Siriyakorn Chansai1,2, Supawadee Yamsri2, Supan Fucharoen2, Goonnapa Fucharoen2 and Nattiya Teawtrakul3.
1
Medical science program, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand 40002.
2
Centre for Research and Development of Medical Diagnostics
Laboratories, Faculty of Associated Medical Sciences, Khon Kaen
University, Khon Kaen, Thailand 40002.
3 Division of
Hematology, Department of Internal Medicine, Srinagarind Hospital,
Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand 40002.
Correspondence to:
Nattiya Teawtrakul, MD, PhD., Internal Medicine Department, Faculty of
Medicine, Khon Kaen University, Mitraphab Road, Maung, Khon Kaen,
Thailand 40002. Tel: 66-43363664, Fax: 66-43204430. E-mail:
nattiya@kku.ac.th
Published: July 1, 2022
Received: February 15, 2022
Accepted: June 15, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022052 DOI
10.4084/MJHID.2022.052
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Thalassemia
is the most common inherited chronic anemia. The patients suffer from
anemia resulting from shortened red blood cell (RBC) survival and
ineffective erythropoiesis (IE). IE is characterized by the premature
death of erythroid precursors in bone marrow or apoptosis of matured
nucleated erythroid cells. Moreover, IE also plays an important role in
the pathophysiology of thalassemia patients. Increased bone marrow
hemopoietic activity from IE frequently leads to the development of
extramedullary hematopoiesis (EMH). Nevertheless, IE is believed to be
the major mechanism that promotes the development of EMH in patients
with thalassemia.[1]
Phosphatidylserine
(PS) is a negatively charged phospholipid located on inner-cell
membranes. Excessive globin chains in thalassemic patients can be
accumulated and precipitated within RBC membranes leading to a flip-out
of the PS phospholipid to the outer RBC membranes. Exposure of the PS
phospholipid on the outer RBC membranes results in RBC destruction at
an early stage in the bone marrow.[2] In thalassemia
disease, it is well established that increased PS-exposed RBC levels
are associated with pulmonary hypertension (PHT), particularly in
splenectomized patients.[3] The correlation of highly PS-exposed RBCs and other complications in thalassemia, however, remains to be elucidated.
Growth
differentiation factor-15 (GDF15) is one of the markers of ineffective
erythropoiesis. It is a regulator of hepcidin expression. In
thalassemia, iron overload and ineffective erythropoiesis induce the
release of GDF15, leading to a high GDF15 level. Increased GDF15 levels
in these patients decrease iron overload by increasing intestinal iron
absorption.[4] High GDF15 levels also correlate with clinical severity in transfusion-dependent thalassemia.[5]
The
soluble transferrin receptor (sTfR) is generated during erythroid cell
maturation. In thalassemia, increased sTfR indicates biomarkers of the
organs' erythropoietic activity and iron status.[6]
Previous studies demonstrated that the correlation of sTfR and EMH
might predict the presence of EMH, particularly in thalassemia patients
with intact spleens.[7]
This study aims to
evaluate the correlation between ineffective erythropoiesis biomarkers
and the development of EMH in patients with thalassemia. It can then be
hypothesized that these results could utilize these biomarkers for
predicting the risk of developing EMH.
Methods
Ethical
approval was obtained from the Institution Review Board (IRB) for human
research at Khon Kaen University, Thailand (HE611361). PS-exposed RBCs,
GDF15, and TfR levels were evaluated before these thalassemia patients
aged >18 who complied with informed consent received blood
transfusion therapy. The study was conducted from April 2019 to January
2020 at Srinagarind Hospital, Khon Kaen University, Thailand. The
history of RBC transfusions, splenectomy, and laboratory data was
reviewed. Spleen length was evaluated by ultrasound technique. Liver
iron concentrations (LIC) and cardiac iron concentrations were
evaluated by the MRI-T2* technique. EMH was confirmed or excluded by
imaging that included ultrasonography, computed tomography (CT) scan,
or magnetic resonance imaging (MRI).
Ineffective erythropoiesis biomarkers.
PS-exposed RBCs were determined by the flow cytometry technique. RBCs
staining and PS exposure were performed as described by Pattanapanyasat
et al.[8] Fixed RBCs were measured using FACSCanto II
flow cytometry and analyzed with the BD FACSDiva version 6.1.3 software
(BD Biosciences). The number of positive cells labeled with
FITC-annexin V and PE-glycophorin A was computed. Isotype
control-positive cells were restricted to < 0.3%. GDF15 and sTfR
levels were determined using enzyme-linked immunosorbent assay (ELISA)
kits, i.e., the GDF-15 Human ELISA kit (Abcam, Cambridge, UK), and the
Human sTfR ELISA (BioVendor, Brno, Czech Republic).
Thalassemia genotypes.
Hemoglobin and DNA analyses were performed in all patients to determine
the thalassemia genotypes. As described previously, common
β-thalassemia and α-thalassemia mutations were detected by
multiplex-gap PCR and allele-specific PCR assays.[9]
Transfusion requirements.
Transfusion-dependent thalassemia (TDT) is a group of patients
requiring a regular blood transfusion at less than six-week intervals.
The remaining patients were classified as non-transfusion-dependent
thalassemia (NTDT).
Statistical analysis.
Independent sample Student's t-tests and the Mann-Whitney U-test were
used to compare continuous data between two groups. Bivariate
correlation analysis was performed with Pearson or Spearman
correlations. A P-value < 0.05 was considered statistically
significant. Logistic regression methods were used to demonstrate the
associations between ineffective erythropoiesis biomarkers and EMH. The
receiver operating characteristic (ROC) curves were constructed to
determine the diagnostic performance of ineffective erythropoiesis
biomarkers to predict the development of EMH. Data analyses were
performed using SPSS 26.0 software (IBM., IL, USA) and STATA 10
statistical software (Stata Corp, College Station, TXP).
Results
One
hundred and thirty-one patients were enrolled in this cohort. The
patients were classified into two groups: β-thalassemia and
α-thalassemia. The clinical characteristics and laboratory data are
summarized in Table 1. The
proportion of patients with splenectomy was more prevalent in patients
with β-thalassemia than those with α-thalassemia (51.1% vs. 25.6%, p =
0.005). More than half of the patients with β- thalassemia were TDT
(54.5%) in contrast to patients with α-thalassemia, of whom most were
NTDT (81.4%). Extramedullary hematopoietic tissues were found in
thirty-four patients (26.0%). EMH was more prevalent among patients
with β-thalassemia (32, 36.4%) than patients with α-thalassemia (2,
4.7%). Serum ferritin and LIC levels were significantly higher among
patients with β-thalassemia compared to patients with α-thalassemia.
The mean spleen length in non-splenectomized patients was not different
in both groups (15.1 vs. 14.5 cm.).
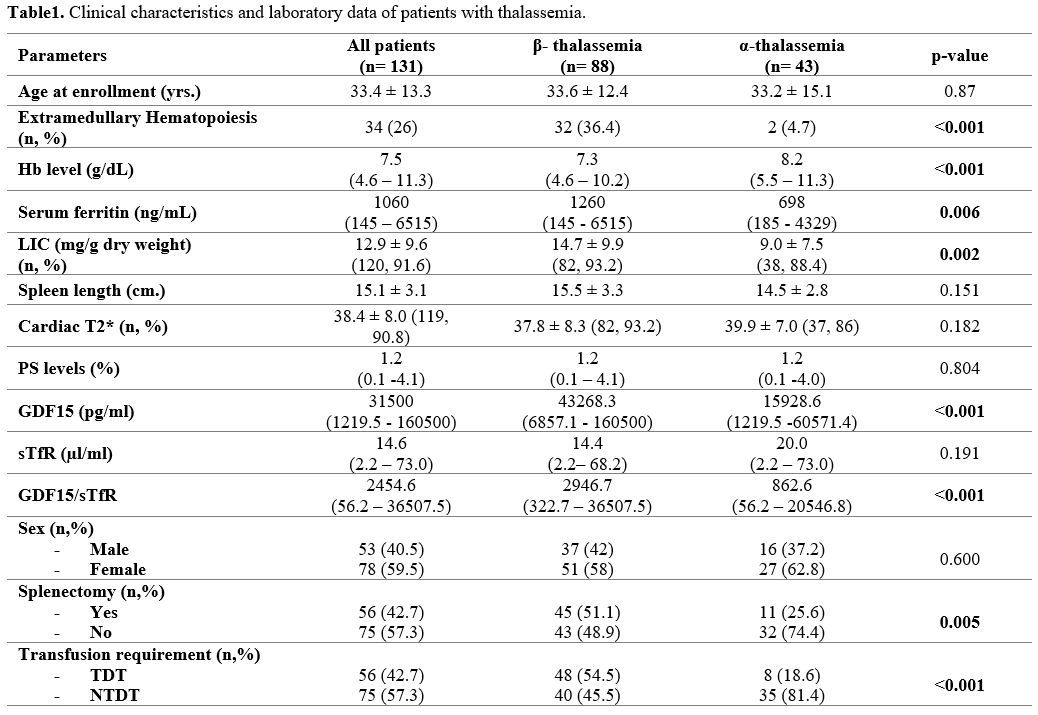 |
Table 1. Clinical characteristics and laboratory data of patients with thalassemia. |
GDF15
levels and GDF15/sTfR ratios in patients with β-thalassemia were
significantly higher than in those with α-thalassemia. On the contrary,
PS-exposed RBCs and sTfR levels were not statistically significantly
different between the two groups.
A multivariate analysis of these risk factors for EMH was performed, as shown in Table 2.
It was found that advanced age and PS-exposed RBC levels remained
significantly associated with EMH after adjustment for other factors
with an adjusted odds ratio of 1.04 (95% CI 1.0 -1.07) p = 0.026 and
1.71 (95% CI 1.05- 2.8) p = 0.032.
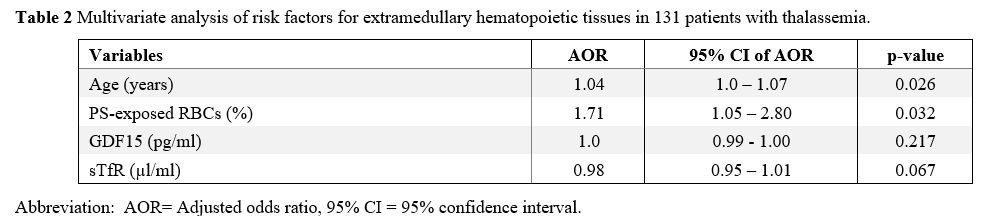 |
Table
2. Multivariate analysis of risk factors for extramedullary hematopoietic tissues in 131 patients with thalassemia. |
The
receiver-operating characteristic (ROC) curve analysis of the
PS-exposed RBC levels and EMH was constructed to identify the optimal
cut-off point (Figure 1). The
cut-off level of PS-exposed RBCs derived from the ROC curve in this
study was 0.45%. Using this cut-off level, the sensitivity and
specificity of PS-exposed RBC prediction of EMH were 94.1% and 80.4%,
with an area under ROC of 0.67 (95%CI 0.57-0.78), p-value = 0.002.
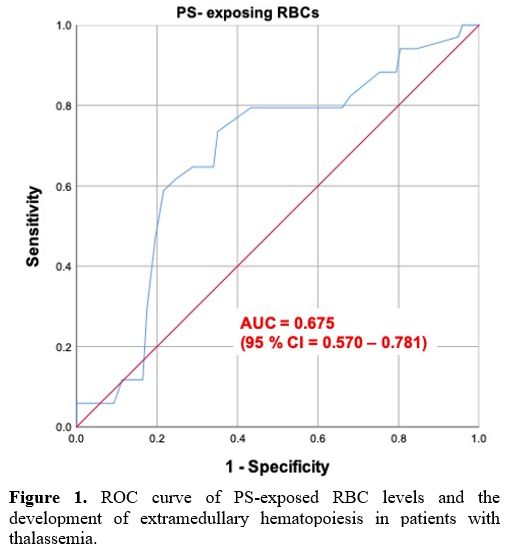 |
Figure 1. ROC curve
of PS-exposed RBC levels and the development of extramedullary
hematopoiesis in patients with thalassemia. |
Discussion
EMH
is one of the main thalassemia-related complications in patients with
thalassemia. It is more prevalent in patients with β-thalassemia
compared to patients with α-thalassemia. Among the ineffective
erythropoiesis biomarkers, PS-exposed RBCs showed a modest correlation
with EMH. The levels of PS-exposed RBCs were not different between
β-thalassemia and α-thalassemia groups. However, the PS-exposed RBC
levels in patients with thalassemia were higher than in normal
controls.[10] Previous studies showed significantly
elevated levels of PS-exposed RBCs in β-thalassemia/Hb E patients who
underwent splenectomy and were associated with pulmonary hypertension.[11] This study demonstrated a correlation between PS-exposed RBCs levels and EMH in patients with thalassemia.
Abnormal
phosphatidylserine (PS) exposure on the surface of RBCs is considered a
principal feature of apoptotic RBC precursors and ineffective
erythropoiesis in thalassemia.[12] The PS-exposed
RBCs may be one of the biomarkers that represent underlying ineffective
erythropoiesis. This study showed that PS-exposed RBCs might be
considered a biomarker to predict the development of EMH in patients
with thalassemia. As shown in Figure 1,
the PS-exposed RBC levels of more than 0.45% can be used to predict the
outcome of EMH with 94.1% sensitivity and 80.4% specificity.
GDF15
is a transforming growth factor-β (TGF- β) superfamily member.
Therefore, increased GDF15 levels were considered a marker of
ineffective erythropoiesis and iron overload.[13]
This current study showed that GDF15 levels in β-thalassemia were
significantly higher than in α-thalassemia, consistent with previous
studies.[14] High GDF15 levels suppressed hepcidin
expression, contributing to increased gastrointestinal iron absorption
and ineffective erythropoiesis.[4,13]
In this study, GDF15 concentration had a weak correlation with EMH.
This finding may be explained by ineffective erythropoiesis and the
iron overload that the GDF15 levels can influence in patients with
thalassemia.
The soluble transferrin receptor is one of the
erythropoiesis biomarkers. Previous studies showed that sTfR levels
could represent a predictive factor for EMH, particularly in NTDT
patients with a spleen.[7] However, in the present
study, sTfR levels between β- and α-thalassemia were not significantly
different and could not predict EMH. In addition, the number of
β-thalassemia patients with splenectomy was markedly higher than
α-thalassemia patients (51.1% vs. 25.6%). This distinction may indicate
that splenectomy is a risk factor for paraspinal EMH that supports the
hypothesis of an association between iron metabolism and erythropoiesis
expansion and the impact of splenectomy on EMH.[7] In
this study, sTfR levels were correlated with GDF15 levels only in
β-thalassemia. A previous study has shown a correlation between log
GDF15 and sTfR level in β- thalassemia intermedia (TI) and β-
thalassemia/Hb E.[14] Nevertheless, this could be due
to a small sample size of α-thalassemia in this study. In addition,
most thalassemia patients were non-transfusion-dependent thalassemia
(NTDT), and GDF15 levels might affect iron overload and sTfR
The
spleen is the most commonly affected organ in compensating for
ineffective erythropoiesis. This study showed that the two groups'
spleen length was not significantly different, but massive splenomegaly
was different. Massive splenomegaly (spleen length ≥ 17 cm)[15]
was more prevalent in patients with β-thalassemia than those patients
with α-thalassemia (13 vs. 7 cases). This result demonstrated that an
enlarged spleen represented ineffective erythropoiesis in patients with
thalassemia.[16] Literature showed that the spleen and liver are the most common sites of EMH in patients with thalassemia.[17,18]
Extramedullary
hematopoiesis is a compensation for the underlying ineffective
erythropoiesis. It is a time-dependent process. Advanced age is a
significant risk factor for developing EMH in patients with
thalassemia. This study also confirms that advanced age is a risk
factor for developing EMH.
The limitation of this study is that
the number of patients with α-thalassemia in this cohort was relatively
small because most of the patients with α-thalassemia were
asymptomatic. Therefore, α-thalassemia is rarely encountered in a
tertiary hospital. Nevertheless, to the best of the current authors'
knowledge, this is the first study demonstrating the association
between PS-exposed RBCs and EMH in patients with thalassemia.
In
conclusion, extramedullary hematopoiesis is more prevalent in patients
with β-thalassemia than in patients with α-thalassemia. Advanced age
and high PS-exposed RBC levels had a significant association with EMH.
Among the ineffective erythropoiesis biomarkers, PS-exposed RBCs showed
a modest correlation with EMH. PS-exposed RBCs may prove useful in
predicting the development of EMH in patients with thalassemia.
Acknowledgements
The
authors would like to thank Emeritus Professor James A. Will,
University of Wisconsin-Madison, for help in preparing the manuscript
via the publication clinic of Khon Kaen University, Thailand. This
study received grant support from the Thailand Research Fund (TRF)
Research Team Promotion Grant (RTA) of the Thailand Science Research
and Innovation (TSRI), Thailand (Contract ID RTA6280005) and the
Faculty of Medicine, Khon Kaen University.
References
- Teawtrakul N, Chansung K, Sirijerachai C, et al. A
Clinical Risk Score for Predicting Paraspinal Extramedullary
Hematopoiesis in Patients with Thalassemia: The KKU-EMH Score. J Med
Assoc Thai 2017; 100 (4):389-95. PMID: 29911832.
- Schrier SL. Thalassemia: Pathophysiology of Red Cell Changes. Annu Rev Med 1994;45:211–218. https://doi.org/10.1146/annurev.med.45.1.211
- Singer
ST, Kuypers FA, Styles L, et al. Pulmonary Hypertension in Thalassemia:
Association with Platelet Activation and Hypercoagulable State. Am J
Hematol 2006;81(9):670–675. https://doi.org/10.1002/ajh.20640
- Tanno
T, Bhanu NV, Oneal PA, et al. High Levels of GDF15 in Thalassemia
Suppress Expression of the Iron Regulatory Protein Hepcidin. Nat Med
2007;13(9):1096–1101. https://doi.org/10.1038/nm1629
- Musallam
KM, Taher AT, Duca L, et al. Levels of Growth Differentiation Factor-15
Are High and Correlate with Clinical Severity in
Transfusion-Independent Patients with β Thalassemia Intermedia. Blood
Cells Mol Dis 2011;47(4):232–234. https://doi.org/10.1016/j.bcmd.2011.07.005
- Cazzola
M, Beguin Y, Bergamaschi G, et al. Soluble Transferrin Receptor as a
Potential Determinant of Iron Loading in Congenital Anaemias Due to
Ineffective Erythropoiesis. Br J Haematol 1999;106(3):752–755. https://doi.org/10.1046/j.1365-2141.1999.01600.x
- Ricchi
P, Ammirabile M, Costantini S, et al. A Useful Relationship between the
Presence of Extramedullary Erythropoeisis and the Level of the Soluble
Form of the Transferrin Receptor in a Large Cohort of Adult Patients
with Thalassemia Intermedia: A Prospective Study. Ann Hematol
2012;91(6):905–909. https://doi.org/10.1007/s00277-011-1385-y
- Pattanapanyasat
K, Noulsri E, Fucharoen S, et al. Flow Cytometric Quantitation of Red
Blood Cell Vesicles in Thalassemia. Cytometry B Clin Cytom
2004;57(1):23–31. https://doi.org/10.1002/cyto.b.10064
- Yamsri
S, Sanchaisuriya K, Fucharoen G, et al. Prevention of Severe
Thalassemia in Northeast Thailand: 16 Years of Experience at a Single
University Center. Prenat Diagn 2010;30(6):540–546. https://doi.org/10.1002/pd.2514
- Chansai
S, Fucharoen S, Fucharoen G, et al. Elevations of Thrombotic Biomarkers
in Hemoglobin H Disease. Acta Haematol 2018;139(1):47–51. https://doi.org/10.1159/000486157
- Atichartakarn
V, Angchaisuksiri P, Aryurachai K, et al. Relationship between
Hypercoagulable State and Erythrocyte Phosphatidylserine Exposure in
Splenectomized Haemoglobin E/Beta-Thalassaemic Patients. Br J Haematol
2002;118(3):893–898. https://doi.org/10.1046/j.1365-2141.2002.03711.x
- Ibrahim HA, Fouda MI, Yahya RS, et al. Erythrocyte Phosphatidylserine Exposure in β-Thalassemia. Lab Hematol 2014;20(2):9–14. https://doi.org/10.1532/LH96.12016
- Kaddah
AM, Abdel-Salam A, Farhan MS, et al. Serum Hepcidin as a Diagnostic
Marker of Severe Iron Overload in Beta-Thalassemia Major. Indian J
Pediatr 2017;84(10):745–750. https://doi.org/10.1007/s12098-017-2375-4
- Porter
JB, Cappellini MD, Kattamis A, et al. Iron Overload across the Spectrum
of Non-Transfusion-Dependent Thalassaemias: Role of Erythropoiesis,
Splenectomy and Transfusions. Br J Haematol 2017;176(2):288–299. https://doi.org/10.1111/bjh.14373
- Taher A, Isma'eel H and Cappellini MD. Thalassemia Intermedia: Revisited. Blood Cells Mol Dis 2006;37(1):12–20. https://doi.org/10.1016/j.bcmd.2006.04.005
- Rivella S. Ineffective Erythropoiesis and Thalassemias. Curr Opin Hematol 2009;16(3):187–194. https://doi.org/10.1097/MOH.0b013e32832990a4
- Ricchi
P, Meloni A, Spasiano A, et al. Extramedullary Hematopoiesis Is
Associated with Lower Cardiac Iron Loading in Chronically Transfused
Thalassemia Patients. Am J Hematol 2015;90(11):1008–1012. https://doi.org/10.1002/ajh.24139
- Ricchi
P, Ammirabile M, Spasiano A, et al. Extramedullary Haematopoiesis
Correlates with Genotype and Absence of Cardiac Iron Overload in
Polytransfused Adults with Thalassaemia. Blood Transfus 2014;12 Suppl
1:s124-130. https://doi.org/10.2450/2013.0287-12
[TOP]


