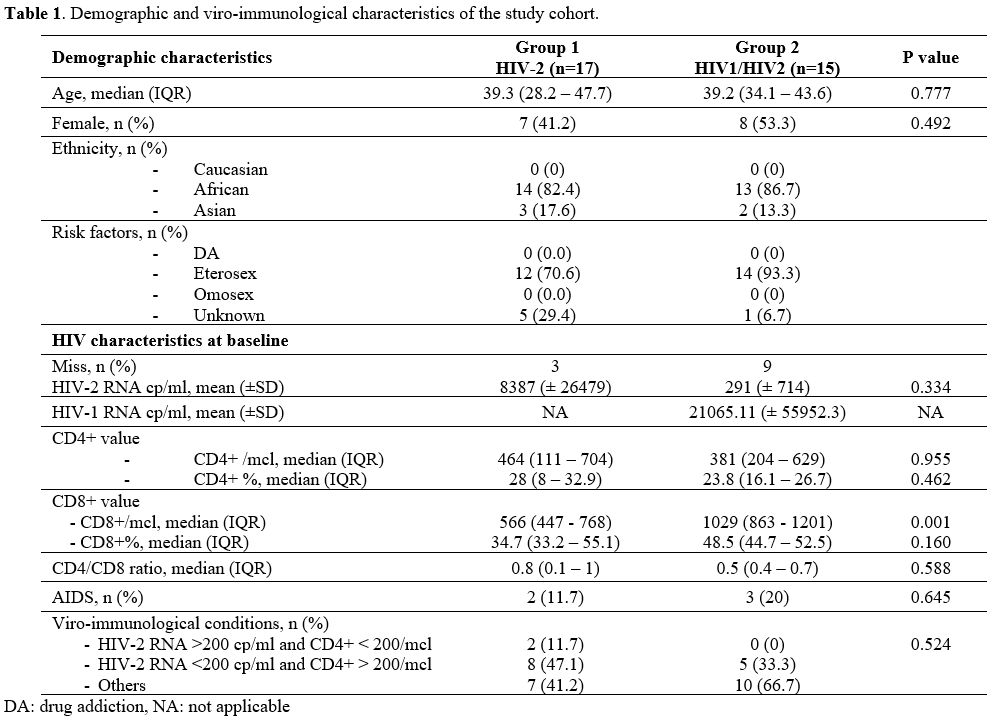
died. The baseline characteristics of our population are summarized in Table 1.
 |
Table
1. Demographic and viro-immunological characteristics of the study cohort. |
Demographic characteristics.
The mean age and sex of patients of the two groups were similar, with
39.3 years old (IQR 28.2 - 47.7) and 58.8% males in the mono-infected
group and 39.2 years old (IQR 34 - 43.6) and 46.7% males in the other
group.
Twenty-seven patients (84.4%) came from Africa, 13 with
HIV-2 infection and 14 with co-infection with HIV-1. We had no South
American or Caucasian patients with HIV-2, and just 15.6% (5/32) came
from Asia, two with co-infection and three with HIV-2 infection.
Viro-immunological profile and AIDS events.
We found a lack of HIV-2 viremia at baseline in 12/32 (37.5%) subjects.
However, between the 20 patients with an available HIV-2 RNA value at
baseline, we did not find any significant difference between the
viro-immunological conditions of the two groups: we found a similar
proportion of subjects with a favorable viro-immunological profile
[8/17 (47%) in the mono-infected group and 5/15 (33.3%) in the
coinfected group]. At baseline, males had a lower CD4/CD8 ratio (mean
CD4/CD8 ratio, 0.52 vs 0. 96, P=0.048) than females.
Likewise, at
baseline we found a similar median number of CD4+ T-cell between HIV-2
and HIV-1/HIV-2 coinfected patients [percentage, 28 % (IQR 8 - 32.9) vs
23.8 % (IQR 16.1 - 26.7), p=0.462; absolute, 464 cell/mcl (IQR 111 -
704) vs 381 cell/mcl (IQR 204 - 629), p=0.955]. Regarding CD8+ T-cell
value, patients infected by both viruses had a higher value of absolute
CD8+ T-cells [median CD8+ 1029 cell/mcl (IQR 863 - 1201) vs 566
cell/mcl (IQR 447 - 768), p=0.001].
Only two patients of Group 1 developed AIDS defining illnesses: one developed Pneumocystis jirovecii
pneumonia and the other was diagnosed with chronic intestinal
isosporidiosis; in both cases, the diagnosis was made when they were
first diagnosed with HIV infection. A similar percentage was found in
the coinfected group: one patient developed only one AIDS event
(pulmonary tuberculosis), two developed two AIDS-defining events (one
pulmonary tuberculosis and non-Hodgkin lymphoma, and the other one
disseminated cytomegalovirosis and neurotoxoplasmosis).
Antiretroviral therapy.
In our cohort, six patients (18.7 %) never started therapy (five with
HIV-2 infection and one with HIV-1/HIV-2 co-infection), five of whom
were lost to follow up after a median of 8 years (IQR 4.5-15) from
diagnosis. At baseline, they all had a favourable viro-immunological
status compared to those who started it, a significant higher value of
CD4+ lymphocyte, both absolute [median CD4 762.5 cell/mcl (IQR
712.5-867.3) vs 324.5 (87.8, 538.3), p<0.001] and percentage [42.6%
(IQR 33.7-47.875) vs 21.8 (9.9-27.4), p<0.001], and a significant
higher CD4+/CD8+ ratio [1.4 (IQR 1-1.8) vs 0.5 (0.16-0.8), p=0.02].
Their viro-immunological status remained favorable during the time, and
they never developed any AIDS event.
Twenty-six patients started
therapy during the observational period, seven of whom were lost to
follow-up after a median of 7 years (IQR 2-12) from diagnosis. They
were all under treatment at the last visit of follow-up recorded. Of
those who started therapy, eight patients (30.1%) started treatment
within one month from diagnosis (early treatment). Among those who did not experience an early treatment,
most (66.7%) started HAART later than 6 months from diagnosis. The most
used first-line cART regimen was a protease-inhibitor (PI) based
regimen, chosen for 8/12 HIV-2 patients and 7/14 HIV-1/HIV-2 coinfected
patients on cART (Table 2). The
most frequent PI-based regimen was lopinavir-ritonavir, prescribed in
3/12 (25 %) HIV-2 patients and 2/14 (14.3 %) HIV-1/HIV-2 coinfected
patients on cART.
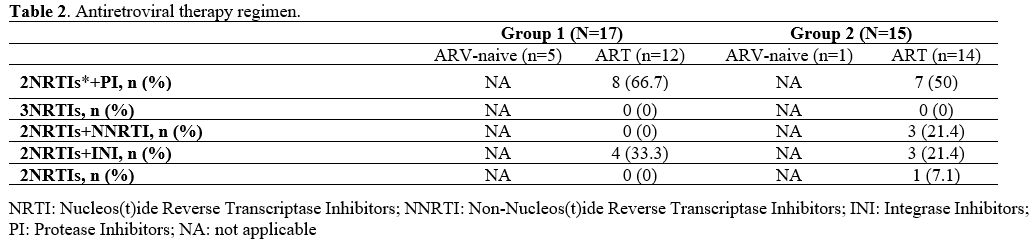 |
Table 2. Antiretroviral therapy regimen. |
NNRTIs
were chosen for 3/14 (21.4%) HIV-1/HIV-2 coinfected patients: one
started with 2 NRTIs plus efavirenz, and two started with 2 NRTIs plus
nevirapine. Two of them improved their immunological conditions during
the follow-up, while one remained stable. None of them developed any
AIDS-defining conditions.
None of the HIV-2 mono-infected
group started with an NNRTI-based or an NRTI-based regimen. An
INI-based regimen was chosen for 4/12 (33.3%) HIV-2 infected and 3/14
(21.4%) HIV-1/HIV-2 coinfected patients on cART. The most prescribed
backbone regimen in HIV-2 infected patients was tenofovir disoproxil or
tenofovir alafenamide plus emtricitabine, initiated in 10/12 (83.3%)
patients. In HIV-1/HIV-2 coinfected patients, the most frequent
first-line backbone regimen was represented by zidovudine plus
lamivudine (42.9%).
Even though the immunological status of the untreated patients remained favorable
over time, we noted that both CD4 absolute and CD4 percentage counts in
subjects who started therapy showed an increasing trend over time
compared to those who did not start it. The difference of the trends
over time between the two groups was statistically significant (CD4 +:
beta 18.96, 95% CI 8.56 - 29.36, p <0.001; CD4% beta 1.12, 95% CI
0.84 - 1.40, p <0.001) (Figure 1).
Lastly,
as we expected, in the multivariable model, CD4+ T-cell count increase
was negatively associated to AIDS (HR 0.34 95%CI 0.13-0.91, p=0.032).
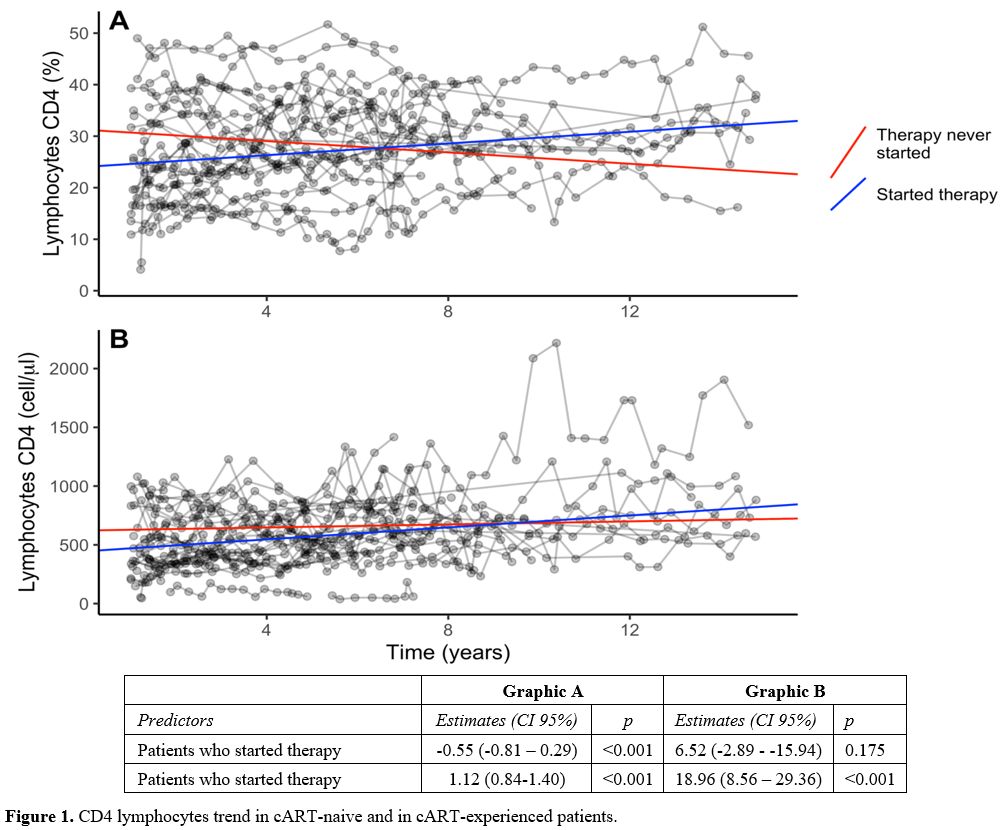 |
Figure
1. CD4 lymphocytes trend in cART-naive and in cART-experienced patients. |
Discussion
In
this study, we found that the benefits of cART are undiscussed even in
patients with a favorable viro-immunological condition at baseline. As
in HIV-1 patients, CD4+ lymphocyte reduction remains the strongest
proxy of death and AIDS also in this population.
At our
outpatients' Clinic, in 2020 we were following 3875 patients with HIV
infection. Despite this number, we could find only a small percentage
of HIV-2 infections or HIV-1/HIV-2 co-infection patients. That is in
line with what literature tells us about the distribution of this
infection globally, with most patients living in West Africa with a
limited spread to other regions.
Data estimate that in West Africa 5 to 10 percent of HIV-1 infected individuals are coinfected with HIV-2.
However,
in our Clinic, as we would expect in a European hospital, we found only
a minimum number of HIV-1 and HIV-2 coinfected patients (0.4 %), and
most of them were coming from Africa.
We did not find any
relevant difference in demographic and viro-immunological
characteristics between HIV-2 patients and HIV 1-2 coinfected patients.
Full amounts of data regarding HIV-2 infection are lacking.
Immunoblot antibody tests used to confirm HIV reactive screening tests
did not contain reagents specific to HIV-2 and, thus, were not reliable
for identifying HIV-2 infections.[19] Serological
tests do not distinguish between HIV-2 and HIV-1 infection, and,
especially in patients infected with both viruses, when the clinical
suspicion is not high, HIV-2 infection could be underestimated.
Additional testing specific to HIV-2 infection should be considered if
HIV-1 test results appear to be atypical or inconsistent with clinical
findings, especially if patients come from endemic Countries. Dual
infection can be proven by the presence of HIV-2 and HIV-1 DNA or RNA
by PCR isolation of both viruses, but plasma HIV-2 RNA may be
undetectable, and HIV-2 proviral DNA may be low or quickly negative in
some persons, making confirmation of HIV-2 infection difficult.[20]
An
early diagnosis is particularly important as HIV-2 results
intrinsically resistant to some drugs that are normally used to treat
HIV-1 infection, and late recognition of HIV-2 infection could lead to
a delayed start of the correct therapy.
Regarding patients with
HIV-1 and HIV-2 co-infection, we found that three patients started
treatment with an NNRTI-based regimen, known to be ineffective against
HIV-2. We think that this choice was made for misdiagnosis of HIV-2
infection. Nonetheless, they all maintained a virological suppression
during the time and had an increase in the CD4+ T-cell count. One of
them died after two years due to a non-AIDS-related event, while the
other two subjects switched the cART regimen due to toxicity.
Although
patients with HIV-2 infection seem to have a slower CD4+ lymphocyte
decline and a slower disease progression, we notice that those who were
taking cART had a CD4+ lymphocyte percentage increase over time and
that the CD4+ T-cell count increase was negatively associated with
death or AIDS, as we usually see in HIV-1 mono-infected individuals.
We
encourage starting cART at diagnosis in HIV-2 patients, regardless of
CD4+ lymphocyte, because even in the new cART era, CD4+ lymphocyte
decrease remains the strongest predictor of death and AIDS also in this
population.
The study's limitations are the lack of data regarding HIV-2 in patients diagnosed before 2000th,
the observational retrospective design of the study, and the diagnostic
technique that are changed during the observational period.
Conclusions
This
study confirmed the benefits of antiretroviral therapy in those who
received cART and demonstrated that the failure to initiate treatment
significantly reduces the circulating CD4+ T cells over time,
increasing the risk of death and AIDS-defining events development.
These findings are important in patients with HIV-2 infection, where
the clinical evolution and worsening of the viro-immunological
parameter are often underrated.
As in HIV-1 infected patients,
the decrease in CD4 T-cells remains the strongest indicator of death
and AIDS events in this population. We can also confirm the importance
of regular follow-up for these patients, with periodic control of
viremia and CD4+ lymphocyte.
Considering the continuous
migratory flows from regions where HIV-2 is endemic, the small number
of patients, and the lack of some data regarding HIV-2, it will be
necessary to carry out further studies, preferably multicentre and
using standardized diagnostic techniques, to characterize this
infection better.
References
- UNAIDS. http://www.unaids.org/en/ (Accessed on July 15, 2020).
- Gao
F, Yue L, White AT, Pappas PG, Barchue J, Hanson AP, et al. Human
infection by genetically diverse SIVSM-related HIV-2 in West Africa.
Nature. 1992;358(6386):495-9. https://doi.org/10.1038/358495a0 PMid:1641038
- Gao
F, Yue L, Robertson DL, Hill SC, Hui H, Biggar RJ, et al. Genetic
diversity of human immunodeficiency virus type 2: evidence for distinct
sequence subtypes with differences in virus biology. J Virol.
1994;68(11). https://doi.org/10.1128/jvi.68.11.7433-7447.1994 PMid:7933127 PMCid:PMC237186
- Ayouba
A, Akoua-Koffi C, Calvignac-Spencer S, Esteban A, Locatelli S, Li H, et
al. Evidence for continuing cross-species transmission of SIVsmm to
humans: Characterization of a new HIV-2 lineage in rural Côte d'Ivoire.
AIDS. 2013;27(15):2488-91. https://doi.org/10.1097/01.aids.0000432443.22684.50 PMid:23939239 PMCid:PMC3881176
- Santiago
ML, Range F, Keele BF, Li Y, Bailes E, Bibollet-Ruche F, et al. Simian
Immunodeficiency Virus Infection in Free-Ranging Sooty Mangabeys
(Cercocebus atys atys) from the Taï Forest, Côte d'Ivoire: Implications
for the Origin of Epidemic Human Immunodeficiency Virus Type 2. J
Virol. 2005;79(19):12515-27. https://doi.org/10.1128/JVI.79.19.12515-12527.2005 PMid:16160179 PMCid:PMC1211554
- Reeves JD, Doms RW. Human immunodeficiency virus type 2. Journal of General Virology. 2002. https://doi.org/10.1099/0022-1317-83-6-1253 PMid:12029140
- Peeters
M, Toure-Kane C, Nkengasong JN. Genetic diversity of HIV in Africa:
Impact on diagnosis, treatment, vaccine development and trials. Vol.
17, AIDS. 2003. p. 2547-60. https://doi.org/10.1097/00002030-200312050-00002 PMid:14685049
- Ariën
KK, Abraha A, Quiñones-Mateu ME, Kestens L, Vanham G, Arts EJ. The
Replicative Fitness of Primary Human Immunodeficiency Virus Type 1
(HIV-1) Group M, HIV-1 Group O, and HIV-2 Isolates. J Virol.
2005;79(14):8979-90. https://doi.org/10.1128/JVI.79.14.8979-8990.2005 PMid:15994792 PMCid:PMC1168791
- Gottlieb
GS, Raugi DN, Smith RA. 90-90-90 for HIV-2? Ending the HIV-2 epidemic
by enhancing care and clinical management of patients infected with
HIV-2. Vol. 5, The Lancet HIV. Elsevier Ltd; 2018. p. e390-9. https://doi.org/10.1016/S2352-3018(18)30094-8
- Centers
for Disease Control and Prevention (CDC). HIV-2 Infection
Surveillance--United States, 1987-2009. MMWR Morb Mortal Wkly Rep
[Internet]. 2011;60(29):985-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21796096
- Costarelli
S, Torti C, Rodella A, Baldanti F, Paolucci S, Lapadula G, et al.
Screening and management of HIV-2-infected individuals in Northern
Italy. AIDS Patient Care STDS. 2008;22(6):489-94. https://doi.org/10.1089/apc.2007.0149 PMid:18582190
- Gottlieb GS. Changing HIV epidemics: What HIV-2 can teach us about ending HIV-1. Vol. 27, AIDS. 2013. p. 135-7. https://doi.org/10.1097/QAD.0b013e32835a11a4 PMid:23221427 PMCid:PMC3726189
- Cock
KM, Adjorlolo G, Ekpini E, Sibailly T, Kouadio J, Maran M, et al.
Epidemiology and Transmission of HIV-2: Why There Is No HIV-2 Pandemic.
JAMA J Am Med Assoc. 1993 Nov 3;270(17):2083-6. https://doi.org/10.1001/jama.270.17.2083 PMid:8147962
- Prince
PD, Matser A, Van Tienen C, Whittle HC, Schim Van Der Loeff MF.
Mortality rates in people dually infected with HIV-1/2 and those
infected with either HIV-1 or HIV-2: A systematic review and
meta-analysis. Vol. 28, AIDS. 2014. p. 549-58. https://doi.org/10.1097/01.SPC.0000432532.87841.78 PMid:23921613
- Schim
van der Loeff MF, Aaby P, Aryioshi K, Vincent T, Awasana AA, Da Costa
C, et al. HIV-2 does not protect against HIV-1 infection in a rural
community in Guinea-Bissau. AIDS. 2001;15(17). https://doi.org/10.1097/00002030-200111230-00012 PMid:11698704
- Esbjörnsson
J, Månsson F, Kvist A, Isberg P-E, Nowroozalizadeh S, Biague AJ, et al.
Inhibition of HIV-1 Disease Progression by Contemporaneous HIV-2
Infection. N Engl J Med. 2012 July 19;367(3):224-32. https://doi.org/10.1056/NEJMoa1113244 PMid:22808957
- Visseaux
B, Damond F, Matheron S, Descamps D, Charpentier C. Hiv-2 molecular
epidemiology. Infect Genet Evol. 2016 December 1;46:233-40. https://doi.org/10.1016/j.meegid.2016.08.010 PMid:27530215
- DHHS
Panel. Guidelines for the Use of Antiretroviral Agents in Adults and
Adolescents with HIV. Dep Heal Hum Serv [Internet]. 2019;298. Available
from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
- De
Cock KM, Porter A, Kouadio J, Maran M, Lafontaine MF, Gershy-Damet GM,
et al. Cross-reactivity on Western blots in HIV-1 and HIV-2 infections.
AIDS. 1991;5(7):859-63. https://doi.org/10.1097/00002030-199107000-00010 PMid:1892591
- Ekouevi DK, Eholie SP. Update on HIV-1 and HIV-2 Dual Infection. In: Encyclopedia of AIDS. Springer New York; 2018. p. 2124-31. https://doi.org/10.1007/978-1-4939-7101-5_49
- Balestre
E, Eholié SP, Lokossue A, Sow PS, Charurat M, Minga A, et al. Effect of
age on immunological response in the first year of antiretroviral
therapy in HIV-1-infected adults in West Africa. AIDS. 2012 May
15;26(8):951-7. https://doi.org/10.1097/QAD.0b013e3283528ad4 PMid:22382142 PMCid:PMC3704338
[TOP]


