This study characterizes pediatric patients admitted for investigation and confirmed diagnosis of VL by Çukurova University Hospital, Pediatric Infectious Diseases Department, Adana, in the south of Turkey. It serves as a research hospital and is an important center where patients come for treatment from the south/southeast regions of Turkey and Syrian immigrants. In addition, other family members of the case were questioned and examined in terms of clinical findings, and simple hematological tests were performed.
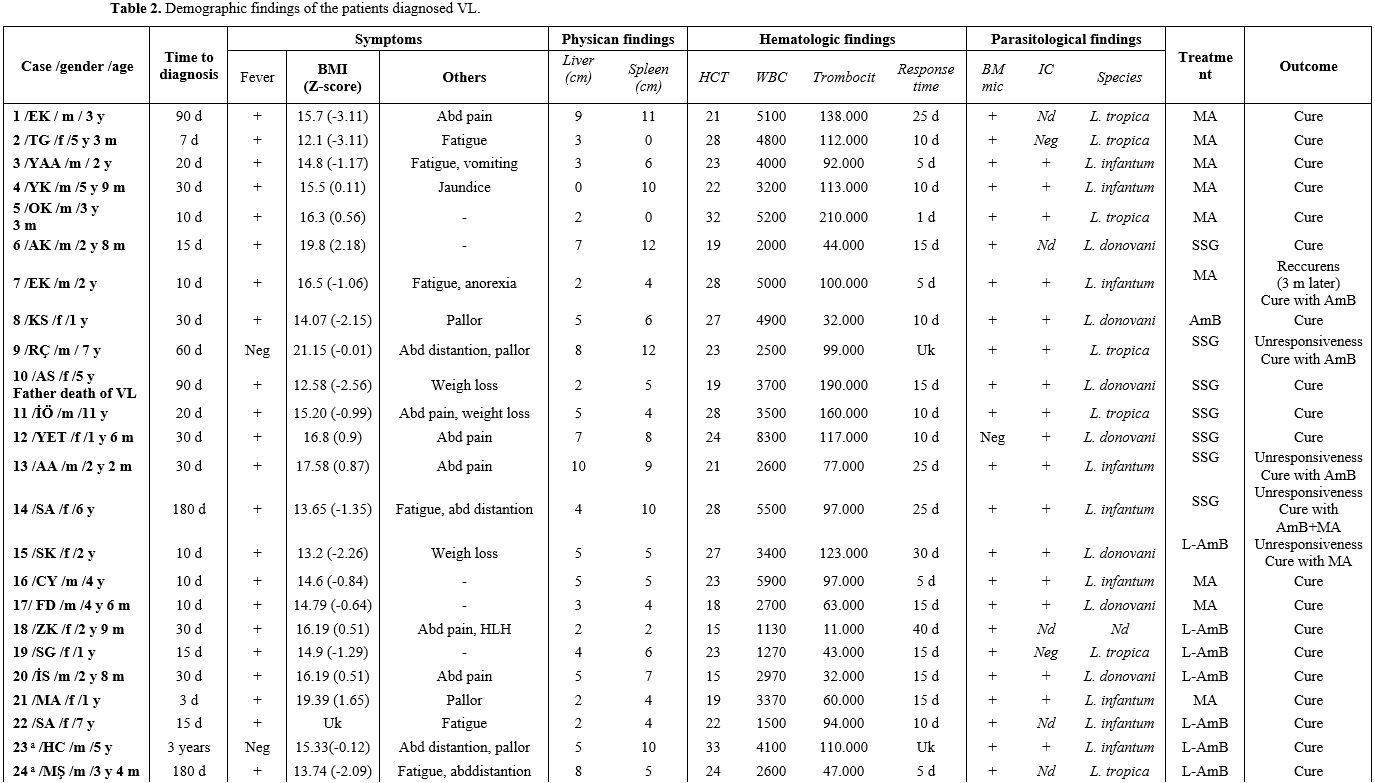
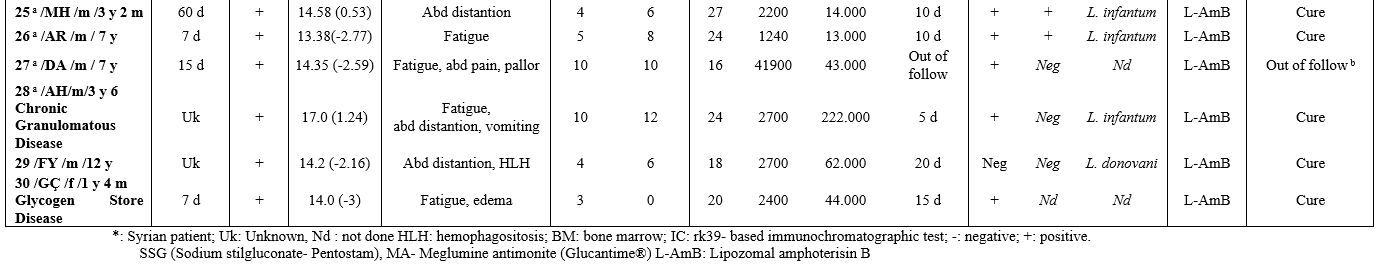
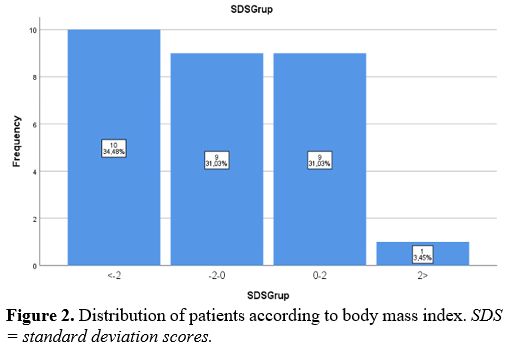
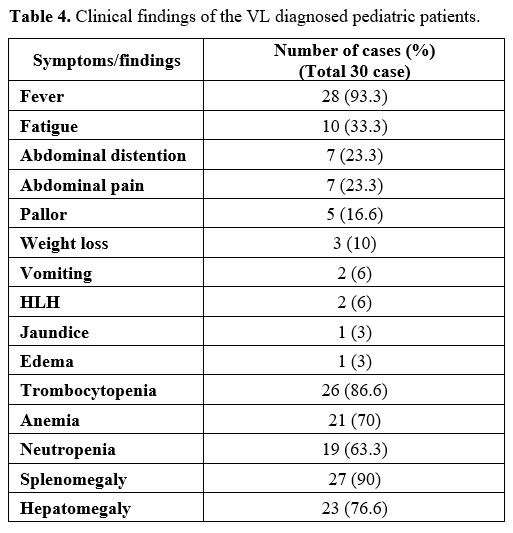
In the clinical examination of the patients included in this study, permanent systemic infections such as fever, fatigue, anorexia, weight loss, and sizes of liver and spleen were investigated. In addition, demographic characteristics of the patients, such as age and gender, were examined in this study. Body mass index (BMI) was determined by dividing weight (kg) by height (m) squared. On WHO charts, the normal range is generally defined as between -2 SD and +2 SD (i.e., Z-scores between -2.0 and +2.0), corresponding to approximately the 2nd and 98th percentiles. In addition, non-specific laboratory data such as full blood count, gamma-globulin concentration, and erythrocyte sedimentation rate (ESR) were reported.
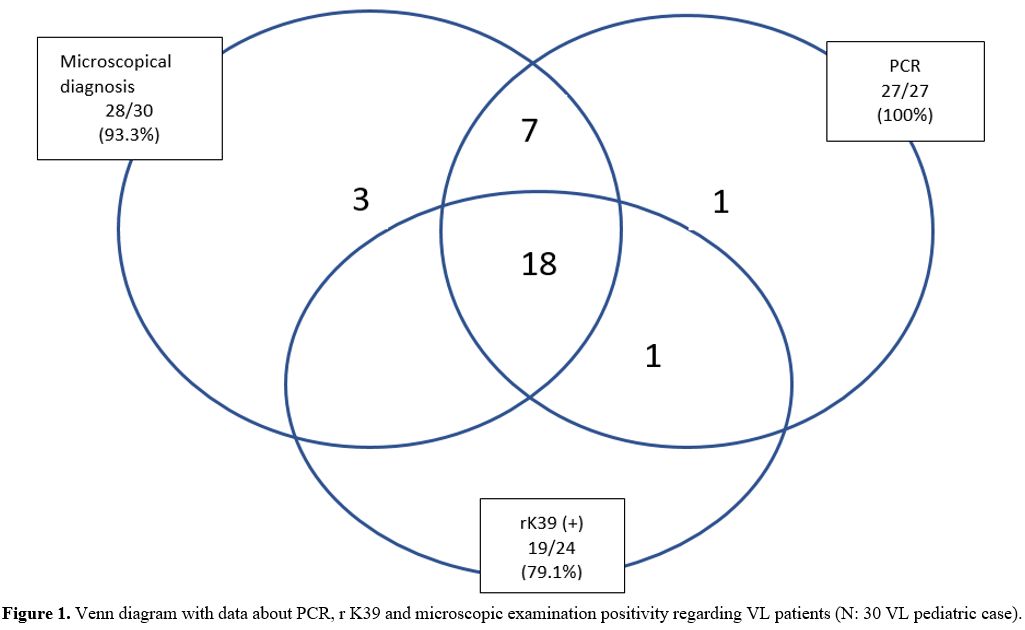
Laboratory Methods. Clinical examination of VL was confirmed by detection of amastigotes in bone-marrow aspirate, and/or rK39, and/or molecular tests (genus-specific PCR, Real-Time PCR, ITS1 PCR-RFLP, DNA sequencing, and Phylogenetic analysis).
Microscopic Examination. The bone marrow sample was smeared onto a glass slide. The prepared bone marrow samples were fixed with absolute methanol, stained with 10% Giemsa, and examined under the light microscope with magnification oil 100X objective. The preparations with amastigote were considered positive, and those without amastigote were considered negative.
The Recombinant Antigen Dipstick Test (rK39). The rapid immune-chromatographic test is based on a recombinant 39-amino acid repeat antigen. The dipstick test was performed according to the manufacturers' instructions (InBios International, Inc. Seattle, WA). Twenty microliters of serum taken from the patients were mixed with two drops of buffer in the rK39 dipstick test. After 10 minutes of incubation, serum samples with two bands on the cellulose strip were considered positive, and serum samples without were considered negative only if the control band appeared.
Molecular tests.[8-10]
Genus-Specific PCR Assay. DNA was extracted from bone marrow samples with an Agencourt DNA kit (Beckman Coulter, Beverly, USA), according to the manufacturer's protocol. The kDNA using the primers 13A (5'-GTG GGG GAG GGG CGT TCT-3') and 13B (5'-ATT TTC CAC CAA CCC CCA GTT-3') was investigated for the presence of Leishmania in clinical samples.[8]. A reaction mix with a final volume of 25 µL consisted of 0.2 mM of dNTP, 1.5 mM MgCl2, 1 unit of Taq polymerase, 1 µM of each primer, and 5 µL DNA samples for use PCR analyses. The DNA was amplified for 35 cycles in a thermocycler, set to run at 94°C for 1 min, 54°C for 1 min, and 72°C for 1 min for each one.
The amplification reactions were analyzed by bromide staining and visualization under UV light. The PCR products with a fragment image of approximately 120 bp under UV light were considered positive.
Real-Time PCR Assay. The kDNA minicircle gene region was targeted to identify Leishmania species, and Leishmania isolates were amplified by melting real-time curve PCR (Real-Time PCR) using primers JW13 and JW14.[9] The Real-Time PCR was performed by hot-start PCR using the LightCycler FastStart DNA Master SYBR Green I Kit in a LightCycler™ (Roche Diagnostics). The 20 µL reaction mixture contained 1 X LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics, Meylan, France), 2 mM MgCl2, 10 µM of each primer and 5 µL of DNA of the parasite.
The Real-Time PCR Reaction and DNA samples were amplified using a 35 cycles thermal cycler program consisting of 10 s at 95°C, 10 s at 53°C, and 10 s at 72°C. The melting program consisted of one cycle of 95°C for 10 s, 67°C for 10 s, and heating at 0.1°C per 1 second to 98°C. The transition rates were 20°C/s except for the extension step and the final step, which had a temperature transition rate of 1°C/s and 0.1°C, respectively. Fluorescence was measured through the slow heating phase. For improved visualization of the melting temperatures (Tm), melting peaks were derived from the initial melting curves (fluorescence [F] versus temperature [T] by plotting the negative derivative of fluorescence over-temperature –dF/dT versus dT). The melting temperatures were 88.5±0.4°C for L. donovani, 89.3±0.2°C for L. tropica, and 90.5±0.2°C for L. infantum, respectively.
ITS1 PCR-RFLP Assay. In this study, to identify Leishmania species according to the study of Schönian et al., the ITS1 gene region was targeted, and LITSR-L5.8S primers were designed.[10] The 40 µL PCR reaction mixture consisted of PCR buffer 1 X (75 mM KCl, pH 8.3 and 20 mM Tris-HCl, 1.5 mM MgCl2, 1U Taq polymerase), 0.2 mM dNTPs, 0.5 pmol of each primer and 5 µL of DNA sample. After the initial denaturation (5 min at 94°C), 35 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 54°C, and elongation for 1 min at 72°C were carried out, and a final extension terminated the PCR at 72°C for 10 min. The PCR products were analyzed in 1% agarose gel by electrophoresis at 100 V in 1 X Tris-boric-EDTA buffer (0.04 mM Tris-boric and 1mM EDTA, pH 8) and visualized by UV light after being stained with ethidium bromide.
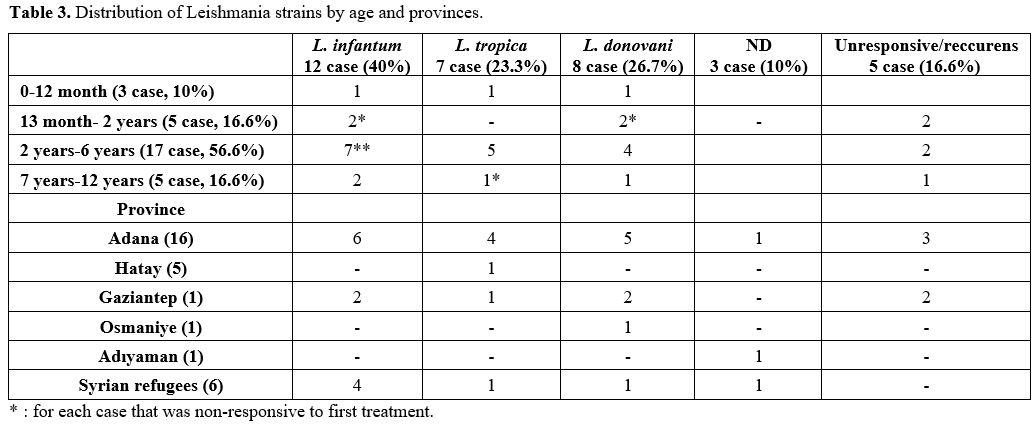
All PCR samples with approximately 350 bp fragment length bands were considered positive according to the agarose gel electrophoresis. ITS1 PCR products (10 µL) were digested with the Hae III restriction enzyme (Promega, Wisconsin, USA) according to the manufacturer's instructions, and the restriction fragments were analyzed by 2% agarose gel electrophoresis. The restriction products were identified as L. donovani, L. infantum, and L. tropica, according to Schönian et al.[10]
DNA Sequencing. ITS1 PCR products were purified for DNA sequencing using a SentroPure DNA purification kit (Sentromer DNA, Istanbul, Turkey). Purified PCR products were sequenced with LITSR-L5.8S primers using BigDye Terminator V 3.1 cycle sequencing kit (Applied Biosystems). This reaction was analyzed according to the instructions for the 3730 DNA analyzer (Applied Biosystems). The ITS1 PCR products of Leishmania isolate sequences were approximately 320 bp in length. The reference strains of Leishmania species (L. donovani ATC 50212, WHOM/IN/80/DD8; L. infantum ATCC 501340, WHOM/TN/80/IPT-1; L. tropica ATCC 50129, WHOM/SU/74/K27) were used in this analysis. The obtained sequences were processed with the available GenBank and checked using basic local alignment search tool (BLAST) analysis software (www.ncbi.nlm.nih.gov/BLAST).
Treatment. The conventional treatment of VL/kala-azar consists of pentavalent antimony salts (PAS- Sbv) - sodium stibogluconate (SSG (Pentostam)) and meglumine antimoniate (MA, (Glucantime®)). In addition, liposomal amphotericin B (L-AMB (AmBisome®)) was used intravenously at a dosage of 3 mg/kg on days 1-5, 10, and 21 (for a cumulative dose of 21 mg/kg). The therapy used in our department was one of the following in Table 1 (choice made according to a supplied drug that day, and/or the patient's suitability, and/or the physician's preference).
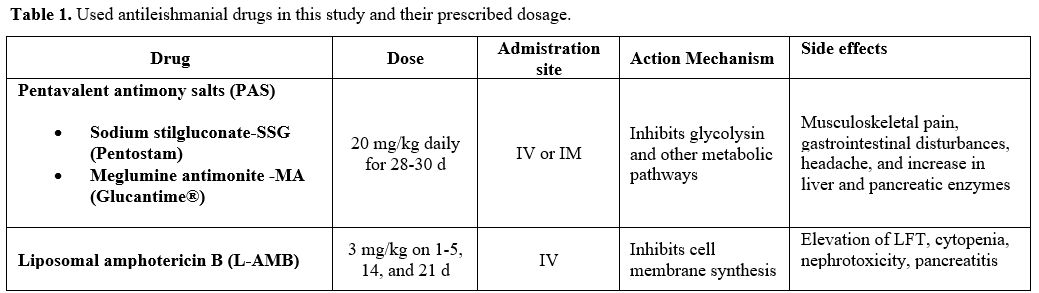 |
Table 1. Used antileishmanial drugs in this study and their prescribed dosage. |
Follow-up. Post-treatment follow-up (at least 1 year, every month for the first 3 months, then every 2 months, and every 3 months after 6 months) included clinical examination, blood cell count, and ESR.
Statistical analysis. The medications used and/or management modalities were collected on a spreadsheet. The Statistical Package for the Social Sciences (SPSS) version 23.0 (IBM, Somers, NY, USA) was used for statistical analysis. Categorical measurements were summarized as numbers and percentages, while continuous measurements were summarized as mean, deviation, and minimum-maximum.
Ethics Statement. Confidentiality and patient privacy were maintained throughout the process. Ethical approval was obtained from Çukurova University Scientific Research Ethics Committee (decision no: 03.04.2022/120) and was performed in accordance with the ethical standards of the Declaration of Helsinki.