Rare immune thrombocytopenia (ITP) cases occurring after both the Pfizer and Moderna vaccines have recently reached public attention.[4-8] It has been reported in patients with previous ITP or other autoimmune diseases and also in individuals with an apparent negative past medical history. However, these cases' management and outcome are still not well investigated and reported in the medical literature.[2,9]
Herein, we report the case of a young woman with a past medical history of ITP who developed a severe ITP recurrence after the second dose of the Pfizer vaccine, administered while in ITP remission and still on treatment with mycophenolate.
A 23-year old female had received a diagnosis of ITP at the age of 14 years; platelet count was 12x109/L; anti-platelets antibodies were negative; immunoglobulin levels and the coagulation profile were normal; a mild positivity for anti-nuclear antibodies (ANA) was detected. An abdomen ultrasound revealed normal liver, spleen, and kidneys sizes. High-dose immunoglobulins (HD-IVIG) were administered with rapid normalization of the platelet count. The patient presented an ITP recurrence (platelets 11x109/L) one year later. ANA, anti-extractable nuclear antigen (ENA), anti-double-stranded DNA (dsDNA), mitochondrial antibodies, and rheumatoid factor were negative. A bone marrow aspirate showed normal hematopoiesis, and the cytogenetic study resulted in a normal female karyotype. The patient received 4 consecutive daily infusions of HD-IVIG (400 mg/kg/day), with a temporary platelet count recovery. Platelet count rapidly decreased (2.0x109/L) one month later. Prednisone (1 mg/kg/day) was started; the symptomatic thrombocytopenia persisted (20.0x109/L) with recurrent epistaxis and metrorrhagia. Rituximab (375 mg/m2/dose/week x 4) was administered. The platelet count normalized and remained in the normal range for one year, when a new ITP recurrence was observed (platelets 12.0x109/L), initially treated again successfully with HD-IVIG (1 g/kg/day x 2). Immunosuppressive treatment with mycophenolate (1gx2/day) was started with a complete platelet count recovery.
In December 2020, when the SARS-CoV-2 vaccine was still not available, the patient's mother and grandmother developed a life-threatening SARS-CoV-2 pneumonitis. In our patient, the real-time polymerase-chain-reaction (RT-PCR) assay for SARS-CoV-2 RNA from nasopharyngeal swabs, repeated three times, proved negative; serological test for SARS-CoV-2 antibodies resulted negative. In complete ITP remission (platelets 220.0x109/L) for 3 years, and still, on mycophenolate treatment, the patient received the first dose of the Pfizer-BioNTech SARS-CoV-2 vaccine on March 20, and the second dose on April 10, 2021. Fifteen days after the second vaccine dose, peripheral blood control showed a decreased platelet count (81.0x109/L). Treatment with mycophenolate was continued, and low-dose prednisone (0.5 mg/kg/day) was added. One week later, the patient was admitted to Emergency Rooms with severe headache, metrorrhagia, and cutaneous hemorrhages. She had not taken medications other than mycophenolate and prednisone.
A complete blood count showed severe thrombocytopenia (platelet count 3.0x109/L) with normal leukocytes and red cells indices. The peripheral blood film did not show platelet clumping or schistocytes. The coagulation profile, renal and liver functions were normal. The brain-computer tomography (CT) ruled out the presence of hemorrhages, and a Chest-X-ray was normal. A viral serology panel, blood cultures and urine analysis were negative for active infections. The patient received HD-IVIG (800 mg/Kg/day) for two consecutive days and prednisone (1 mg/kg/day) and continued mycophenolate. The platelet count slowly increased to >20x109/L in five weeks. Prednisone was tapered and discontinued in 4 weeks. The platelet count fully recovered after 3 months. The patient remains in ITP remission with a normal platelet count (platelets > 200.0x109/L) and on mycophenolate therapy (Figure 1).
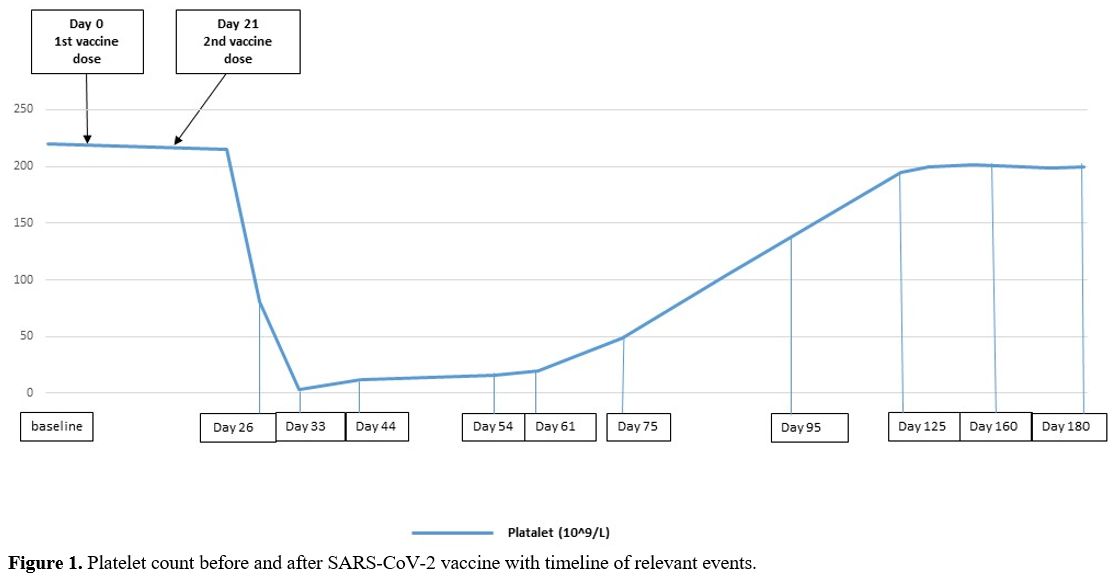 |
Figure
1. Platelet count before and after SARS-CoV-2 vaccine with timeline of relevant events. |
Vaccine-induced autoimmunity and, in particular, ITP has been described following vaccinations against various infectious agents: measles-mumps-rubella (MMR), Hemophilus influenzae, hepatitis B, varicella-zoster, polio, and pneumococcus.[10] More recently, cases of ITP after SARS-CoV-2 mRNA vaccination have been reported in patients with a prior ITP, other autoimmune disorders, or conditions with an underlying inflammatory state that may have contributed to the ITP development.[4,6,8,11] However, other cases of ITP following a SARS-CoV-2 mRNA vaccination did not report any condition predisposing to thrombocytopenia, recent viral infections, or a family history of ITP. It is unclear if the ITP in our patient was secondary to the vaccination or whether it was a recurrence of the ITP that coincided with the vaccine administration; she had had a recurrent ITP, but she had achieved a platelet count normalization that had lasted for 3 years on mycophenolate therapy. Thrombocytopenia recurrence coincides with the timing of vaccine antibody production; serological test for SarS-CoV2 and autoimmunity tests were not performed because of emergency treatment with IV-IG and steroids. Three months after the vaccine, at platelets recovery and steroid discontinuation but still on mycophenolate immunosuppressive therapy, serological test for SARS-CoV-2 antibodies resulted negative. Recently other reports, as single cases or series from the Centers for Diseases Control and Prevention (CDC), FDA, and agencies of the U.S. Department of Health and Human Services (HHS) Vaccine Adverse Events Reporting System (VAERS), have described both healthy patients or patients with previous autoimmune disorders developing thrombocytopenia following SARS-CoV-2 mRNA vaccines (0.8 cases per million doses).[4-6] At our Center, from March to April 2021, 77 ITP patients, age ≥ 18 years, on immunosuppressive treatment and platelet count ranging from 24.0 to 220.0 x 109/L, received the SARS-CoV-2 mRNA vaccine as "fragile category"; platelet count was evaluated 7-10 days after the first and second dose, and none of them developed such a severe thrombocytopenic recurrence. A slight deflection of the platelet count was observed in 15 asymptomatic cases, resolved within two weeks, without further treatment.
An immune-mediated mechanism can be hypothesized, particularly evident in patients with a past medical history of ITP. In the currently available literature, ITP secondary to SARS-CoV-2 mRNA vaccination in patients with a prior history of ITP is a rarely reported adverse effect; it is not yet possible to establish whether the incidence of ITP secondary to mRNA vaccine is higher in these patients compared to the general population; in healthy people, asymptomatic thrombocytopenia may not be recognized. However, in our Center, in 15/77 (19%) "fragile patients" with ITP on immunosuppressive therapy, vaccinated for SARS-CoV-2 and carefully monitored, a platelets count reduction was detected; this data is in agreement with another report in literature.[7]
Most authors believe that ITP is not an absolute contraindication for the COVID-19 vaccination, given a much higher rate of severe ITP linked with the natural infection.[9] The reasons to strongly suggest the vaccination in our patient were the stable ITP phase, the ongoing immunosuppressive treatment, and the family history of severe SARS-CoV-2 infections. In addition, recent data show that post-vaccination ITP is highly responsive to IV-IG treatment;[7,9] it has also been suggested that corticosteroids and IV-IG are the most effective treatment for thrombocytopenia correction in order to control or prevent severe hemorrhages.[9] Our patient had a benefit from this combined treatment with rapid and complete disappearance of hemorrhagic symptoms and a full recovery of the platelet count.
The benefits of the vaccination largely outweigh the risk of SARS-CoV-2 infection in ITP patients. A long-term follow-up of large cohorts of patients receiving mRNA vaccine will answer the question as to whether it increases the risk of autoimmune conditions. Until then, given the severe health situation created by the pandemic, healthcare providers are encouraged to prescribe mRNA vaccines, continuing to monitor possible side effects.