Aditi Singh1, Ishaq Asghar2, Laura Kohler1, Daniel Snower2, Hosam Hakim1 and Daniel Lebovic1.
1 Department of Hematology and Oncology, Ascension St. John Hospital.
2 Department of Pathology, Ascension St. John Hospital.
Correspondence to: Aditi
Singh, MD, Hematology-Oncology Fellow, Department of Hematology and
Oncology, Ascension St. John Hospital. E-mail:
aditisingh0215@gmail.com
Published: March 1, 2022
Received: September 28, 2021
Accepted: February 7, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022018 DOI
10.4084/MJHID.2022.018
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
In
the modern era, classification of neoplasms not only depends on
immunomorphological features but also on specific disease-defining
genetic events. Translocations/rearrangements of MYC/8q24 locus
combined with BCL-2 or BCL6 translocations (double/triple hit) are
considered hallmarks of high-grade B-cell lymphoma (HGBL), a type of
aggressive mature B-cell lymphoma. When cases with immature
immunophenotypes present these rearrangements, diagnosis becomes very
difficult. We herein report an unusual case of an aggressive B-cell
lymphoma/leukemia that presented with immature morphology and
immunophenotype with triple hit gene rearrangements. This case
highlights the difficulty in classifying and appropriately treating
these patients. The novel aspect is the treatment and outcome with
chimeric antigen receptor or CAR T-cell therapy.
|
Introduction
Classification
of hematological malignancies is an important and evolving process.
These malignancies are classified according to lineage and further
subclassified into mature and immature neoplasms based on morphology,
immunohistochemistry, and cytogenetics.[1] The
distinction is of utmost importance in determining correct treatment
modalities that differ significantly for mature and immature lymphoid
neoplasms.
When classifying lymphoid neoplasms, terminal
deoxynucleotidyl transferase (TdT) expression, CD34 expression, lack of
surface immunoglobulin light chains, and morphology can help
differentiate immature neoplasms from mature B cell neoplasms.[2]
Similarly, specific genetic alterations can be considered pathognomonic
for certain lymphomas aiding in their classification. Triple
hit/double-hit lymphomas are characterized by
translocations/rearrangements of MYC/8q24 locus in combination with
BCL-2 and/or BCL6 translocations. They are considered hallmarks of
high-grade B-cell lymphoma (HGBL).[3] HGBL is usually
TdT negative. TdT positive neoplasms with MYC and BCL2 and/or BCL6
rearrangements are classified as acute lymphoblastic lymphoma (ALL)
under the current 2016 World Health Organization (WHO) classification.[1]
Significant controversy exists about this classification. We report a
rare case of a TdT positive triple hit neoplasm with concomitant
follicular lymphoma, treated with CAR T-cell therapy.
Case
A
48-year-old Caucasian male with a past medical history of
gastroesophageal reflux and mitral valve prolapse presented with
fatigue, malaise, intermittent abdominal pain, and loose stools which
started about one week prior to presentation. Vital signs were stable
and physical examination was unremarkable. Laboratory evaluation
revealed hemoglobin of 14.7gm/dL; white blood cell counts 32920 cells/μL and platelet count 44000 cells/μL. Differential count showed absolute neutrophil count (ANC) 13830 cells/μL, absolute lymphocyte count (ALC) 6910 cells/μL, absolute monocyte counts 2300 cell/μL, and 30% blasts (Figure 2 a).
Lactate dehydrogenase (LDH) >1800IU/L and uric acid 8.8mg/dL. CT
scan of the chest, abdomen, pelvis showed few non-enlarged axillary
lymph nodes, few non-enlarged axillary lymph noded, enlarged
paratracheal and subcarinal lymph nodes measuring 1.3 cm and 4.9 cm
respectively, gastrohepatic ligament lymph node measuring 3 cm, the
portal caval lymph node measuring 2.3 cm, peripancreatic lymph node
measuring 2.8 cm, retroperitoneal lymph node measuring 2.9 x 4.5 cm,
left renal hilar lymph node measuring 3.7 cm, enlarged common iliac
lymph node measuring 2 cm, and mildly enlarged external iliac lymph
nodes measuring 1.1 cm short axis and bilaterally. Enlarged bilateral
inguinal lymph nodes were also noted (Figure 1.a and 1.b).
HIV (human immunodeficiency virus) and EBV (Epstein-Barr virus) tests
were negative. A right iliac bone marrow biopsy was performed (Figure 2.b and 2.c).
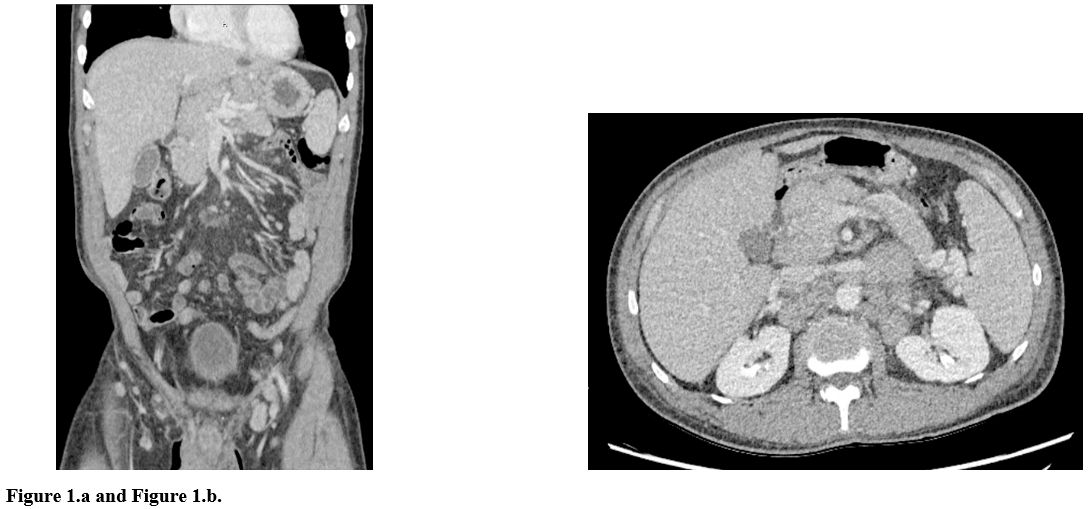 |
Figure 1.a and Figure 1.b. |
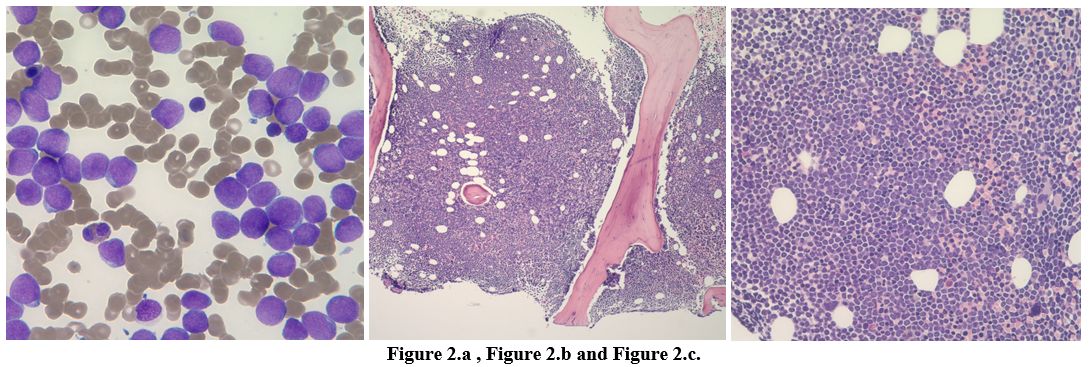 |
Figure 2.a, Figure 2.b and Figure 2.c. |
The bone marrow core biopsy was hypercellular for age (>85% cellularity), with 90% blast-like cells infiltrating the marrow (Figures 2.b, 2.c).
The neoplastic cells were large with fine nuclear chromatin, prominent
nucleoli, and light basophilic cytoplasm. No Auer rods were identified.
Flow cytometry showed monoclonal B-cells positive for CD10, CD19, CD38,
and HLA-DR. Immunohistochemical staining showed blasts to be positive
for TdT, c-MYC, BCL2, and BCL6(dim); negative for CD20 (Figure 3).
A diagnosis of acute B-cell lymphoblastic leukemia (B-ALL) was made.
BCR-ABL by polymerase chain reaction (PCR) and molecular testing was
negative. B-ALL specific FISH (fluorescence in situ hybridization)
analysis was performed which included TCF3/PBX1 (E2A/PBX1) t(1;19),
trisomy or tetrasomy 4, 6, 10, 17 (Cen 4, Cen 6, Cen 10, Cen 17), MYC
(8q24), BCR/ABL1/ASS1 t(9;22), MLL (11q23), IgH (14q32). Only IgH
rearrangement was positive.
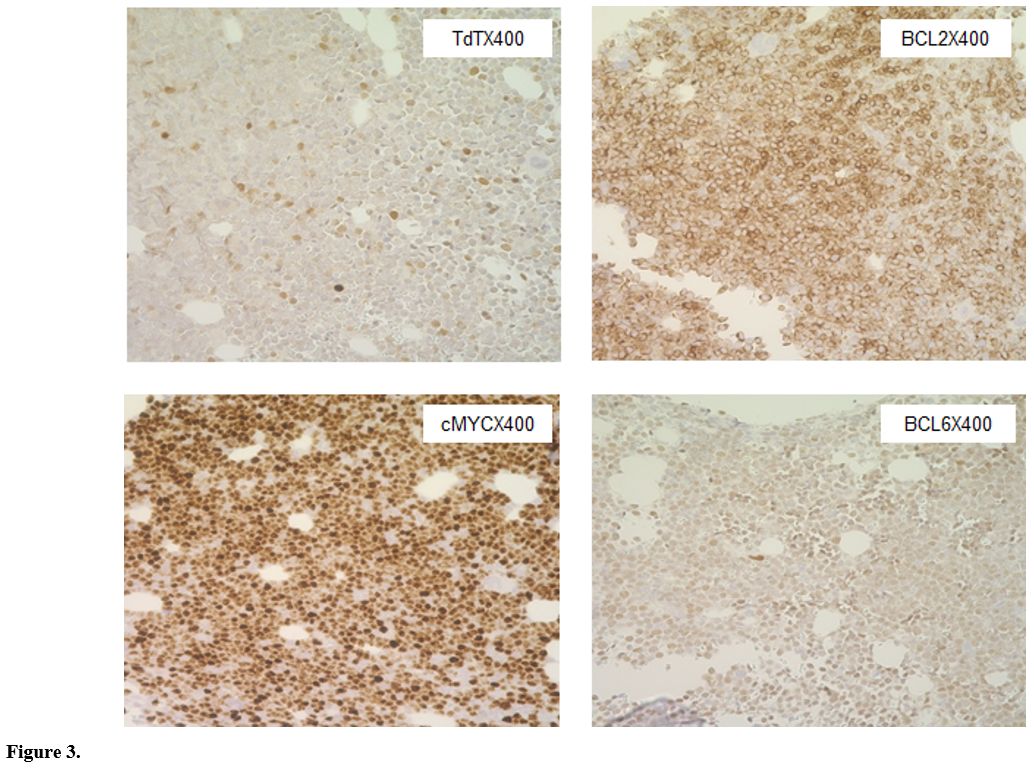 |
Figure 3. |
Given
the initial diagnosis of Philadelphia-negative B-ALL, he was started on
induction chemotherapy with hyper-CVAD, without rituximab, as CD20 was
negative.
Subsequent cytogenetic analysis showed abnormal male
karyotype, dup (1)(q11q44), t(3,6)(q25; q13), t(8;22)(24.1; q11.2), and
t(14;18)(q32; q21) in fourteen of twenty examined cells. This was a
very atypical karyotype for B-ALL. The t(3;6)(q25; q13) was near, but
not at the locus for BCL6 or MECOM rearrangements, and t(8;22)(24.1;
q11.2) rearrangement is a variant of MYC-IGL. However, t(14;18)(q32;
q21) was consistent with IGH-BCL2 rearrangement. FISH for cMYC, BCL2,
and BCL6 were positive. These findings were suspicious for a
double/triple hit HGBL rather than pre-B ALL.
Due to the dilemma
in diagnosis, a right inguinal lymph node excisional biopsy was
performed. At this time patient was still admitted inpatient, and
therefore, a PET scan could not be obtained to assist in the diagnosis.
The right inguinal lymph node biopsy was performed as it was thought to
be the least invasive to perform. The pathology showed disruption of
normal architecture by the proliferation of follicles with small
lymphocytes having irregular nuclear contours. These cells were
positive for CD10, CD20, CD79a, PAX-5, BCL6, and BCL2 by
immunohistochemistry. They were negative for cMYC, cyclin D1, MuM-1,
and TdT. The Ki-67 was 10%. Flow cytometry identified a monoclonal
B-cell population positive for CD10, CD19, and CD20, with monoclonal
kappa light chain restriction. No cells in metaphase were present
for cytogenetic analysis.
This was consistent with follicular lymphoma (FL).
Hyper-CVAD
was continued due to overlapping efficacy for ALL and HGBL. However,
after completing one course of each hyper-CVAD and HD-MTX-Ara-C, he had
persistent blasts in his marrow. The immunophenotype was positive for
CD22 during this time. A second induction with rituximab, methotrexate,
vincristine, pegylated L-asparaginase, and dexamethasone (R-MOAD) was
given. Bone marrow biopsy after this second induction showed persistent
disease. He then received one cycle of inotuzumab ozogamicin but still
had no disease response. The patient was so far treated with ALL
regimens without any response. Although the CD19-directed chimeric
antigen receptor (CAR) T-cell therapy was not approved for ALL patients
above 25 years, he was evaluated for CAR T-cell therapy and was
qualified to receive it due to overlapping features with HGBL for which
CAR T-cell therapy was approved.
He underwent
lymphocyte-depleting chemotherapy followed by tisagenlecleucel, a
CD19-directed chimeric antigen receptor (CAR) T-cell therapy. The post
CAR T-cell infusion course was complicated by grade 3 cytokine release
syndrome (CRS), grade 2 immune effector cell-associated neurotoxicity
syndrome (ICANS), and macrophage activation syndrome requiring
intensive care admission. He was treated with corticosteroids,
tocilizumab, and anakinra. He made a full recovery and was discharged
home. A follow-up bone marrow aspiration and biopsy performed one month
later demonstrated a complete response. Unfortunately, another month
later, a repeat marrow biopsy demonstrated recurrent high-grade B-cell
lymphoma/leukemia with 90% blasts. His immunophenotype after the CAR-T
was noted to be CD45(dim), CD10(heterozygous), CD38(heterozygous). It
was negative for CD19, CD20, CD22, CD5, CD34, smKappa, smLambda, CD56,
CD66b. Given the lack of effective treatment options, he was
transitioned to supportive care and succumbed to the disease. His
overall survival was eight months.
Discussion
Overlapping
morphologies and phenotypes are commonly seen in lymphomas. Morphology
alone is insufficient to differentiate between lymphoblastic leukemia
or high-grade B-cell lymphoma. Blastoid morphology has been well
described in high-grade B-cell lymphomas. Uchida and colleagues
identified 47 patients with the initial manifestation of bone marrow
infiltration by blastoid B cells with MYC and BCL2 and/or BCL6
rearrangements (MBR) and classified them as acute lymphoblastic
leukemia (ALL)-like disease of HGBL-MBR (AL-HGBL-MBR). However, it
should be noted that these patients had negative TdT expression.[4]
Although TdT may be useful in identifying immature cells, a single
marker alone may not be appropriate for characterizing a cell type. The
degree of differentiation usually determines the immunophenotype of
lymphoid malignancies. TdT and CD34 antigens are the hallmarks of
immature lymphoid neoplasms in the earliest stage blasts (pro-B ALL
cells). CD34 and TdT expressions are usually noted parallel to one
another. However, TdT negative B-ALL, CD34 negative B-ALL, and dual
negative B-ALL are well-described in literature.[5,6]
Similarly, TdT expression has also been seen in mature lymphoid neoplasms, including high-grade B-cell neoplasms.[7,8]
Neoplastic cells, in our case, were positive for TdT but negative for
CD34 expression. The absence of surface light chains is considered a
feature of immature leukemias/lymphomas but can be seen in up to 20% of
cases of high-grade B-cell lymphoma.[8] Of note, our
patient did not have CD-20 expression on the neoplastic cells extracted
from the bone marrow. CD20-negative de-novo mature diffuse large B cell
lymphomas are rarely reported. Most cases include primary effusion
lymphoma, plasmablastic lymphoma, ALK-positive large B-cell lymphoma,
and large B-cell lymphoma arising in HHV8 (human herpesvirus-8).[25] Gaur et al. described two unclassifiable CD20-negative high-grade lymphomas refractory to chemotherapy.[26]
Molecular analysis using cytogenetics or FISH (fluorescent in-situ
hybridization) to detect rearrangements or translocations of Bcl-2,
Bcl-6, and MYC have been proposed to aid in diagnosing such cases.[27] CD20 expression is variable in B-ALL/LBL and can be found in neoplastic cells beyond the pro-B cell maturation stage.
40-50% of ALL cases have been noted to be CD20 positive which portends a poor prognosis.[28] Therefore, even immunophenotype in conjunction with morphology may not be enough to classify a neoplastic cell immature.[5,6]
Khalnari et al. have recently proposed a scoring system to help
classify challenging blastoid lymphoid malignancies. The scoring system
is conceptually sound but needs external validation. They found that
only the CD34 marker is sensitive and specific to make such a
distinction. A score greater than or equal to 3 supports the diagnosis
of blastoid HGBL. Our case scores 2 points if the scoring system is
strictly applied and favors B-ALL.[29]
In the
era of molecular genomics, the classification of lymphoid malignancies
no longer relies predominantly on morphology or immunophenotype. The
development of sophisticated techniques like fluorescence in situ
hybridization (FISH), next-generation sequencing of cancer genomes,
genome-wide analysis of copy number variations, and gene expression
profiling has enriched our knowledge of distinct cytogenetic
abnormalities associated with specific types of hematological
malignancies. The importance of these new findings in the molecular
landscape of lymphoid malignancies was highlighted in the 2016 revision
of the World Health Organization classification of lymphoid neoplasms.[1]
MYC dysregulation along with that of BCL2 and less frequently BCL6 are
characteristic oncogenic events of lymphomagenesis in diffuse large B
cell lymphomas.[3,9,10] These
rearrangements can be seen in up to 20% of HGBL cases but are
exceedingly rare in de novo ALL. In 2016, WHO updated all B-cell
lymphomas with MYC and BCL2 and/or BCL6 rearrangements, which were
included in a single category of HGBL, except for cases that express
TdT, which are classified as B-ALL. Given that this updated
classification relies on genetic findings over immunomorphological
findings, the use of TdT positivity to classify these cases as B-ALL
needs further discussion.[11-13] In a recently
published series of TdT positive lymphomas, Ok et al. propose that TdT
positive cases with MYC and BCL2 and/or BCL6 rearrangements should be
classified as HGBL with TdT positivity.[8]
Clinical
characteristics, including the prognosis of these cases, seem to align
more closely with HGBL than with B-ALL. Our patient had an extremely
elevated LDH and international prognostic index (IPI) score, as well as
bone marrow involvement—all poor prognostic factors in HGBL. HGBL
typically presents as an advanced disease with frequent extranodal and
bone marrow involvement, as seen in our case.[11]
Treatment
of these patients, either double/triple hit lymphoma or B-ALL, presents
a bigger challenge. Treatment of double/triple hit HGBL with standard
chemotherapy R-CHOP is associated with a dismal outcome with a median
overall survival of 12 months from diagnosis.[11]
Evidence to support the use of "Burkitt-like" intensified regimens like
R-EPOCH, hyper-CVAD, and CODOX-M/IVAC comes from small retrospective
studies.[14,15] Unfortunately, no molecularly
targeted approach has yet shown any success but is an area of active
investigation. Autologous stem cell transplantation (ASCT) has shown no
benefit in either overall survival or disease-free survival in a small
number of patients who achieve complete response.[14]
The disease course is marked by progression or early relapse for most
patients. Standard salvage treatment success rates for patients with
relapsed/refractory disease with non-cross-resistant chemotherapy
R-DHAP or R-ICE and ASCT are low.[16-18] Similarly, cases treated with ALL-type regimens, like hyper-CVAD, had similar poor outcomes.[4,8]
Our patient was treated with several lines of ALL regimens with no
response. He et al. reported a similar case about a 39-year-old male
with retroperitoneal mass and lymphadenopathy. Biopsy of mass revealed
malignant cells positive for TdT, CD99, CD10, PAX-5, BCL2 (70%), MYC
(70%) with Ki-67 of 80%. FISH studies demonstrated 8q24/MYC
rearrangement and IGH/BCL2 gene fusion. The patient had an excellent
response to hyper-CVAD.[30]
For our patient,
tisagenlecleucel appeared to be the only effective line of treatment.
Tisagenlecleucel is a CD19-directed genetically modified autologous
T-cell immunotherapy approved for the treatment of relapsed/refractory
DLBCL and patients with B-cell precursor ALL that is refractory or in
second or later relapse. In the Juliet trial, which included 19
patients with double/triple hit gene rearrangement, median overall
survival of 12 months was observed.[19] For patients
with relapsed/refractory B-ALL, treatment with CAR T-cell therapy has
shown a median time to progression of about 5.5 months and median
survival of 7.4 months.[20] Unfortunately, CAR T-cell
therapy was not approved for patients above the age of 25 years at the
time of treatment for our patient. However, due to a dilemma in his
exact diagnosis and suspicion of an alternate diagnosis of HGBL, he was
qualified to receive CAR T-cell therapy.
Our patient achieved
complete response after CAR T-cell therapy but suffered a relapse in 30
days. This is the first reported case of TdT+ triple hit
lymphoma/leukemia treated with CAR T-cell therapy. In addition,
Brexucabtagene autoleucel is now approved for the treatment of adult
patients (18 years and older) with relapsed or refractory B-cell
precursor acute lymphoblastic leukemia (ALL) after the ZUMA3 trial
showed a high and durable response in a heavily pretreated population
of B-ALL.[31]
Of interest, our case also had
synchronously diagnosed follicular lymphoma. Although we were unable to
perform clonal studies, it is possible that this high-grade
lymphoma/leukemia transformed from follicular lymphoma. Well-defined
cases of HGBL transformed from a preexisting or concomitant follicular
lymphoma by the acquisition of c-MYC exist in the literature.[21]
As seen in our case, this antecedent FL are usually BCL2 positive.
Several cases of follicular lymphoma transformation to TdT- HGBL and
subsequently to TdT+ high-grade B-cell lymphoma/leukemia have also been
proposed.[8,22]
Similarly,
cases with composite morphology with co-existing TdT+ immature neoplasm
and TdT- mature neoplasm has been reported, raising a possibility of an
intermediate stage of TdT- HGBL in the development of TdT+ HGBL. It has
been shown that mature neoplasms are capable of de-differentiating into
immature cells.[23] Alternatively, a common
progenitor cell (CPC) theory has also been proposed. It has been
hypothesized that a CPC resides in the bone marrow niche and can evolve
into FL, DLBCL, HGBL, and B-ALL.[24]
The
current WHO classification of lymphoid neoplasms precludes patients
such as ours from being involved in already scarce trials for the
treatment of double/triple hit lymphoma. These TdT+ double/triple hit
lymphoma/leukemia cases require further research for better
understanding of the disease biology and therapeutic exploration of
these aggressive neoplasms.
Conclusions
The
clinical course of patients with ALL-like morphology with MYC and BCL2
and/or BCL6 rearrangements is aggressive. Unfortunately, the disease is
refractory to the current standard treatments for both HGBL and B-ALL.
As these cases are extremely rare and heterogeneous, large clinical
trials exploring their treatment options are unlikely. Therefore, we
believe that such cases should be reported.
References
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein
H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES.
The 2016 revision of the World Health Organization classification of
lymphoid neoplasms. Blood. 2016;127(20):2375-90. https://doi.org/10.1182/blood-2016-01-643569 PMid:26980727 PMCid:PMC4874220
- Donlon
JA, Jaffe ES, Braylan RC. Terminal Deoxynucleotidyl Transferase
Activity in Malignant Lymphomas. New Engl J Medicine.
1977;297(9):461-4. https://doi.org/10.1056/NEJM197709012970901 PMid:301987
- Karube K, Campo E. MYC Alterations in Diffuse Large B-Cell Lymphomas. Semin Hematol. 2015;52(2):97-106. https://doi.org/10.1053/j.seminhematol.2015.01.009 PMid:25805589
- Uchida
A, Isobe Y, Uemura Y, Nishio Y, Sakai H, Kato M, Otsubo K, Hoshikawa M,
Takagi M, Miura I. De novo acute lymphoblastic leukemia-like disease of
high grade B-cell lymphoma with MYC and BCL2 and/or BCL6
rearrangements: a case report and literature review. Bmc Clin
Pathology. 2017;17(1):21. https://doi.org/10.1186/s12907-017-0060-1 PMid:29151814 PMCid:PMC5679186
- Zhou
Y, Fan X, Routbort M, Yin CC, Singh R, Bueso-Ramos C, Thomas DA, Milton
DR, Medeiros LJ, Lin P. Absence of terminal deoxynucleotidyl
transferase expression identifies a subset of high-risk adult
T-lymphoblastic leukemia/lymphoma. Modern Pathol. 2013;26(10):1338-45. https://doi.org/10.1038/modpathol.2013.78 PMid:23702731
- Hassan
M, Abdullah HMA, Wahid A, Qamar MA. Terminal deoxynucleotidyl
transferase (TdT)-negative T-cell lymphoblastic lymphoma with loss of
the T-cell lineage-specific marker CD3 at relapse: a rare entity with
an aggressive outcome. Bmj Case Reports. 2018;2018:bcr-2018-224570. https://doi.org/10.1136/bcr-2018-224570 PMid:29884716 PMCid:PMC6011547
- Moench
L, Sachs Z, Aasen G, Dolan M, Dayton V, Courville EL. Double- and
triple-hit lymphomas can present with features suggestive of
immaturity, including TdT expression, and create diagnostic challenges.
Leukemia Lymphoma. 2016;57(11):1-10. https://doi.org/10.3109/10428194.2016.1143939 PMid:26892631 PMCid:PMC6292197
- Ok
CY, Medeiros LJ, Thakral B, Tang G, Jain N, Jabbour E, Pierce SA,
Konoplev S. High-grade B-cell lymphomas with TdT expression: a
diagnostic and classification dilemma. Modern Pathol. 2019;32(1):48-58.
https://doi.org/10.1038/s41379-018-0112-9 PMid:30181564
- Nguyen
L, Papenhausen P, Shao H. The Role of c-MYC in B-Cell Lymphomas:
Diagnostic and Molecular Aspects. Genes-basel. 2017;8(4):116. https://doi.org/10.3390/genes8040116 PMid:28379189 PMCid:PMC5406863
- Harrington
AM, Olteanu H, Kroft SH, Eshoa C. The Unique Immunophenotype of
Double-Hit Lymphomas. Am J Clin Pathol. 2011;135(4):649-50. https://doi.org/10.1309/AJCPL11MAHISIJBQ PMid:21411790
- Liu
Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis,
risk stratification, and treatment. Am J Hematol. 2019;94(5):604-16. https://doi.org/10.1002/ajh.25460 PMid:30859597
- Ok
CY, Medeiros LJ. High-grade B-cell lymphoma: a term re-purposed in the
revised WHO classification. Pathology. 2020;52(1):68-77. https://doi.org/10.1016/j.pathol.2019.09.008 PMid:31735344
- Midha
S, Chavez JC, Saeed H, Bello C, Khimani F, Bunting ST, Shah B. The Grey
Zone: Characterization of Aggressive High-Grade B-Cell Malignancy with
TdT Expression and IGH/BCL2 and MYC Rearrangement. Blood.
2019;134(Supplement_1):5347-5347. https://doi.org/10.1182/blood-2019-130721
- Petrich
AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, Shah KA,
Whyman JD, Lansigan F, Hernandez-Ilizaliturri FJ, Lee LX, Barta SK,
Melinamani S, Karmali R, Adeimy C, Smith S, Dalal N, Nabhan C, Peace D,
Vose J, Evens AM, Shah N, Fenske TS, Zelenetz AD, Landsburg DJ, Howlett
C, Mato A, Jaglal M, Chavez JC, Tsai JP, Reddy N, Li S, Handler C,
Flowers CR, Cohen JB, Blum KA, Song K, Sun H (Linda), Press O, Cassaday
R, Jaso J, Medeiros LJ, Sohani AR, Abramson JS. Impact of induction
regimen and stem cell transplantation on outcomes in double-hit
lymphoma: a multicenter retrospective analysis. Blood.
2014;124(15):2354-61. https://doi.org/10.1182/blood-2014-05-578963 PMid:25161267
- Howlett
C, Snedecor SJ, Landsburg DJ, Svoboda J, Chong EA, Schuster SJ, Nasta
SD, Feldman T, Rago A, Walsh KM, Weber S, Goy A, Mato A. Front‐line,
dose‐escalated immunochemotherapy is associated with a significant
progression‐free survival advantage in patients with double‐hit
lymphomas: a systematic review and meta‐analysis. Brit J Haematol.
2015;170(4):504-14. https://doi.org/10.1111/bjh.13463 PMid:25907897
- Herrera
AF, Mei M, Low L, Kim HT, Griffin GK, Song JY, Merryman RW, Bedell V,
Pak C, Sun H, Paris T, Stiller T, Brown JR, Budde LE, Chan WC, Chen R,
Davids MS, Freedman AS, Fisher DC, Jacobsen ED, Jacobson CA, LaCasce
AS, Murata-Collins J, Nademanee AP, Palmer JM, Pihan GA, Pillai R,
Popplewell L, Siddiqi T, Sohani AR, Zain J, Rosen ST, Kwak LW,
Weinstock DM, Forman SJ, Weisenburger DD, Kim Y, Rodig SJ, Krishnan A,
Armand P. Relapsed or Refractory Double-Expressor and Double-Hit
Lymphomas Have Inferior Progression-Free Survival After Autologous
Stem-Cell Transplantation. J Clin Oncol. 2016;35(1):24-31. https://doi.org/10.1200/JCO.2016.68.2740 PMid:28034071 PMCid:PMC5455688
- Allen
JM, Mendez ALR, Rybicki LA, Jagadeesh D, Dean RM, Pohlman BL, Smith MR,
Hsi ED, Hill BT. Outcomes of patients with relapsed/refractory diffuse
large B-cell lymphoma treated with R-ICE based on dual expression of
MYC and BCL2. J Clin Oncol. 2016;34(15_suppl):e19043-e19043. https://doi.org/10.1200/JCO.2016.34.15_suppl.e19043
- Herrera
AF, Rodig SJ, Song JY, Kim Y, Griffin GK, Yang D, Nikolaenko L, Mei M,
Bedell V, Cin PD, Pak C, Alyea EP, Budde LE, Chen R, Chen Y-B, Chan WC,
Cutler CS, Ho VT, Koreth J, Krishnan A, Murata-Collins JL, Nikiforow S,
Palmer J, Pihan GA, Pillai R, Popplewell L, Rosen ST, Siddiqi T, Sohani
AR, Zain J, Kwak LW, Weisenburger DD, Weinstock DM, Soiffer RJ, Antin
JH, Forman SJ, Nademanee AP, Armand P. Outcomes after Allogeneic Stem
Cell Transplantation in Patients with Double-Hit and Double-Expressor
Lymphoma. Biol Blood Marrow Tr. 2018;24(3):514-20. https://doi.org/10.1016/j.bbmt.2017.11.023 PMid:29196080 PMCid:PMC5826860
- Schuster
SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U,
Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR,
Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R,
Chu J, Anak Ö, Salles G, Maziarz RT, Investigators J. Tisagenlecleucel
in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New Engl
J Med. 2019;380(1):45-56. https://doi.org/10.1056/NEJMoa1804980 PMid:30501490
- Wudhikarn
K, Flynn JR, Rivière I, Gonen M, Wang X, Senechal B, Curran KJ, Roshal
M, Maslak PG, Geyer MB, Halton EF, Diamonte C, Davila ML, Sadelain M,
Brentjens RJ, Park J. Interventions and Outcomes of Adult Patients with
B-ALL Progressing After CD19 Chimeric Antigen Receptor T Cell Therapy.
Blood. 2021;138(7):531-543. https://doi.org/10.1182/blood.2020009515 PMid:33851211
- Lossos IS, Gascoyne RD. Transformation of follicular lymphoma. Best Pract Res Cl Ha. 2011;24(2):147-63. https://doi.org/10.1016/j.beha.2011.02.006 PMid:21658615 PMCid:PMC3112479
- Geyer
JT, Subramaniyam S, Jiang Y, Elemento O, Ferry JA, Leval L de,
Nakashima MO, Liu Y-C, Martin P, Mathew S, Orazi A, Tam W.
Lymphoblastic transformation of follicular lymphoma: a
clinicopathologic and molecular analysis of 7 patients. Hum Pathol.
2015;46(2):260-71. https://doi.org/10.1016/j.humpath.2014.10.021 PMid:25529125
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313-7. https://doi.org/10.1038/nature05934 PMid:17554338
- Carlotti
E, Wrench D, Matthews J, Iqbal S, Davies A, Norton A, Hart J, Lai R,
Montoto S, Gribben JG, Lister TA, Fitzgibbon J. Transformation of
follicular lymphoma to diffuse large B-cell lymphoma may occur by
divergent evolution from a common progenitor cell or by direct
evolution from the follicular lymphoma clone. Blood.
2009;113(15):3553-7. https://doi.org/10.1182/blood-2008-08-174839 PMid:19202129
- Anand
K, Ensor AM, Ensor JE. CD20-negative diffuse large B-cell lymphoma
subtype: a look at Texas Cancer Registry: Hematology Reports 2020
/09/15;12(s1)
- Gaur S, Padilla O, Nahleh
Z. Clinical Features and Prognosis of CD20 Negative Aggressive B-Cell
Non-Hodgkins Lymphoma. Lymphoma 2013 /01/03;2013:e290585. https://doi.org/10.1155/2013/290585
- Katchi T, Liu D. Diagnosis and treatment of CD20 negative B cell lymphomas. Biomark Res 2017 -2-7;5. https://doi.org/10.1186/s40364-017-0088-5 PMid:28191314 PMCid:PMC5297138
- Thomas
DA, O'Brien S, Jorgensen JL, Cortes J, Faderl S, Garcia-Manero G, et
al. Prognostic significance of CD20 expression in adults with de novo
precursor B-lineage acute lymphoblastic leukemia. Blood 2009
-06-18;113(25):6330-6337. https://doi.org/10.1182/blood-2008-04-151860 PMid:18703706 PMCid:PMC2943753
- Khanlari
M, Medeiros LJ, Lin P, Xu J, You MJ, Tang G, et al. Blastoid high-grade
B-cell lymphoma initially presenting in bone marrow: a diagnostic
challenge. Modern Pathology 2021 Oct 4,:1-8. https://doi.org/10.1038/s41379-021-00909-4
- He
L, Li Z, Fan X, Chen J, Wu H, Fu Y. "Double hit" B-lymphoblastic
lymphoma with concurrent IGH/BCL2 and 8q24/MYC translocations: a case
report. Translational Cancer Research 2021 Mar;10(3):1594-1598. https://doi.org/10.21037/tcr-20-2748 PMid:35116484 PMCid:PMC8797657
- Shah
BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al.
KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic
leukaemia: phase 2 results of the single-arm, open-label, multicentre
ZUMA-3 study. The Lancet 2021 /08/07;398(10299):491-502. https://doi.org/10.1016/S0140-6736(21)01222-8
[TOP]


