Mehran Karimi1, Tahereh Zarei1, Sezaneh Haghpanah1, Azita Azarkeivan2, Maryam Naderi3, Sara Matin4, Asghar Bazrafshan1, Zohreh Zahedi1, Afshan Shirkavand5, Parisa Pishdad6 and Vincenzo De Sanctis7.
1 Hematology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
2
Zafar Adult Thalassemia Clinic, Blood Transfusion Research Center, High
Institute for Research and Education in Transfusion Medicine, Tehran,
Iran.
3 Iranian Blood Transfusion Organization Research Center, Thalassemia Clinic, Tehran, Iran.
4 Pediatric Department, Jahrom University of Medical Sciences, Jahrom, Iran.
5
Laser Medicine Research group, MLRC, Yara institute, ACECR; Medical
physicist, Pardis Noor Medical Imaging Center, Tehran, Iran.
6
Medical Imaging Research Center, Department of Radiology, School of
Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
7
Coordinator of ICET-A Network (International Network of Clinicians for
Endocrinopathies in Thalassemia and Adolescence Medicine) and Pediatric
and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
Correspondence to: Mehran
Karimi, MD. Professor Emeritus of Pediatric Hematology and Oncology,
Hematology Research Center Shiraz University of Medical Sciences,
Shiraz, Iran. Tel/fax: +987136122263. E-mail:
mkarimi820@gmail.com
Published: March 1, 2022
Received: November 16, 2021
Accepted: February 11, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022026 DOI
10.4084/MJHID.2022.026
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: The
ongoing COVID-19 pandemic, caused by severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2), has led to high morbidity and mortality
worldwide. Vaccination against SARS-CoV-2 is a leading strategy to
change the course of the COVID-19 pandemic.
Aims of the study: Our
aim was to investigate the efficacy and side effects of the Sinopharm
vaccine in patients with hemoglobinopathies in Iran and the frequency
of breakthrough infection after a full course of vaccination.
Methods: A
multicenter cross-sectional study of 434 patients with
hemoglobinopathies (303 β-thalassemia major, 118 β-thalassemia
intermedia, and 13 sickle-thalassemia) were conducted from March to
July 2021 in IRAN. All patients have received the first dose of the
China Sinopharm vaccine and received the second dose of the vaccine 28
days apart.
Antibody testing: Detection
of immunity after vaccination was evaluated by commercial enzyme-linked
immunosorbent assay (Pishtazteb ELISA commercial kit), including a
surrogate virus neutralization test (sVNT), for detection of SARS-CoV-2
immunoglobulins (IgA, IgM, IgG), total neutralizing antibody (NAb).
Results: The
mean age of patients was 35.0 ± 8.5 (from 18 to 70) years, and 55.6%
were positive for the antibody. Overall, 48.2% of the studied
population had at least one side effect after vaccination. The most
frequent side effects were fever and chills, dizziness, and body pain.
A total of 90 (20.7%) vaccinated patients developed breakthrough
infections after two doses of Sinopharm vaccination. Disease severity
was recorded, and it was classified as mild in 77.8%, moderate in
13.6%, and severe in 7.4% of patients. One 28-year-old woman with β-
thalassemia major died eight days after diagnosing a breakthrough
SARS-CoV-2 infection.
Conclusion: No
safety concerns were identified in patients who received two doses of
the Sinopharm vaccine. Its efficacy was not optimal due to the lack of
effect on new variations of the virus. However, our data show that it
seems to be protective against the severity of COVID-19 infection in
patients with hemoglobinopathies. The frequency of breakthrough
infections after two doses of Sinopharm vaccination supports the
evolving dynamic of SARS-CoV-2 variants requiring special challenge
since such infection may represent a risk for vulnerable patients.
|
Introduction
The
coronavirus disease (COVID-19) is caused by a positive-stranded RNA
virus called severe acute respiratory syndrome coronavirus-2
(SARS-CoV-2), belonging to the Coronaviridae family. In humans, the
virus causes COVID-19, a disease characterized by fever, shortness of
breath, and pneumonia, which can be fatal in vulnerable individuals.[1]
The most severe cases of COVID-19 with admission to the Intensive Care
Unit (ICU) are generally more frequent in males and the elderly,
especially those with comorbidities such as diabetes mellitus, obesity,
chronic cardiovascular and/or respiratory disease.[2]
The prevalence and mortality rates of SARSCOV-2 are changing on a daily basis.[2]
According to World Health Organization (WHO), as of October 2021, the
COVID-19 pandemic involved 216 countries and had affected 240,260,449
people, and that caused the death of 4,890,424.[3]
Therefore,
a prophylactic approach is crucial to control the disease, although the
specific management against COVID-19 is still under investigation. To
date, multiple vaccines have been developed worldwide utilizing
different technologies, including messenger ribonucleic acid (mRNA)
vaccines and classic inactivated virus vaccines. Each vaccine exhibits
a different potency and duration of efficacy, as determined by the
antigen design, adjuvant molecules, vaccine delivery platforms, and
immunization method. Large-scale clinical studies found that COVID-19
vaccines prevented most people from getting COVID-19 illness, but like
most other vaccines, they are not 100% effective.[4-8]
Sinopharm Beijing and Wuhan Institute of Biological Products have
produced two inactive SARS-CoV-2 virus vaccines (CoronaVac vaccine
developed by Sinovac and Sinopharm BBIBP-CorV) using chemical
β-propiolactone. Both were treated with aluminum-based adjuvant to
increase immunogenicity. A schedule of two doses (0/21-28-days) of the
vaccine that can be stored at 2°C – 8°C is recommended to prevent
SARS-CoV- 2 infection.[9]
According to China
National Biotec Group Company study, more than 40,000 people in the
United The Arab Emirates and Bahrain, aged 18 and above without a known
history of COVID-19, participated in the trials. The vaccine showed an
efficacy rate of 79% against symptomatic COVID-19 cases and reported
serious adverse events (AEs) after vaccination were rare.[10,11]
On
May 7, 2021, the World Health Organization (WHO) approved the vaccine,
and it is used in many countries, including Iran, but the European
Medicines Agency (EMA) has not yet reviewed its use for the European
Union.
In Iran, a country with a population of around 85,451,701,
COVID-19 affected 5,987,814 people and 127,299 mortalities were
reported up to Nov. 8, 2021.
In patients with hemoglobinopathies
(thalassemias and sickle cell disease), several factors (iron overload,
frequent hospital visits and admissions, immunodeficiency related to
the disease itself, continuous use of medications, or associated
complications) could predispose subjects to an increased risk for
acquiring COVID-19 and consequent complications.[12-15] Moreover, higher mortality has been reported in subjects with thalassemias than the general infected population.[12] For all these reasons, patients with hemoglobinopathies were registered as the first group to get the vaccine in Iran.
We
undertook this study to determine the efficacy and side effects of the
Sinopharm vaccine in patient with hemoglobinopathies in Iran, and the
frequency of breakthrough infection after two doses of Sinopharm
vaccination.
Patients and Methods
Study design.
This cross-sectional study was performed from March to July 2021 among
434 patients with hemoglobinopathies (303 β-thalassemia major, 118
β-thalassemia intermedia, and 13 sickle-thalassemia), followed in
Shiraz (331 patients) and Tehran (103 patients) who received COVID-19
vaccine.
Patients' data.
All patients were diagnosed based on their clinical and laboratory data
(clinical history, complete blood count and hemoglobin
electrophoresis). Patients with transfusion-dependent thalassemia (TDT)
were those requiring regular lifelong blood transfusions, starting
before the age of 2 years with a hemoglobin level below 7 g/dL.
Patients with non-transfusion-dependent thalassemia (NTDT) and
sickle-thalassemia were Transfusions free or receiving occasional blood
transfusions for a very limited period, such as surgery. All patients
received two doses of Sinopharm vaccination. In subjects with a
previous history of SARS-CoV-2 infection, the first Sinopharm dose was
given three months after COVID-19. The interval between blood
transfusion and vaccine injection was three days at least.
Patients
under 18 years old, pregnant women, patients with fever and acute phase
of the COVID-19 disease have not been included in the present report.
A
designed questionnaire was made to collect all demographic, clinical,
and laboratory data, including age, sex, age of diagnosis of a
hemoglobinopathy, type of thalassemia, blood transfusion requirement
(type and interval of blood transfusion), splenectomy status, serum
ferritin level, preexisting comorbidities (diabetes, chronic heart
failure, hypothyroidism, hypoparathyroidism, hypogonadism, cirrhosis,
HCV positivity, osteoporosis, extramedullary hematopoiesis, thrombosis,
liver, kidney and cardiac diseases, and pulmonary hypertension). In
addition, a serum ferritin > 2,500 ng/mL was considered an indirect
index of severe iron overload.
Patients' outcome and AEs after vaccination were recorded via interview or after reviewing patients' personal histories.
Breakthrough SARS-CoV-2 infection.
"A breakthrough infection" was suspected in presence of clinical
defined signs and diagnosed by detection of SARS-CoV-2 on
reverse-transcriptase-polymerase-chain- reaction (RT-PCR) assay, two
weeks or more after the second dose of Sinopharm vaccine[8] and were
classified into asymptomatic/mild, moderate, and severe, according to
WHO.[16]
Antibody testing.
Detection of immunity after vaccination was evaluated by commercial
enzyme-linked immunosorbent assay (Pishtazteb ELISA commercial kit),
including a surrogate virus neutralization test (sVNT), for detection
of SARS-CoV-2 immunoglobulins (IgA, IgM, IgG), total and neutralizing
antibody (NAb). The assays were performed according to the
manufacturer's protocol. Elisa assay has a sensitivity and specificity
of 94.1% and 98.3%, respectively. A negative result can occur if the
quantity of antibodies for the SARS-CoV-2 virus present in the specimen
is below the assay's detection limit, or the virus has undergone minor
amino acid mutation(s) in the epitope recognized by the antibody
utilized in the test. The tests were performed one month after the
second dose of Sinopharm vaccination.[17]
Statistical analysis.
Data were analyzed by IBM SPSS version 23. Normality of data was
checked by Kolmogorov-Smirnov test. Descriptive data were presented as
mean, standard deviation, median, range, and percentage. Inferential
analysis was performed by Student t-test or Mann-Whitney test to
compare quantitative data between the two groups. Comparison of
qualitative variables among different groups was made by the Chi-square
test. A P-value less than 0.05 was considered statistically
significant.
Ethical statement.
The study protocol was approved by the Ethical Committee of Shiraz
University of Medical Sciences (I.R.SUMS.REC.1400.248 (which covered
both centers. Written informed consent was obtained from the patients
before starting the study.
Results
Four
hundred thirty-four patients with hemoglobinopathies received two doses
of Sinopharm vaccine up to July 2021. The mean age of patients was 35.0
± 8.5 (range:18 - 70) years; 269 were females, and 165 were males. The
demographic and clinical characteristics of the patients are summarized
in table 1. Most of our
patients got the SARS-CoV-2 infection from their parents, friends, or
public areas. One hundred fifty-three patients (35.3%) SARS-CoV-2
antibody levels were checked, 85 patients (55.6%) resulted positive.
 |
Table
1. Summary of clinical characteristics and laboratory data of vaccinated patients with hemoglobinopathies. |
Reported post-vaccination AEs and their frequencies are summarized in table 2.
Overall, 48.2% of enrolled patients had at least one adverse effect.
The most frequent were fever and chills (18.0%), dizziness (15.7%), and
body pain (12.4%).
 |
Table 2. Adverse events (AEs) reported in order of frequency in vaccinated patients with hemoglobinopathies. |
Ninety
vaccinated patients (20.7%) developed breakthrough coronavirus
infection at least one month after the second dose of the Sinopharm
vaccine. In 22 out of 90 patients (24.4%), SARS-CoV-2 antibodies were
checked; seven (31.8%) resulted positive.
The severity of
breakthrough coronavirus infection in 81 out of 90 patients (90%) was
classified as mild in 77.8%, moderate in 13.6%, and severe in 7.4%. The
most common mutations of TDT were IVS-I-II and IVS-I-V with the
genotype of β0/β0, and the most common mutations of NTDT were -101 and -92 with β+/β+ genotype without co-inherited α-thalassemia.
The mean pre-transfusion hemoglobin levels in TDT and hemoglobin levels
in NTDT and sickle-thalassemia were 9.9 ± 1.1 g/dL and 9.7 ± 1 g/dL,
respectively. We did not find any relationship of COVID-19 severity
disease with the severity of thalassemia itself (P=0.717), hemoglobin
levels (P=0.956), and patients' genetic mutations.
A 28-year-old female with β-thalassemia major (blood group O+,
regularly transfused with washed packed red blood cells) presented with
fever, dry cough, and dyspnea 120 days after the second dose of the
Sinopharm vaccine. She had been compliant with chelation therapy with
Deferasirox (dose of 20 mg/kg, based on the serum ferritin level).
Moreover, the median ferritin level during the past six months was
about 1,000 ng/mL, and the last recorded serum ferritin was 500 ng/mL.
No relevant associated comorbidities were found, and the spleen was
palpable 2 centimeters below the costal margin. The patient was
hospitalized with a positive PCR COVID-19 test, and Remdesivir and
thromboprophylaxis with Enoxaparin (40 mg /day) were immediately
started as a severe case. Laboratory data showed that prothrombin time
(PT, 15.4 Sec), International Normalized Ratio (INR, (1.12), activated
partial- thromboplastin time (APTT, 31.6 Sec), D dimer (less than 500
mg/mL), fibrinogen (200 mg/dL) and platelet count (390×103/µL), were normal at admission.
On the 4th
day of admission, she developed a severe and persistent headache
requiring a brain computed tomography (CT) scan. No relevant changes
were documented; however, a second brain CT scan, carried out 12 hours
later, showed an intraventricular and left frontal lobe parenchymal
hemorrhage with midline shift and herniation (Figure 1). Moreover, the CT venography detected a subarachnoid hemorrhage (Figure 2)
without evidence of intracranial vascular thrombosis. Therefore, the
patient underwent surgery due to impending herniation and midline
shift. Unfortunately, a few hours after surgery, she developed
hypotension and finally died. Coagulation assays (PT, INR, APTT, and
platelet count) were normal prior to surgery, but PT and INR were
prolonged after surgery (32.6 Sec, Control:13.8 Sec and 2.5, Reference
range: 0.9-1, respectively), while PTT and platelet count were normal.
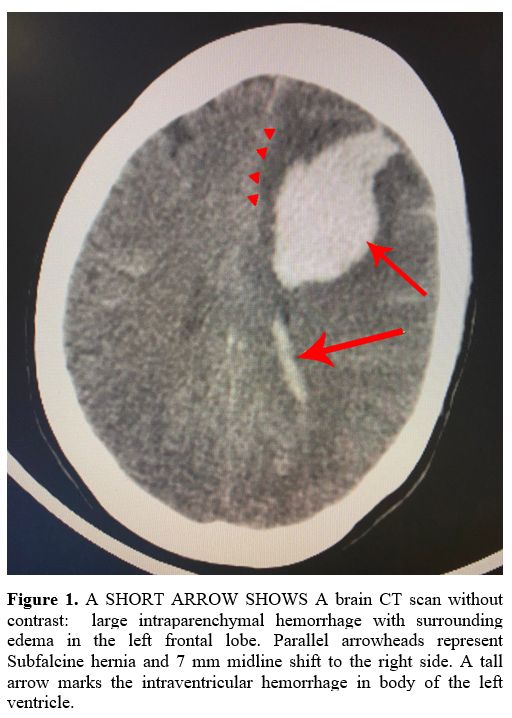 |
Figure 1. A SHORT ARROW
SHOWS A brain CT scan without contrast: large intraparenchymal
hemorrhage with surrounding edema in the left frontal lobe. Parallel
arrowheads represent Subfalcine hernia and 7 mm midline shift to the
right side. A tall arrow marks the intraventricular hemorrhage in body
of the left ventricle. |
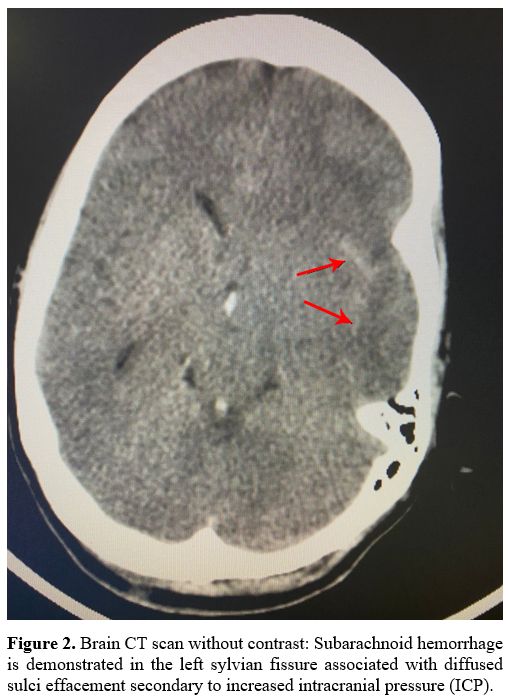 |
Figure
2. Brain CT scan without contrast: Subarachnoid hemorrhage is
demonstrated in the left sylvian fissure associated with diffused sulci
effacement secondary to increased intracranial pressure (ICP). |
Chest CT scan showed diffuse bilateral lung involvement, typical for COVID-19 pneumonia. (Figure 3).
The patient had no family history of bleeding tendency and
was not receiving hormone replacement therapy
with oral estrogen/progesterone.
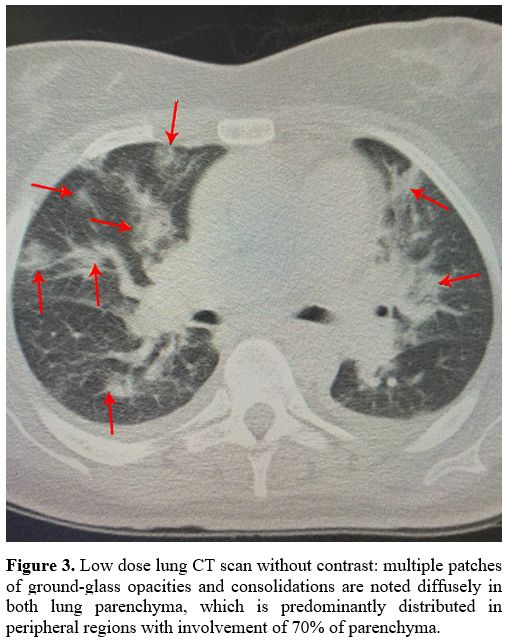 |
Figure 3. Low dose lung CT
scan without contrast: multiple patches of ground-glass opacities and
consolidations are noted diffusely in both lung parenchyma, which is
predominantly distributed in peripheral regions with involvement of 70%
of parenchyma. |
Table 3
shows the association of some clinical and laboratory parameters
related to the antibody status of vaccinated patients. They were
divided into two groups based on positive or negative antibodies. The
results revealed a significantly lower incidence of breakthrough
coronavirus infection after full vaccination in the positive antibody
group (8.2%) compared to the negative group (22.1%; P = 0.02). The
other evaluated factors were comparable between the two groups (P=
˃0.05).
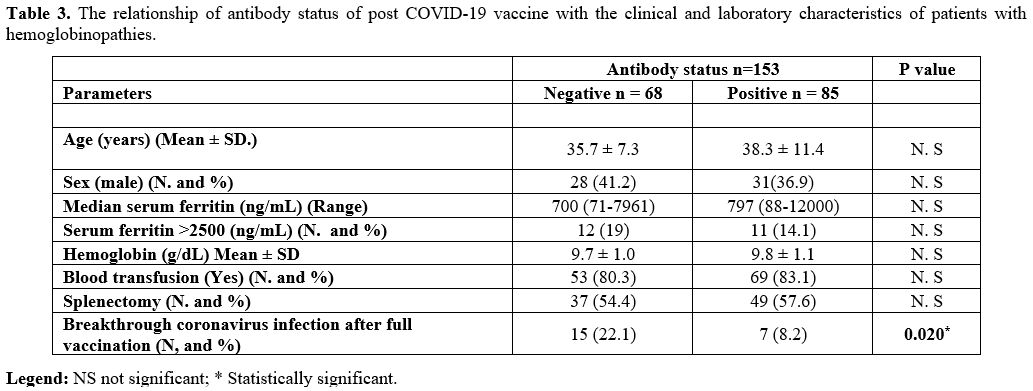 |
Table 3. The relationship
of antibody status of post COVID-19 vaccine with the clinical and
laboratory characteristics of patients with hemoglobinopathies. |
Moreover, we evaluated the laboratory, and clinical features of post vaccinated patients with and without Sars-CoV-2 infection (Table 4).
Only one parameter resulted statistically significant: the median of
serum ferritin level in patients who developed a breakthrough
coronavirus infection (Table 4; P = 0.013).
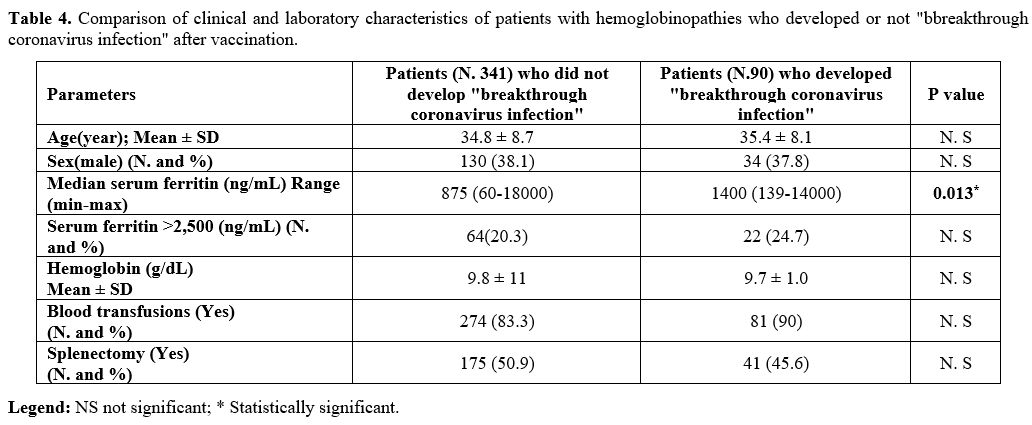 |
Table 4. Comparison of
clinical and laboratory characteristics of patients with
hemoglobinopathies who developed or not "bbreakthrough coronavirus
infection" after vaccination. |
Discussion
Since
there is no definitive therapy for COVID-19, vaccination is the only
appropriate approach for controlling the disease. So, vaccines are a
critical tool in managing the COVID-19 pandemic.
According to the type of vaccine, variable immune responses and side effects are seen.[6-8]
Several vaccines have been rapidly developed and approved by many countries for emergency use.
One
of these vaccines is Sinopharm (Beijing Institute of Biological
Products Co., Ltd, China) that is an inactive SARS-CoV-2 virus vaccine.[12] An interim recommendation for its use has been reported by the WHO Strategic Advisory Group of Experts (SAGE).
Most studies have assessed post-vaccination AEs of Pfizer–BioNTech, Moderna, and AstraZeneca vaccines,[18-21] but limited data are available on Sinopharm vaccine[22,23] knowledge no comprehensive published studies are available in subjects with hemoglobinopathies.
A
cross-sectional study of 1,102 attendants (aged ≥18 years) in the UAE
who received two doses of Sinopharm vaccine showed that, in general, it
was tolerated, and post-vaccination AEs were mild without any serious
reported adverse event.[10,11,24] Our data confirm these observations in our patients with hemoglobinopathies too.
Based on antibody assessment, the reported efficacy rate of the Sinopharm vaccine is between 73% - 86%,[11,23]
but our preliminary data have shown a lower level of immunity or
protection from COVID-19 (55%) after two doses of vaccine with 28 days
apart. Twenty-one of vaccinated patients developed breakthrough
coronavirus infection at least one month after the second dose of
Sinopharm vaccine. Although in the vast majority of patients with
hemoglobinopathies, who developed post-vaccine COVID-19 disease, the
symptomatology was mild-moderate, in 7.4% of them, the disease was
severe, and one female patient died, three months after the second dose
of vaccine, for a breakthrough coronavirus infection. Therefore, the
efficacy of Sinopharm against SARS-CoV-2 infection (COVID-19) seems to
be partial and less protective compared to other types of vaccines, as
reported in the general population.[18-23] However,
specific studies are needed to assess the immunogenicity, protective
efficacy, and durability of immune response in subjects with
hemoglobinopathies.
A breakthrough infection post
coronavirus vaccine was also observed among the positive antibody
testing group, although the incidence was significantly lower (8.2%)
than the negative group (22.1%), reaffirming its suboptimal
immunogenicity and protective effect in our patients with
hemoglobinopathies. Since several variants of SARS-CoV-2 have been
described during the pandemia, only a few are considered variants of
concern (VOCs) by the WHO; it is pertinent to think that one of these
variants may have decreased the vaccination effectiveness.
SARS-CoV-2,
like other RNA viruses, is prone to genetic evolution while adapting to
their new human hosts with the development of mutations over time,
resulting in the emergence of multiple variants that may have different
characteristics compared to its ancestral strains. These adaptive
mutations in the viral genome can alter the virus's pathogenic
potential, and even a single amino acid exchange can drastically affect
a virus's ability to evade the immune system.[25,26]
One
more interesting finding documented in our patients was the
significantly higher median of serum ferritin level in the group of
subjects with breakthrough coronavirus infection, supporting the
potential role of iron overload on the immune system that may
predispose patients to severe iron overload to SARS-CoV-2 infection (Table 4).[12-15]
Therefore, the impact of iron overload and the benefit of strict
adherence to iron chelation therapy should be recommended in these
vulnerable patients in association to close heart monitoring, as
reported in a previous study showing that heart failure and pulmonary
hypertension are significant risk factors for COVID-19 severity in
thalassemia patients.[27]
Nevertheless, a
28-year-old β-thalassemia major woman, with no significant past medical
history of comorbidities or bleeding tendency, died during breakthrough
coronavirus disease while she got the second dose of vaccine four
months prior to COVID-19 infection. Hypercoagulability is a known
complication of COVID-19. Although severe cases are usually reported in
older populations and in those with underlying comorbidities, our case
emphasizes that this may occur in young without significant medical
history. Intracerebral hemorrhage (ICH) was reported in a 5-case series
with COVID-19 without having an underlying vascular abnormality, as we
observed in our case.
All cases were on anticoagulants, and the
time between symptom onset and ICH identification was 14 to 38 days in
this case series, but it was shorter in our case (one week after
starting COVID-19 symptoms).[28]
The exact
mechanism of COVID-19 associated ICH is not obvious at this young age
case series, and our case supports the hypothesis that endothelial
toxicity and disruption of the renin-angiotensin system might play a
role in COVID-19-mediated ICH.[28-30]
The
recent emergence of multiple variants of SARS-CoV-2 has become a
significant concern for public health worldwide. New variants have been
classified either as VOCs or variants of interest (VOIs) by the CDC
(USA) and WHO. VOCs are associated with enhanced transmissibility or
virulence, reduction in neutralization by antibodies obtained through
natural infection or vaccination, the ability to evade detection, or a
decrease in therapeutics or vaccination effectiveness. The common
feature of these variants is that they share the N501Y mutation
involving the SARS-CoV-2 spike (S) protein, which is precisely the
target of most COVID-19 vaccines. Furthermore, mutations such as N501Y,
E484K, and K417N in the S protein may affect viral fitness and
transmissibility. However, current research on the impact of these
variants on COVID-19 vaccines is still lacking.[31]
SARS-CoV-2
and its variants continue to cause great damages across the world. We
advise that even fully vaccinated people continue to follow all safety
precautions. Since the beginning of the pandemic, a preventive
infection program has been recommended by the Iranian Ministry of
Health that includes social distancing, wearing a mask, ward hospital
isolation of infected patients during blood transfusion, and hand
washing. Continued viral surveillance of new variants must be performed
at regular intervals with viral genomic sequencing, given the
possibility that more highly transmissible, more virulent variants and
treatment-resistant variants could emerge.[32] Moreover, getting a booster shot may help prevent a breakthrough infection or have symptoms, as recommended by the CDC.[33]
Conclusions
Although the safety concern of Astra Zeneca was evaluated in three patients with S/B0 thalassemia,[34]
the current research is the first study that has evaluated the safety
and efficacy of the COVID-19 vaccine in a large population of patients
with hemoglobinopathies. No safety concerns were identified in our
patients who received two doses of the Sinopharm vaccine. However, its
efficacy is not optimal and is associated with a relatively low level
of antibodies against COVID-19 (55%), which indicates a non-full
protective effect. However, it is well-tolerated and seems to reduce
the risk of severe breakthrough COVID-19 infection among patients with
hemoglobinopathies. The frequency of breakthrough infections after full
Sinopharm vaccination supports the evolving dynamic of SARS-CoV-2
variants requiring special challenge since such infection could
represent a risk for vulnerable patients.
References
- Atzrodt CL, Maknojia I, McCarthy RDP, Oldfield TM,
Po J, Ta KTL, Stepp HE, Clements TP. A Guide to COVID-19: a global
pandemic caused by the novel coronavirus SARS-CoV-2. FEBS J. 2020;
287:3633-3650. https://doi.org/10.1111/febs.15375 PMid:32446285 PMCid:PMC7283703
- Lai
CC, Shih TP, Ko WC, Tang H-J, Hsueh PR. Severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019
(COVID-19): The epidemic and the challenges. Int J Antimicrob Agents.
2020; 55(3):105924. https://doi.org/10.1016/j.ijantimicag.2020.105924 PMid:32081636 PMCid:PMC7127800
- World Health Organization (WHO). Coronavirus disease (COVID-19) outbreak situation. Available at: https://www.who.int/emergencies/diseases/novel- coronavirus-2019 cited date Oct 2021.
- Anderson
EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell
JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin
BC, Doria-Rose NA, O'Dell S, Schmidt SD, Corbett KS, Swanson PA 2nd,
Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K,
Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad
CA, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. Safety
and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N
Engl J Med. 2020;383:2427-2438. https://doi.org/10.1056/NEJMoa2028436 PMid:32991794 PMCid:PMC7556339
- Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines:
comparison of biological, pharmacological characteristics and adverse
effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol
Sci. 2021; 25:1663-1669.
- Knoll M.D., Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021; 397:72-74. https://doi.org/10.1016/S0140-6736(20)32623-4
- Hotez
PJ, Nuzhath T, Callaghan T, Colwell B. COVID-19 Vaccine Decisions:
Considering the Choices and Opportunities. Microbes Infect. 2021;
23:104811. https://doi.org/10.1016/j.micinf.2021.104811 PMid:33744495 PMCid:PMC7968147
- Alishaq
M, Nafady-Hego H, Jeremijenko A, Al Ajmi JA, Elgendy M, Vinoy S, Fareh
SB, Veronica Plaatjies J, Nooh M, Alanzi N, Kaleeckal AH, Latif AN,
Coyle P, Elgendy H, Abou-Samra AB, Butt AA. Risk factors for
breakthrough SARS-CoV-2 infection in vaccinated healthcare workers.
PLoS ONE.2021;16(10): e0258820. https://doi.org/10.1371/journal.pone.0258820 PMid:34653228 PMCid:PMC8519462
- Baraniuk C. What do we know about China's covid-19 vaccines? BMJ. 2021;373: n912. https://doi.org/10.1136/bmj.n912 PMid:33836994
- Al
Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al
Nusair M, Hassany M, Jawad JS, Abdalla J, Hussein SE, Al Mazrouei SK,
Al Karam M, Li X, Yang X, Wang W, Lai B, Chen W, Huang S, Wang Q, Yang
T, Liu Y, Ma R, Hussain ZM, Khan T, Saifuddin Fasihuddin M, You W, Xie
Z, Zhao Y, Jiang Z, Zhao G, Zhang Y, Mahmoud S, ElTantawy I, Xiao P,
Koshy A, Zaher WA, Wang H, Duan K, Pan A, Yang X. Effect of 2
Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in
Adults: A Randomized Clinical Trial. JAMA. 2021; 326:35-45. https://doi.org/10.1001/jama.2021.8565 PMid:34037666 PMCid:PMC8156175
- Saeed
BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and
perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis.
2021; 111:219-226. https://doi.org/10.1016/j.ijid.2021.08.013 PMid:34384899 PMCid:PMC8351310
- Haghpanah
S, Hosseini-Bensenjan M, Sayadi M, Karimi M. Incidence Rate of COVID-19
Infection in Hemoglobinopathies: A Systematic Review and Meta-analysis.
Hemoglobin. 2021:1-9. https://doi.org/10.1080/03630269.2021.1927751
- Karimi
M, De Sanctis V. Implications of SARS-CoV 2 infection in thalassemias:
Do patients fall into the "high clinical risk" category? Acta Biomed.
2020;91:50-56.
- Karimi M, Haghpanah S,
Azarkeivan A, Zahedi Z, Zarei T, Akhavan Tavakoli M, Bazrafshan A,
Shirkavand A, De Sanctis V. Prevalence and mortality in β-thalassaemias
due to outbreak of novel coronavirus disease (COVID-19): the nationwide
Iranian experience. Br J Haematol. 2020;190:e137-e140. https://doi.org/10.1111/bjh.16911 PMid:32484906 PMCid:PMC7300954
- Karimi
M, Haghpanah S, Zarei T, Azarkeivan A, Shirkavand A, Matin S, Tavakoli
MA, Zahedi Z, De Sanctis V. Prevalence and severity of Coronavirus
disease 2019 (COVID-19) in Transfusion Dependent and Non-Transfusion
Dependent β-thalassemia patients and effects of associated
comorbidities: an Iranian nationwide study. Acta Biomed.
2020;91(3):e2020007.
- Son KB, Lee TJ,
Hwang SS. Disease severity classification and COVID-19 outcomes,
Republic of Korea. Bulletin of the World Health Organization.
2021;99:62-66. https://doi.org/10.2471/BLT.20.257758 PMid:33658735 PMCid:PMC7924894
- Okba
NMA, Müller MA, Li W, Wang C, Geurtsvan Kessel CH, Corman VM, Lamers
MM, Sikkema RS, de Bruin E, Chandler FD, Yazdanpanah Y, Le Hingrat Q,
Descamps D, Houhou-Fidouh N, Reusken CBEM, Bosch BJ, Drosten C,
Koopmans MPG, Haagmans BL. Severe Acute Respiratory Syndrome
Coronavirus 2-Specific Antibody Responses in Coronavirus Disease
Patients. Emerg Infect Dis. 2020; 26:1478-1488. https://doi.org/10.3201/eid2607.200841 PMid:32267220 PMCid:PMC7323511
- El-Shitany
NA, Harakeh S, Badr-Eldin SM, Bagher AM, Eid B, Almukadi H, Alghamdi
BS, Alahmadi AA, Hassan NA, Sindi N, Alghamdi SA, Almohaimeed HM,
Mohammedsaleh ZM, Al-Shaikh TM, Almuhayawi MS, Ali SS, El-Hamamsy M.
Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine
Among Saudi Residents: A Retrospective Cross-Sectional Study. Int J Gen
Med. 2021;14:1389-1401. https://doi.org/10.2147/IJGM.S310497 PMid:33907443 PMCid:PMC8068468
- Chapin-Bardales J, Gee J, Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA. 2021;325:2201-2202. https://doi.org/10.1001/jama.2021.5374 PMid:33818592
- Kadali
RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA
COVID-19 vaccine: A randomized, cross-sectional study with detailed
self-reported symptoms from healthcare workers. Int J Infect Dis.
2021;106:376-381. https://doi.org/10.1016/j.ijid.2021.04.047 PMid:33866000 PMCid:PMC8049195
- Menni
C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen
LH, Drew DA, Merino J, Hu C, Selvachandran S, Antonelli M, Murray B,
Canas LS, Molteni E, Graham MS, Modat M, Joshi AD, Mangino M, Hammers
A, Goodman AL, Chan AT, Wolf J, Steves CJ, Valdes AM, Ourselin S,
Spector TD. Vaccine side-effects and SARS-CoV-2 infection after
vaccination in users of the COVID Symptom Study app in the UK: a
prospective observational study. Lancet Infect Dis. 2021;21:939-949. https://doi.org/10.1016/S1473-3099(21)00224-3
- Jayadevan R, Shenoy RS, Anithadevi T. Survey of symptoms following COVID-19 vaccination in India. medRxiv. 2021.02.08.21251366 https://doi.org/10.1101/2021.02.08.21251366
- Hatmal
MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM,
Mohamud R. Side Effects and Perceptions Following COVID-19 Vaccination
in Jordan: A Randomized, Cross-Sectional Study Implementing Machine
Learning for Predicting Severity of Side Effects. Vaccines (Basel).
2021; 9 (6) :556. https://doi.org/10.3390/vaccines9060556 PMid:34073382 PMCid:PMC8229440
- Zahid
MN, Moosa MS, Perna S, Buti EB A review on COVID-19 vaccines: stages of
clinical trials, mode of actions and efficacy. Arab J Basic Appl Sci.
2021;28:225-33. https://doi.org/10.1080/25765299.2021.1903144
- Xia
S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z,
Huang W, Xu W, Huang B, Wang H, Wang W, Zhang W, Li N, Xie Z, Ding L,
You W, Zhao Y, Yang X, Liu Y, Wang Q, Huang L, Yang Y, Xu G, Luo B,
Wang W, Liu P, Guo W, Yang X. Safety and immunogenicity of an
inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind,
placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39-351.
https://doi.org/10.1016/S1473-3099(20)30831-8
- Jahromi
M, Al Sheikh MH. Partial protection of Sinopharm vaccine against SARS
COV2 during recent outbreak in Bahrain. Microb Pathog. 2021;158:105086.
https://doi.org/10.1016/j.micpath.2021.105086 PMid:34260903 PMCid:PMC8272690
- Karimi
M, Haghpanah S, Azarkeivan A, Matin S, Safaei A, De Sanctis V.
Coronavirus Disease 2019 (COVID-19) Severity in Patients with
Thalassemias: A Nationwide Iranian Experience. Mediterr J Hematol
Infect Dis. 2021;13(1):e2021008. https://doi.org/10.4084/mjhid.2021.008 PMid:33489047 PMCid:PMC7813286
- Benger
M, Williams O, Siddiqui J, Sztriha L. Intracerebral haemorrhage and
COVID-19: Clinical characteristics from a case series. Brain Behav
Immun. 2020;88:940-4. https://doi.org/10.1016/j.bbi.2020.06.005 PMid:32525049 PMCid:PMC7276127
- Ronaldson
PT, Davis TP. Mechanisms of Endothelial Injury and Blood-Brain Barrier
Dysfunction in Stroke. Primer on Cerebrovascular Diseases. 2nd Ed.,
Elsevier; 2017; 220-6. https://doi.org/10.1016/B978-0-12-803058-5.00045-X
- Divani
AA, Andalib S, Di Napoli M, Lattanzi S, Hussain MS, Biller J,
McCullough LD, Azarpazhooh MR, Seletska A, Mayer SA, Torbey M.
Coronavirus Disease 2019 and Stroke: Clinical Manifestations and
Pathophysiological Insights. J Stroke Cerebrovasc Dis.
2020;29(8):104941. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.104941 PMid:32689643 PMCid:PMC7214348
- Jia
Z, Gong W. Will Mutations in the Spike Protein of SARS-CoV-2 Lead to
the Failure of COVID-19 Vaccines? J Korean Med Sci. 2021 May
10;36(18):e124. https://doi.org/10.3346/jkms.2021.36.e124 PMid:33975397 PMCid:PMC8111046
- Aleem
A, Akbar Samad AB, Slenker AK. Emerging Variants of SARS-CoV-2 And
Novel Therapeutics Against Coronavirus (COVID-19). In: StatPearls
[Internet]. Treasure Island (FL): StatPearls Publishing; 2022. PMID:
34033342.
- COVID-19 Vaccine Booster Shots. www.cdc.gov/vaccines. Accessed on Jan. 19, 2022.
- Alkindi
S, Elsadek RA, Pathare AV. Safety Warning for ChAdOx1 nCov-19 Vaccine
in Patients with Sickle Cell Disease. Mediterr J Hematol Infect Dis.
2021;13(1): e2021059. https://doi.org/10.4084/MJHID.2021.059 PMid:34527211 PMCid:PMC8425344
[TOP]






