Panagiota Zikidou1, Christina Tsigalou2, Gregorios Trypsianis3, Alexandros Karvelas2, Aggelos Tsalkidis1 and Elpis Mantadakis1.
1 Department of Pediatrics, Democritus University of Thrace, University General Hospital of Alexandroupolis, Thrace, Greece
2 Laboratory of Microbiology, Democritus University of Thrace, University General Hospital of Alexandroupolis, Thrace, Greece
3 Department of Medical Statistics, Democritus University of Thrace Faculty of Medicine, Alexandroupolis, Thrace, Greece
Correspondence to:
Elpis Mantadakis, MD, PhD Professor of Pediatrics-Pediatric
Hematology/Oncology. Department of Pediatrics, Hematology/Oncology
Unit, University General Hospital of Alexandroupolis. 68100
Alexandroupolis, Thrace, Greece. Tel: +30-25513-51411, Fax:
+30-25510-30340, E-mail:
emantada@med.duth.gr
Published: July 1, 2022
Received: March 11, 2022
Accepted: June 16, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022054 DOI
10.4084/MJHID.2022.054
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objective:
Iron deficiency (ID) is a major public health problem with high
prevalence in early childhood. We assessed the prevalence of anemia,
ID, and iron deficiency anemia (IDA) in healthy children of Thrace,
Greece, its correlation with several factors, and evaluated the
diagnostic performance of hematologic and biochemical markers of
sideropenia.
Patients and Methods:
For 202 healthy children 1-5 years old, a questionnaire was filled out
describing their nutritional habits during infancy and early childhood.
Venous hemograms along with serum ferritin, TIBC, %TS, and CRP were
obtained from all studied children. In a subset of 156 children, the
concentration of sTfR was also determined.
Results: Children with ID and IDA had significantly lower beef consumption than children without sideropenia (p=0.044).
Using the WHO cutoff values of Hb <11g/dl and ferritin <12μg/l,
the prevalence of anemia, ID, and IDA was 9.41%, 6.44%, and 3.47%,
respectively. If Hb <12g/dl and ferritin<18μg/l were used as
cutoffs, the prevalence of anemia, ID, and IDA was 26.73%, 16.33%, and
5.94%, respectively. ROC analysis revealed that at ferritin <12μg/l,
the AUC of sTfR alone (0.827) was substantially better than that of
TIBC (0.691), while at serum ferritin cutoff of 18μg/l, the AUC of TIBC
(0.770) was better than that of sTfR (0.716).
Conclusions:
The prevalence of ID and IDA in children 1-5 years old in Thrace is
like in other developed countries. The chosen cutoff of serum ferritin
affects the evaluation of the diagnostic significance of the different
sideropenia markers.
|
Introduction
Iron
deficiency (ID) is the most common micronutrient deficiency in all
countries and is a major public health problem with high prevalence in
early childhood.[1] Initially, ID leads to decreased
body iron stores without anemia. However, when the iron stores are
eventually depleted, iron deficiency anemia (IDA) occurs, i.e., a drop
in hemoglobin (Hb) is noticed.[1] Anemia is a major public health problem worldwide, and approximately 50% of it is due to ID.[2]
According to the World Health Organization (WHO), about 35% of the
world's population, i.e., > 2 billion people, suffer from anemia.[3] The prevalence of ID worldwide is estimated to be 2 to 2.5 times higher than that of IDA.[4]
Three
key questions arise when dealing with the diagnosis of IDA, i.e., which
children should be screened for, with what hematologic and biochemical
markers, and with what diagnostic cutoff values. The WHO recommends
targeted screening for IDA in children before iron administration if
the prevalence of anemia is >5%.[2] The American
Academy of Pediatrics recommends universal screening for IDA at one
year of age.[5] However, the US Preventive Services Task Force questions
the value of IDA screening in asymptomatic children 6-24 months old.[6] Finally, the U.S. Centers for Disease Control and Prevention recommends targeted screening in children at high risk for IDA.[7]
Hb
concentration is used for the diagnosis of anemia. However, it cannot
be used as the sole marker of IDA as it lacks specificity and
sensitivity.[8-10] Serum ferritin concentration is the
most widely used marker of ID, as it reflects the body's iron stores
with high specificity but moderate sensitivity because it increases in
the presence of inflammation.[6,11]
Transferrin is a hepatic glycoprotein that carries nutritional iron
from the gut to sites of iron storage and the bone marrow. Transferrin
saturation (%TS) is the percentage of transferrin occupied by iron.[2,11] Total iron-binding capacity (TIBC) is the maximum amount of iron that can bind to transferrin and is increased in IDA.[11]
Transferrin allows the intracellular transport of iron by binding to
transferrin receptors, which are transmembrane proteins found on the
surface of most body cells. Soluble transferrin receptors (sTfR) are
portions of transferrin receptors that circulate in the blood. When
cellular iron uptake is insufficient, an elevation of TfR occurs that
allows the cell to compete more efficiently for circulating iron, thus
resulting in more circulating sTfR. sTfR are typically elevated in IDA
and are less affected by inflammation than serum ferritin.[2,5,11]
In addition, they signal the transition of subclinical ID from depleted
iron stores to ineffective erythropoiesis and do not increase in serum
until the body's iron stores are exhausted.[5]
Therefore, the ratio of sTfR to the common logarithm of serum ferritin
concentration, also known as the sTfR/Fer index, has a greater
diagnostic value for IDA than the use of sTfR and ferritin alone,
especially in patients with inflammatory conditions.[12]
However, the above ratio has not been adequately studied in infants and
children, and limited studies have been performed to determine its
reference range and cutoff values for ID.[10,12-15]
In
Greece, the prevalence of anemia, ID, and IDA is confounded by the high
prevalence of heterozygous thalassemia and has not been well-studied
during the last decade in infants and toddlers. The Thrace region is
one of the least developed areas of Greece, with lower income than the
rest of the country. This prospective study aimed to assess the
prevalence of anemia, ID, and IDA in healthy children 1-5 years old in
Thrace and correlate it with several factors. We also evaluated the
diagnostic performance of hematologic and biochemical markers of ID and
IDA when different cutoff values of serum ferritin and Hb were used to
define ID and IDA.
Patients and Methods
From
March 2019 to August 2021, we prospectively studied the prevalence of
anemia, ID, and IDA in healthy children 1-5 years old. For the sample
size calculation, we assumed the prevalence of ID to be around 10%.
Hence, with an accuracy of ± 4% and with a confidence interval of 95%,
about 200-250 children had to be studied.
Our study population
included 202 healthy children 1-5 years old who lived permanently in
Thrace and visited the University General Hospital of Alexandroupolis
or the General Hospital of Didymoteicho for well-child visits during
the study period. Children with chronic diseases, infections [serum
C-reactive protein (CRP) >0.5mg/dl)], bleeding disorders, known
anemia due to other causes beyond ID, and permanent residence outside
Thrace were excluded. The Scientific Institutional Review Boards
approved the study of both participating hospitals. The parents or
guardians signed a written informed consent to provide detailed
demographic and medical information and to allow laboratory testing of
their children. The study's questionnaire included demographic
information (child's age and sex) and information regarding parental
socioeconomic status and nutritional habits during infancy and early
childhood. Venous blood sampling was performed for complete blood count
(CBC) measurement along with serum ferritin, TIBC, %TS, and CRP, to
assess the prevalence of anemia, ID, and IDA. In a subset of 156
patients with an adequate amount of available serum, the concentration
of sTfR was also determined. The Sysmex 5000 analyzer (Sysmex
Corporation, Kobe, Japan) was used for CBC determination, while the
Immulite 1000 analyzer (Siemens Healthcare, Erlangen, Germany) was used
for serum ferritin measurement. The Targa 1500 analyzer (Biotecnica
Instruments S.p.A., Rome, Italy) and the FERENE direct colorimetric
method were used for TIBC and TS measurement. Finally, the ADVIA 2400
analyzer (Siemens Healthcare, Erlangen, Germany) and the
immunoturbidimetry method were used to determine CRP and sTfR. The
definition of WHO, i.e., Hb concentration <11g/dl for children 1-5
years old, was used to define anemia.[2] The National Health and Nutrition Examination Survey (NHANES) serum ferritin cutoff of <12μg/l was used to delimit ID.[16]
The combination of low Hb and serum ferritin was used to define IDA.
Finally, the sTfR/Fer index was calculated, as previously described.[17,18]
Statistical
analysis was performed using the Statistical Package for the Social
Sciences (SPSS), version 19.0 (IBM Corporation, Armonk, NY, USA). The
normality of quantitative variables was tested with Kolmogorov-Smirnov
or Shapiro-Wilks tests (for small samples). Normally distributed
quantitative variables were expressed as mean ± standard deviation,
while non-normally distributed variables were expressed as medians and
ranges. Qualitative variables were expressed as absolute and relative
(%) frequencies. For the correlation between the two independent groups
(healthy children versus children with ID), the Unpaired t-test was
used for variables that follow a normal distribution. Mann-Whitney
U-test was used for the remaining variables. Chi-square and Fisher's
exact tests were used to evaluate potential associations between
qualitative variables. Receiver operating characteristic (ROC) analysis
was used to evaluate the diagnostic significance of the hematologic and
biochemical parameters tested. The area under the ROC curve (AUC),
sensitivity, specificity, positive and negative predictive values were
calculated, while Cohen's kappa was used to assess agreement. The
optimal cutoff values were derived according to Youden Index. All tests
were two-tailed, and statistical significance was set at P<0.05.
Results
The demographics, nutritional status, and laboratory tests of healthy children and those with ID are presented in Table 1.
The children's median age was 40 months. The family's annual income
ranged from 0 to 40,000 euros, with a median of 16,000 euros. Overall,
57.43% of the children were boys. Only 4.95% of the children belonged
to the Muslim minority (including Pomaks) of Greek Thrace and 12.87% to
the Roma minority. The median duration of exclusive breastfeeding was
150 days. The median consumption of beef was twice a week. During
infancy, 19.80% of children were breastfed, 67.33% were formula-fed,
and 12.37% were on a mixed diet. As shown, the median value of Hb was
12.50g/dl (7.69-15), of MCV 79.55fl (53.10-93.20), of MCH 26.90pg
(17.40-36.50), and of RDW 14.70% (11.30-27.10). The mean serum ferritin
was 35.60μg/l (2.18-325), of sTfR 1.20mg/l (0.70-5.99) and of sTfR/Fer
index 0.73 (0.31-17.70). The mean TIBC and TS% were 367.70μg/dl
(±70.21) and 19.79% (±9.56), respectively.
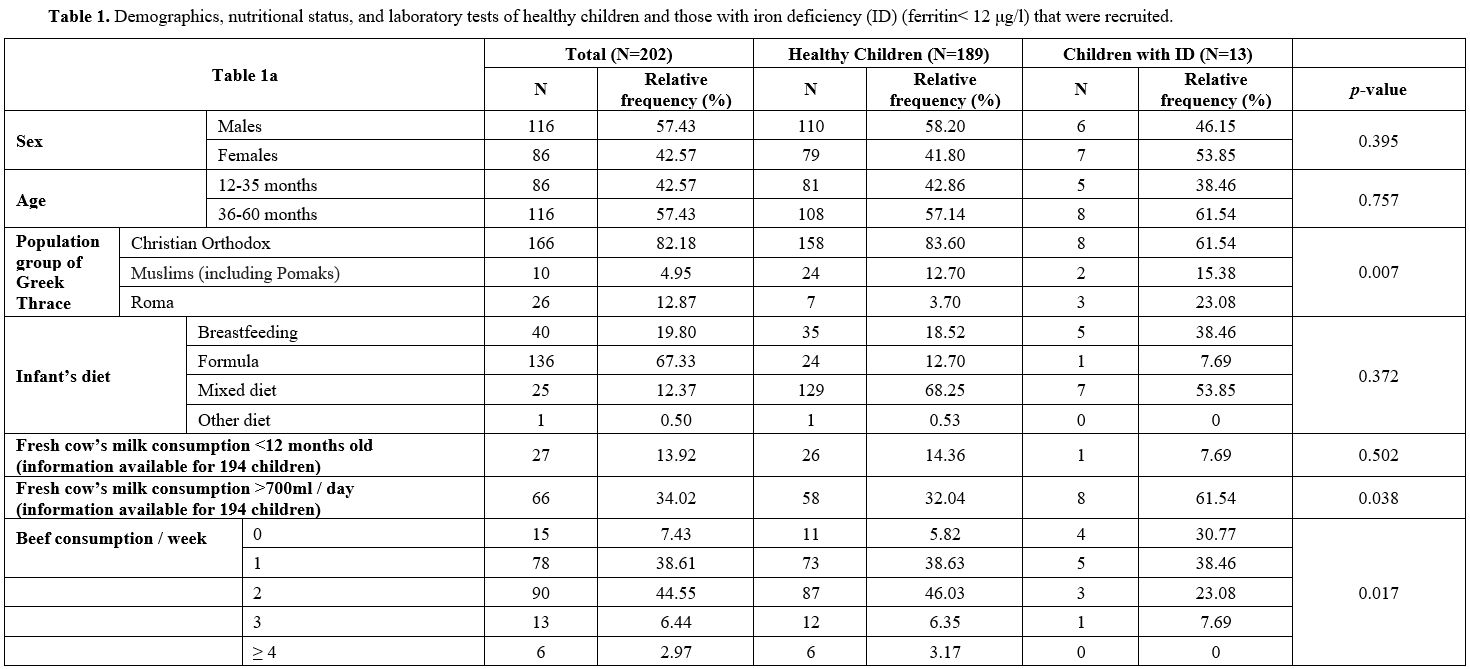 |
Table
1. Demographics, nutritional status, and laboratory tests of healthy
children and those with iron deficiency (ID) (ferritin< 12 μg/l)
that were recruited. |
Overall, 23.08% of children with ID belonged to the Roma minority compared to only 3.70% of healthy children (p=0.007).
Healthy children were found to have higher beef consumption than
children with ID [median two meals per week (0-7) versus 1 (0–3), p=0.044]. Remarkably, 30.77% of children with ID did not include beef in their diet compared to 5.82% of healthy children (p=0.017).
The
overall prevalence of anemia based on the WHO definition was 9.41%. If
a cutoff of Hb<12g/dl was used, the overall prevalence of anemia was
26.73%. The overall prevalence of ID was 6.44%. If a cutoff value of
ferritin<18μg/l was used, then the prevalence of ID was 16.33%. The
overall prevalence of IDA was 3.47%. If Hb <12g/dl and
ferritin<18μg/l were used as cutoffs, then the prevalence of IDA was
5.94%. The differences observed in the prevalence of anemia, ID, and
IDA between age groups were not significant, except for the prevalence
of anemia using a cutoff Hb value of 12g/dl (Table 2).
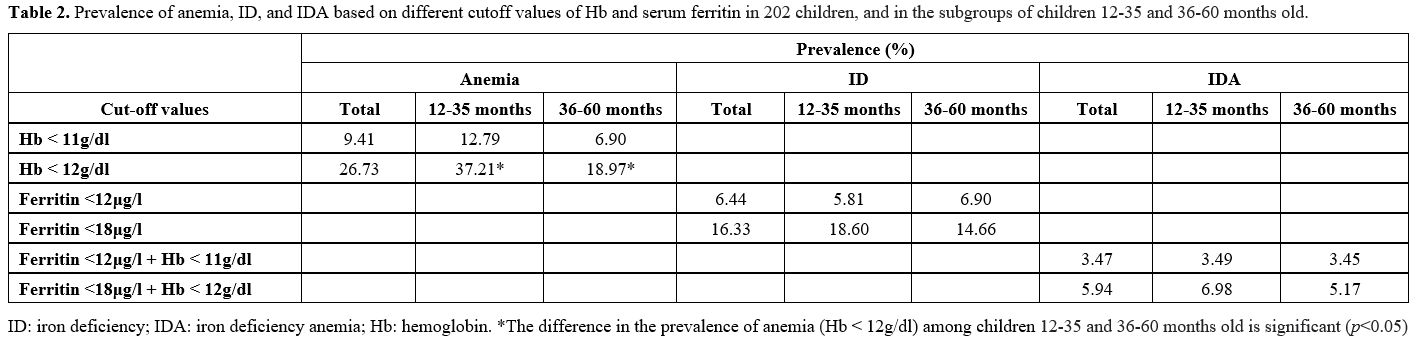 |
Table
2.
Prevalence of anemia, ID, and IDA based on different cutoff values of
Hb and serum ferritin in 202 children, and in the subgroups of children
12-35 and 36-60 months old. |
Table 3 and Figures 1 and 2
depict the results of the ROC analysis for the evaluation of
specificity and sensitivity of sideropenia biomarkers, i.e., %TS, TIBC,
sTfR, sTfR/Fer index, and Hb in children 1-5 years old, when ferritin
< 12μg/l and <18μg/l were used to define ID. When ferritin <
12μg/l was used to delimit ID, the biomarker with the highest
specificity but the lowest sensitivity was sTfR (93% and 69.2%,
respectively). In contrast, the biomarker with the highest sensitivity
(100%) was sTfR/Fer index. sTfR were found to have the highest positive
predictive value (PPV) (47.4%), while Ηb was found to have the lowest
PPV (16.7%). Conversely, the sTfR/Fer index was found to have the
highest negative predictive value (NPV) (100%). The AUC was highest for
the sTfR/Fer index (0.971), followed by sTfR (0.827). When ferritin
<18μg/l was used to define ID, the sTfR/Fer index had the highest
AUC (0.946).
At serum ferritin 12μg/l, as the cutoff of ID, the
AUC of sTfR alone (0.827) was substantially better than that of TIBC
(0.691), as shown by the green line of sTfR in Figure 1.
On the other hand, at a serum ferritin cutoff of 18μg/l, the AUC of
TIBC (0.770) was better than that of sTfR (0.716), as depicted by the
blue line of TIBC in Figure 2.
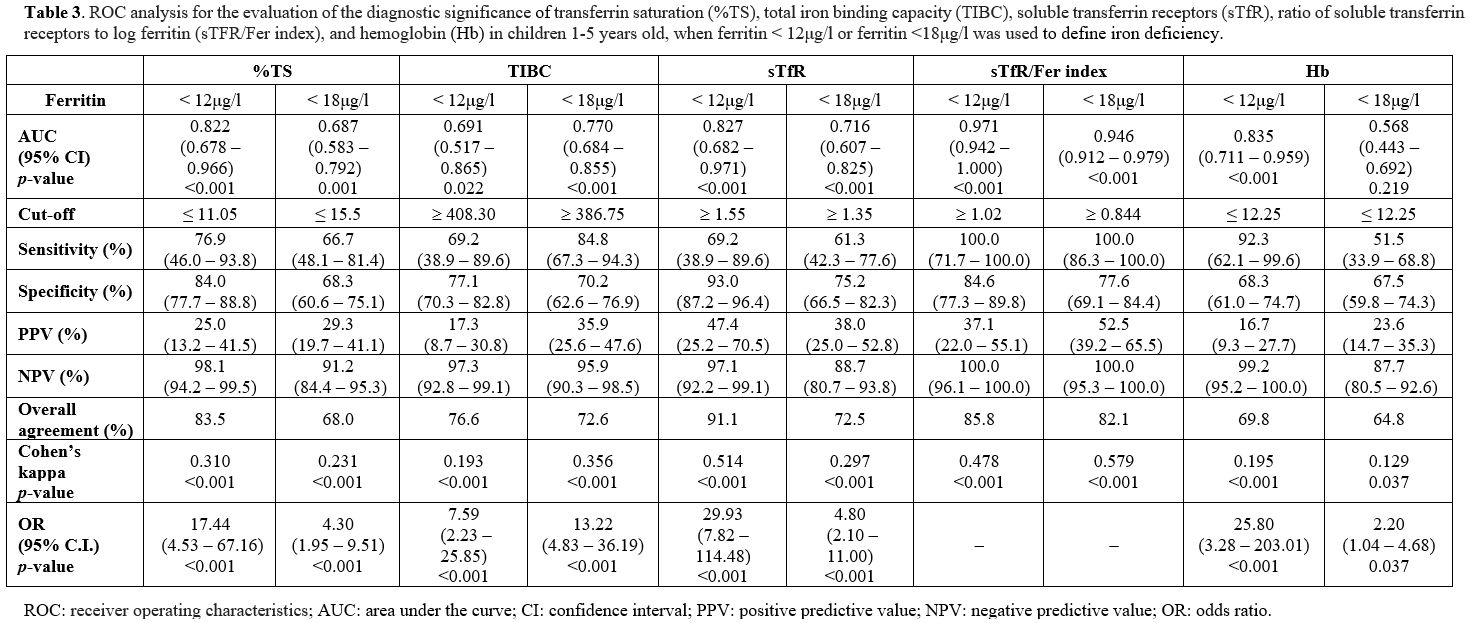 |
Table 3. ROC analysis for
the evaluation of the diagnostic significance of transferrin saturation
(%TS), total iron binding capacity (TIBC), soluble transferrin
receptors (sTfR), ratio of soluble transferrin receptors to log
ferritin (sTFR/Fer index), and hemoglobin (Hb) in children 1-5 years
old, when ferritin < 12μg/l or ferritin <18μg/l was used to
define iron deficiency. |
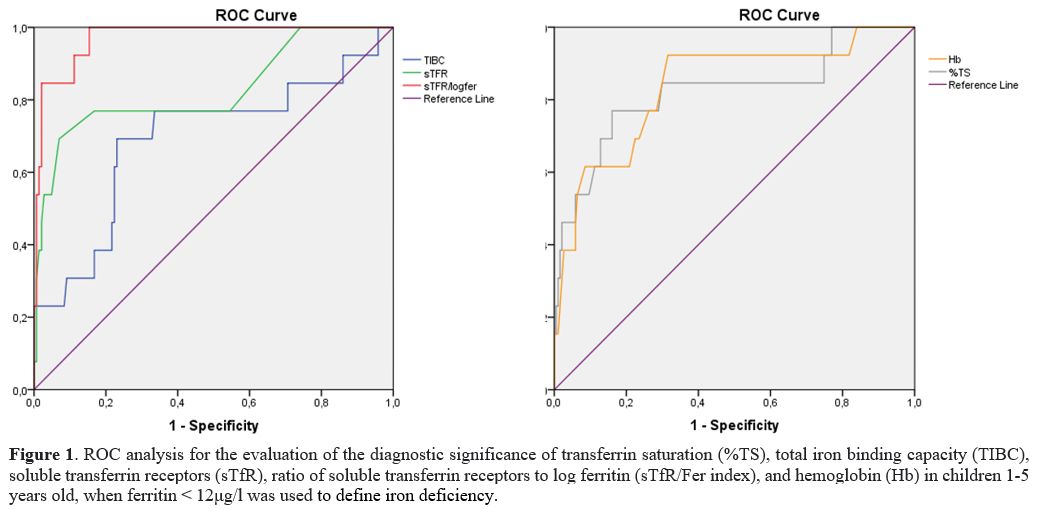 |
Figure 1. ROC analysis for
the evaluation of the diagnostic significance of transferrin saturation
(%TS), total iron binding capacity (TIBC), soluble transferrin
receptors (sTfR), ratio of soluble transferrin receptors to log
ferritin (sTfR/Fer index), and hemoglobin (Hb) in children 1-5 years
old, when ferritin < 12μg/l was used to define iron deficiency. |
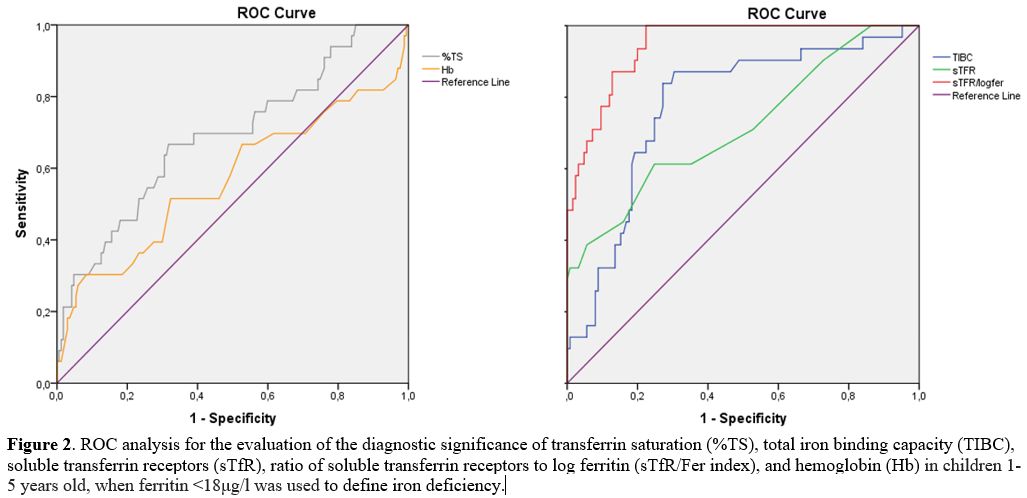 |
Figure 2. ROC analysis for
the evaluation of the diagnostic significance of transferrin saturation
(%TS), total iron binding capacity (TIBC), soluble transferrin
receptors (sTfR), ratio of soluble transferrin receptors to log
ferritin (sTfR/Fer index), and hemoglobin (Hb) in children 1-5 years
old, when ferritin <18μg/l was used to define iron deficiency. |
Discussion
ID
is the most common nutritional deficiency worldwide and a public health
problem in late infancy and in children 2-5 years old.[1]
When left untreated, IDA occurs, negatively affecting preschoolers'
motor, emotional, and social development and their subsequent
intellectual performance and learning abilities. Therefore, preventing
ID in early childhood is a public health priority.[19]
Our study used two serum ferritin thresholds, 12 μg/l and 18 μg/l, to
define ID and two Hb thresholds (11g/dl and 12g/dl) to define anemia.
Using the WHO cutoff value of Hb <11g/dl and of ferritin
<12μg/l, we showed the prevalence of anemia, ID, and IDA in healthy
children 1-5 years old in Thrace to be 9.41%, 6.44%, and 3.47%,
respectively. Hence, only approximately 37% of anemia in healthy
children 1-5 years old was IDA in our sample, an almost identical
figure to the US, where 60% of anemia in toddlers is not IDA.[5]
Using the higher cutoffs, the prevalence of anemia, ID, and IDA
increased to 26.73%, 16.33%, and 5.94%, respectively. Higher cutoffs
allow earlier intervention, i.e., dietary changes or administration of
iron supplements, although their diagnostic accuracy needs to be
determined prospectively via epidemiologic methods.[19]
In the ROC analyses, the chosen cutoff of serum ferritin affects the
evaluation of the diagnostic significance of the different markers of
sideropenia. More specifically, sTfR alone was a better biomarker than
TIBC when serum ferritin cutoff of 12μg/l was used to define ID, while
TIBC was slightly better than sTfR at serum ferritin cutoff of 18μg/l.A
prospective long-term study in 2001 conducted in 11 European countries
found the prevalence of anemia in 12-month-old infants to be 9.4%, ID
7.2%, and IDA 2.3%.[20] A review of 44 studies
conducted in 19 European countries showed that ID occurred in 3-48% of
children 12-36 months old, while the prevalence of IDA was close to 50%
in Eastern Europe but less than 5% in Southern and Western Europe.[21]
A US study published in 1997 found that the prevalence of ID and IDA in
children 12-24 months old was 9% and 3%, respectively, while in
children older than three years of age, the prevalence of ID and IDA
was ≤3% and <1%, respectively.[22] Similarly, in a
more recent US study published in 2016, the prevalence of anemia in
toddlers 1-2 years old was 2.7%, but only half of the anemic children
suffered from ID. In the same study, the prevalence of ID, anemia, and
IDA in healthy children 1-5 years old was 7.1%, 3.2%, and 1.1%,
respectively.[23]Regarding
Greece, in a prospective study conducted in 2007 of 3,100 children aged
8 months to 15 years in Northern Greece, the prevalence of ID and IDA
was 14% and 2.9%, respectively, with these rates being substantially
higher in children <2 years old (34.1% and 16.1%, respectively).[24]
A cross-sectional study in 2008 from Thessaly, in Central Greece, with
938 children aged 12-24 months, found the prevalence of IDA to be
approximately 8%.[25,26] Notably, we did not find
that the prevalence of ID and IDA was significantly different among
children aged 12-35 and 36-60 months.Hb concentration alone cannot be used to define ID or IDA, as it lacks sensitivity and specificity.[8-10] Recent
studies confirm the need for combined Hb and serum ferritin testing for
ID screening and verify the nonlinear relationship between them.[27,28]
More importantly, these studies propose raising the diagnostic serum
ferritin threshold in one-year-olds to 18μg/l from the currently
accepted NHANES threshold of 10-12μg/l because, at the 18μg/l serum
ferritin inflection, the Hb level is 12g/dl, i.e., much higher than the
long-established WHO threshold for anemia of 11g/dl. Moreover, an
anemia threshold Hb of 11g/dl corresponded to serum ferritin <5μg/l.[27,28]
Thus, by allowing serum ferritin to drop to values much lower than
10-12μg/l if Hb remains >11g/dl, we lose time in correcting ID,
which has potentially long-lasting neurodevelopmental consequences.[19]
A single-institution study in a high-resource setting found that higher
serum ferritin has been associated with higher cognitive function, with
serum ferritin of 17μg/l corresponding to the maximum level of
cognition at 24 months of age. However, maternal education was not
included in the author's model when previous studies on cognitive
outcomes of ID suggest poor maternal education and low socioeconomic
status to be additional risk factors for the ID. Hence, these findings
cannot be generalized to lower-income settings, and further research is
essential to validate them in more diverse low- and medium-income sets.[29]sTfR,
when combined with the serum ferritin, is a useful indicator of ID and
erythropoietic activity with increased specificity and sensitivity.[2,5,11]
In the ROC analyses, the AUC of sTfR alone was substantially better
than that of TIBC at a serum ferritin cutoff of 12μg/l, while at a
serum ferritin cutoff of 18μg/l, the AUC of TIBC was slightly better
than that of sTfR (0.716). Our findings are consistent with older
reports in adults and children.[14,30-39]
The sTfR/Fer index incorporates the high sensitivity of sTfR, which
indicate cellular oxygen needs, and the high specificity of ferritin,
which represents iron stores.[30] In a meta-analysis,
the overall sensitivity, specificity, and positive and negative
likelihood ratios of sTfR in a set of studies were 86%, 75%, 3.85, and
0.19, respectively, with an AUC of 0.912.[31] In
another study, sTfR and sTfR/Fer index had the highest AUC (0.75 and
0.76, respectively). They were the most sensitive markers for detecting
ID (83% and 75%, respectively) in children living in areas with a high
prevalence of infections, although with moderate specificity (50% and
56%, respectively).[32] In children with inflammatory
bowel diseases (IBD), the biomarkers that better-predicted ID and IDA
were also the sTfR and sTfR/Fer index,[33,34] something that has been confirmed in adults with IBD as well.[35] In
our study, children with ID were found to have lower beef consumption
than healthy children. In addition, 30.77% of children with ID did not
include beef in their diet compared to only 5.82% of healthy children.
Several studies evaluated the association between meat consumption and
iron status in infants and young children, leading to conflicting
results. Some found no differences in iron status when high meat
consumers were compared to low meat consumers or when meat consumers
were compared to cereal or milk consumers.[40] On the
other hand, other studies support our findings; thus, in Northern
European, healthy infants and toddlers, meat and fish consumption is
associated with better iron status.[41] In a cross-sectional study of 263 Israeli healthy 1.5- to 6-year-old
children, extremely low red meat consumers had a 4-fold higher rate of
ID than those who consumed red meat twice per week, whereas poultry
consumption was not associated with ID.[42] Moreover,
a 20-week randomized placebo-controlled trial in 12-20-months-old
children showed that in comparison with the control group, serum
ferritin was significantly higher in the red meat group.[43]
In addition, a randomized interventional trial from Denmark identified
a difference in Hb but not serum ferritin when high meat consumers were
compared to low meat consumers in the first year of life.[44]
Finally, in a Canadian cross-sectional study of 12-36 months-old
healthy children, eating meat or meat alternatives was not associated
with serum ferritin but with decreased odds of ID.[40]
Therefore, pediatricians should be encouraged to advocate earlier meat
consumption in infants to prevent ID / IDA. However, this may not apply
to low-income countries, where meat is scarce and/or too expensive to
obtain regularly.In
our study, children with ID were found to have higher fresh cow's milk
consumption (>700ml/24h) than healthy ones, which is consistent with
current knowledge.[45] In two studies performed in
Iceland, iron status at 12 months of age was negatively associated with
fresh cow's milk consumption between 9 and 12 months of age. The iron
status of infants consuming higher amounts of fresh cow's milk was
significantly worse than that of infants in the lowest quintile of milk
consumption, suggesting the dose-dependent negative effect of fresh
cow's milk on iron status.[46,47]Our
study has several limitations. First, we studied a relatively small
number of children to assess the prevalence of anemia, ID, and IDA. For
safer conclusions, more children had to be enrolled; but unfortunately,
the recruitment period coincided with the COVID-19 pandemic, which
severely limited the number of children visiting both study hospitals
for well-child visits. Second, regarding most of the established
environmental risk factors for sideropenia studied, no statistically
significant differences were found between healthy children and those
with ID, likely due to the small sample size. Third, children from the
Muslim minority of Thrace were likely under-represented, although Roma
children were likely over-represented. The prevalence of anemia, ID,
and IDA is probably higher in minority populations. Finally,
determination of sTfR was not available in all studied children.
Conclusions
We found
that the current prevalence of anemia, ID, and IDA in children of
Thrace 1-5 years old does not significantly differ from that of other
developed countries. However, in the future, it is crucial to carefully
choose the cutoff values of Hb and serum ferritin to define ID and IDA,
as the goal is for fewer toddlers and preschoolers with sideropenia to
remain undiagnosed and untreated. In this regard, the chosen cutoff of
serum ferritin may affect the evaluation of the diagnostic significance
of the different sideropenia markers.
References
- Mantadakis E, Chatzimichael E, Zikidou P. Iron
Deficiency Anemia in Children Residing in High and Low-Income
Countries: Risk Factors, Prevention, Diagnosis and Therapy. Mediterr J
Hematol Infect Dis. 2020;12(1):e2020041. doi: 10.4084/MJHID.2020.041.
Epub 2020 Jul 1. https://doi.org/10.4084/mjhid.2020.041 PMid:32670519
PMCid:PMC7340216
- Assessing the iron status of
populations: including literature reviews. Report of a Joint World
Health Organization/Centers for Disease Control and Prevention
Technical Consultation on the Assessment of Iron Status at the
Population Level. Geneva, Switzerland. 6-8 April 2004. 2nd edition.
- WHO (2000). Nutrition for health and development. A global agenda for combating malnutrition. Geneva.
- ACC/SCN
(2001). What Works? A Review of the Efficacy and Effectiveness of
Nutrition Interventions, Allen LH and Gillespie SR. ACC/SCN: Geneva in
collaboration with the Asian Development Bank, Manila.
- Baker
RD, Greer FR. Committee on Nutrition American Academy of Pediatrics.
Diagnosis and prevention of iron deficiency and iron-deficiency anemia
in infants and young children (0-3 years of age). Pediatrics.
2010;126(5):1040-1050. doi: 10.1542/peds.2010-2576. Epub 2010 Oct 5.
https://doi.org/10.1542/peds.2010-2576 PMid:20923825
- Cappellini
MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern
Med. 2020;287(2):153-170. doi: 10.1111/joim.13004. Epub 2019 Nov 12.
https://doi.org/10.1111/joim.13004 PMid:31665543
- Centers
for Disease Control and Prevention. Recommendations to prevent and
control iron deficiency in the United States. MMWR Recomm Rep.
1998;47(RR-3):1-29.
https://www.cdc.gov/mmwr/preview/mmwrhtml/00051880.htm
- Dupont
C. Prevalence of iron deficiency. Arch Pediatr. 2017;24(5S):5S45-5S48.
doi: 10.1016/S0929-693X(17)24009-3.
https://doi.org/10.1016/S0929-693X(17)24009-3
- Kohli-Kumar
M. Screening for anemia in children. Pediatrics. 2001;108(3):E56. doi:
10.1542/peds.108.3.e56. https://doi.org/10.1542/peds.108.3.e56
PMid:11533374
- Burke RM, Leon JS, Suchdev PS.
Identification, prevention and treatment of iron deficiency during the
first 1000 days. Nutrients. 2014;6(10):4093-4114. doi:
10.3390/nu6104093. Epub 2014 Oct 10. https://doi.org/10.3390/nu6104093
PMid:25310252 PMCid:PMC4210909
- Cameron BM,
Neufeld LM. Estimating the prevalence of iron deficiency in the first
two years of life: technical and measurement issues. Nutr Rev.
2011;69(Suppl 1):S49-S56. doi: 10.1111/j.1753-4887.2011.00433.x.
https://doi.org/10.1111/j.1753-4887.2011.00433.x PMid:22043883
- Vázquez-López
MA, López-Ruzafa E, Lendinez-Molinos F, Ortiz-Pérez M, Ruiz-Tudela L,
Martín-González M. Reference values of serum transferrin receptor
(sTfR) and sTfR/log ferritin index in healthy children. Pediatr Hematol
Oncol. 2016;33(2):109-120. doi: 10.3109/08880018.2015.1138007. Epub
2016 Mar 7. https://doi.org/10.3109/08880018.2015.1138007
PMid:26950203
- Malope BI, MacPhail AP, Alberts M, Hiss DC.
The ratio of serum transferrin receptor and serum ferritin in the
diagnosis of iron status. Br J Haematol. 2001;115(1):84-89. doi:
10.1046/j.1365-2141.2001.03063.x.
https://doi.org/10.1046/j.1365-2141.2001.03063.x PMid:11722416
- Vázquez-López
MA, López-Ruzafa E, Ibáñez-Alcalde M, Martín-González M,
Bonillo-Perales A, Lendínez-Molinos F. The usefulness of reticulocyte
haemoglobin content, serum transferrin receptor and the sTfR-ferritin
index to identify iron deficiency in healthy children aged 1-16 years.
Eur J Pediatr. 2019;178(1):41-49. doi: 10.1007/s00431-018-3257-0. Epub
2018 Sep 27. https://doi.org/10.1007/s00431-018-3257-0
PMid:30264352
- Ooi CL, Lepage N, Nieuwenhuys E,
Sharma AP, Filler G. Pediatric reference intervals for soluble
transferrin receptor and transferrin receptor-ferritin index. World J
Pediatr. 2009;5(2):122-126. doi: 10.1007/s12519-009-0024-3. Epub 2009
Jul 9. https://doi.org/10.1007/s12519-009-0024-3 PMid:19718534
- WHO
guideline on use of ferritin concentrations to assess iron status in
individuals and populations. Geneva: World Health Organization. 2020.
Licence: CC BY-NC-SA 3.0 IGO.
- Punnonen K, Irjala K,
Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin
in the diagnosis of iron deficiency. Blood. 1997;89(3):1052-1057.
https://doi.org/10.1182/blood.V89.3.1052 PMid:9028338
- Dimitriou
H, Stiakaki E, Markaki EA, Bolonaki I, Giannakopoulou C, Kalmanti M.
Soluble transferrin receptor levels and soluble transferrin
receptor/log ferritin index in the evaluation of erythropoietic status
in childhood infections and malignancy. Acta Paediatr.
2000;89(10):1169-1173. doi: 10.1080/080352500750027510.
https://doi.org/10.1080/080352500750027510 PMid:11083370
- Mantadakis
E. Editorial: Serum Ferritin Threshold for Iron Deficiency Screening in
One-Year-Old Children. J Pediatr. 2022. https://doi.org/10.1016/
j.jpeds.2022.02.050. Epub 2022 Feb 26.
- Male C,
Persson LA, Freeman V, Guerra A, van't Hof MA, Haschke F. Prevalence of
iron deficiency in 12-mo-old infants from 11 European areas and
influence of dietary factors on iron status (Euro-Growth Study). Acta
Paediatr. 2001;90(5):492-498. doi: 10.1080/080352501750197601.
https://doi.org/10.1080/080352501750197601 PMid:11430706
- Eussen
S, Alles M, Uijterschout L, Brus F, van der Horst-Graat J. Iron intake
and status of children aged 6-36 months in Europe: a systematic review.
Ann Nutr Metab. 2015;66(2-3):80-92. doi: 10.1159/000371357. Epub 2015
Jan 21. https://doi.org/10.1159/000371357 PMid:25612840
- Looker
AC, Dallman PR, Carroll MD, Gunter EW, Johnson CJ. Prevalence of Iron
Deficiency in the United States. JAMA. 1997;277(12):973-976. doi:
10.1001/jama.1997.03540360041028.
https://doi.org/10.1001/jama.1997.03540360041028 PMid:9091669
- Gupta
PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency
anemia among young children in the United States. Nutrients.
2016;8(6):330. doi: 10.3390/nu8060330.
https://doi.org/10.3390/nu8060330 PMid:27249004 PMCid:PMC4924171
- Gompakis
N, Economou M, Tsantali C, Kouloulias V, Keramida M, Athanasiou-Metaxa
M. The effect of dietary habits and socioeconomic status on the
prevalence of iron deficiency in children of northern Greece. Acta
Haematol. 2007;117(4):200-204. doi: 10.1159/000098273. Epub 2007 Jan 3.
https://doi.org/10.1159/000098273 PMid:17199080
- Tympa-Psirropoulou
E, Vagenas C, Dafni O, Matala A, Skopouli F. Environmental risk factors
for iron deficiency anemia in children 12-24 months old in the area of
Thessalia in Greece. Hippokratia. 2008;12(4):240-250.
- Tympa-Psirropoulou
E, Vagenas C, Psirropoulos D, Dafni O, Matala A, Skopouli F.
Nutritional risk factors for iron-deficiency anaemia in children 12-24
months old in the area of Thessalia in Greece. Int J Food Sci Nutr.
2005;56(1):1-12. doi: 10.1080/09637480500081183.
https://doi.org/10.1080/09637480500081183 PMid:16019310
- Mukhtarova
N, Ha B, Diamond CA, Plumb AJ, Kling PJ. Serum Ferritin Threshold for
Iron Deficiency Screening in One-Year-Old Children. J Pediatr.
2022:S0022-3476(22)00081-6. doi: 10.1016/j.jpeds.2022.01.050. Online
ahead of print. https://doi.org/10.1016/j.jpeds.2022.01.050
PMid:35114287
- Abdullah K, Birken CS, Maguire JL,
Fehlings D, Hanley AJ, Thorpe KE, Parkin PC. Re-Evaluation of Serum
Ferritin Cut-Off Values for the Diagnosis of Iron Deficiency in
Children Aged 12-36 Months. J Pediatr. 2017;188:287-290. doi:
10.1016/j.jpeds.2017.03.028. Epub 2017 Apr 18.
https://doi.org/10.1016/j.jpeds.2017.03.028 PMid:28431746
- Parkin
PC, Koroshegyi C, Mamak E, Borkhoff CM, Birken CS, Maguire JL, Thorpe
KE. Association between Serum Ferritin and Cognitive Function in Early
Childhood. J Pediatr. 2020;217:189-191.e2. doi:
10.1016/j.jpeds.2019.09.051. Epub 2019 Nov 2.
https://doi.org/10.1016/j.jpeds.2019.09.051 PMid:31685227
- Phiri
KS, Calis JCJ, Siyasiya A, Bates I, Brabin B, Boele van Hensbroek M.
New cut-off values for ferritin and soluble transferrin receptor for
the assessment of iron deficiency in children in a high infection
pressure area. J Clin Pathol. 2009;62(12):1103-1106.
doi:10.1136/jcp.2009.066498. https://doi.org/10.1136/jcp.2009.066498
PMid:19946096 PMCid:PMC2776133
- Infusino I, Braga F,
Dolci A, Panteghini M. Soluble Transferrin Receptor (sTfR) and sTfR/log
Ferritin Index for the Diagnosis of Iron-Deficiency Anemia. A
Meta-Analysis. Am J Clin Pathol. 2012;138(5):642-649. doi:
10.1309/AJCP16NTXZLZFAIB
https://doi.org/10.1309/AJCP16NTXZLZFAIB PMid:23086764
- Aguilar
R, Moraleda C, Quintó L, Renom M, Mussacate L, Macete E, Aguilar JL,
Alonso PL, Menéndez C. Challenges in the Diagnosis of Iron Deficiency
in Children Exposed to High Prevalence of Infections. PLoS ONE.
2012;7(11):e50584. doi:10.1371/journal.pone.0050584. Epub 2012 Nov 27.
https://doi.org/10.1371/journal.pone.0050584 PMid:23209786
PMCid:PMC3507793
- Krawiec P, Pac-Kozuchowska E.
Soluble transferrin receptor and soluble transferrin receptor/log
ferritin index in diagnosis of iron deficiency anemia in pediatric
inflammatory bowel disease. Dig Liv Dis. 2019;51(3):352-357. doi:
10.1016/j.dld.2018.11.012. Epub 2018 Nov 22.
https://doi.org/10.1016/j.dld.2018.11.012 PMid:30538074
- Krawiec
P, Pac-Kozuchowska E. Biomarkers and Hematological Indices in the
Diagnosis of Iron Deficiency in Children with Inflammatory Bowel
Disease. Nutrients. 2020;12(5):1358. doi:10.3390/nu12051358.
https://doi.org/10.3390/nu12051358
PMid:32397525 PMCid:PMC7284745
- Oustamanolakis
P, Koutroubakis IE, Messaritakis I, Niniraki M, Kouroumalis EA. Soluble
transferrin receptor-ferritin index in the evaluation of anemia in
inflammatory bowel disease: a case-control study. Ann Gastroenterol.
2011;24(2):108-114.
- Angeles Vázquez López M,
Molinos FL, Carmona ML, Morales AC, Muñoz Vico FJ, Muñoz JL, Muñoz
Hoyos A. Serum transferrin receptor in children: usefulness for
determinating the nature of anemia in infection. J Pediatr Hematol
Oncol. 2006;28(12):809-815. doi: 10.1097/MPH.0b013e31802d751a.
https://doi.org/10.1097/MPH.0b013e31802d751a PMid:17164650
- Grant
FK, Martorell R, Flores-Ayala R, Cole CR, Ruth LJ, Ramakrishnan U,
Suchdev PS. Comparison of indicators of iron deficiency in Kenyan
children. Am J Clin Nutr. 2012;95(5):1231-1237. doi:
10.3945/ajcn.111.029900. Epub 2012 Mar 28.
https://doi.org/10.3945/ajcn.111.029900 PMid:22456661 PMCid:PMC4697948
- Jonker
FA, Boele van Hensbroek M, Leenstra T, Vet RJ, Brabin BJ, Maseko N,
Gushu MB, Emana M, Kraaijenhagen R, Tjalsma H, Swinkels DW, Calis JC.
Conventional and novel peripheral blood iron markers compared against
bone marrow in Malawian children. J Clin Pathol. 2014;67(8):717-723.
doi: 10.1136/jclinpath-2014-202291. Epub 2014 Jun 10.
https://doi.org/10.1136/jclinpath-2014-202291 PMid:24915849
- Chen
YC, Hung SC, Tarng DC. Association between transferrin
receptor-ferritin index and conventional measures of iron
responsiveness in hemodialysis patients. Am J Kidney Dis.
2006;47(6):1036-1044. doi: 10.1053/j.ajkd.2006.02.180.
https://doi.org/10.1053/j.ajkd.2006.02.180 PMid:16731299
- Cox
KA, Parkin PC, Anderson LN, Chen Y, Birken CS, Maguire JL, Macarthur C,
Borkhoff CM; TARGet Kids! Collaboration. Association Between Meat and
Meat-Alternative Consumption and Iron Stores in Early Childhood. Acad
Pediatr. 2016;16(8):783-791. doi: 10.1016/j.acap.2016.01.003. Epub 2016
Jan 20. https://doi.org/10.1016/j.acap.2016.01.003 PMid:26804490
- Holmlund-Suila
EM, Hauta-Alus HH, Enlund-Cerullo M, Rosendahl J, Valkama SM, Andersson
S, Mäkitie O. Iron status in early childhood is modified by diet, sex
and growth: Secondary analysis of a randomized controlled vitamin D
trial. Clin Nutr. 2022;41(2):279-287. doi: 10.1016/j.clnu.2021.12.013.
Epub 2021 Dec 13. https://doi.org/10.1016/j.clnu.2021.12.013
PMid:34999321
- Moshe G, Amitai Y, Korchia G, Korchia
L, Tenenbaum A, Rosenblum J, Schechter A. Anemia and iron deficiency in
children: association with red meat and poultry consumption. J Pediatr
Gastroenterol Nutr. 2013;57(6):722-727. doi:
10.1097/MPG.0b013e3182a80c42.
https://doi.org/10.1097/MPG.0b013e3182a80c42 PMid:24280989
- Szymlek-Gay
EA, Ferguson EL, Heath AL, Gray AR, Gibson RS. Food-based strategies
improve iron status in toddlers: a randomized controlled trial12. Am J
Clin Nutr. 2009;90(6):1541-1551. doi: 10.3945/ajcn.2009.27588. Epub
2009 Oct 14. https://doi.org/10.3945/ajcn.2009.27588 PMid:19828711
- Engelmann
MD, Sandström B, MichaelsenKF. Meat intake and iron status in late
infancy: an intervention study. J Pediatr Gastroenterol Nutr.
1998;26(1):26-33. doi: 10.1097/00005176-199801000-00005.
https://doi.org/10.1097/00005176-199801000-00005 PMid:9443116
- Powers
JM, Buchanan GR. Disorders of Iron Metabolism: New Diagnostic and
Treatment Approaches to Iron Deficiency. Hematol Oncol Clin North Am.
2019;33(3):393-408. doi: 10.1016/j.hoc.2019.01.006. Epub 2019 Mar 29.
https://doi.org/10.1016/j.hoc.2019.01.006 PMid:31030809
- Thorsdottir
I, Gunnarsson BS, Atladottir H, Michaelsen KF, Palsson G. Iron status
at 12 months of age - effects of body size, growth and diet in a
population with high birth weight. Eur J Clin Nutr. 2003;57(4):505-513.
doi: 10.1038/sj.ejcn.1601594.
https://doi.org/10.1038/sj.ejcn.1601594 PMid:12700611
- Thorisdottir
AV, Ramel A, Palsson GI, Tomassson H, Thorsdottir I. Iron status of
one-year-olds and association with breast milk, cow's milk or formula
in late infancy. Eur. J. Nutr. 2013;52(6):1661-1668. doi:
10.1007/s00394-012-0472-8. Epub 2012 Dec 2.
https://doi.org/10.1007/s00394-012-0472-8 PMid:23212531
[TOP]





