Bedrettin Orhan1, Fahir Özkalemkaş1, Vildan Özkocaman1, Büşra Gürbüz2, Tuba Ersal1, İbrahim Ethem Pınar1, Cumali Yalçin1, Ömer Candar1, Sinem Çubukçu1, Tuba Güllü Koca1 and Rıdvan Ali1.
1 Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Bursa Uludag University, Bursa, Turkey.
2 Department of Internal Medicine, Faculty of Medicine, Bursa Uludag University, Bursa, Turkey..
Correspondence to:
Bedrettin Orhan, MD. Faculty of Medicine, Department of Internal
Medicine, Division of Hematology, Uludag University, 16059 Bursa,
Turkey. Tel: +905052409503, Fax: +902242951141. E-mail:
borhan18@gmail.com
Published: July 1, 2022
Received: February 9, 2022
Accepted: June 14, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022051 DOI
10.4084/MJHID.2022.051
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Background and Objective:
Infections are the most common cause of anal and perianal pathologies
in patients with hematological malignancies. Perianal infection
diagnosis in this group of patients is difficult; thus, a careful
anorectal examination is necessary with imaging modalities. In
addition, the literature reveals a knowledge gap in the approach to
anal pathologies in patients with neutropenia during diagnosis or
chemotherapy. This study aimed to examine our institutional data on
perianal complications and investigate the relationship between the
white blood cell-neutrophil count, perianal lesion, and the type of
treatment in patients with hematologic malignancies during the
neutropenic period.
Methods:
Patients with a hematologic malignancy, hospitalized for cytotoxic
chemotherapy, complicated by perianal pathology, documented by at least
one imaging method, were included in the study.
Results:
A total of 42 patients were included in the study. Most of them had
acute leukemia, 31 were affected by acute myeloid leukemia (AML),
and 7 by Acute lymphoid leukemia (ALL). There was no statistically
significant relationship between the anal abscess formation, the
neutrophil count, and a previous perianal pathology. Anal abscess
development was significantly more frequent in acute myeloid leukemia.
An inverse relationship was found between the total white blood cell
number at onset and having a surgical intervention for anal pathology.
In
conclusion, this article has shown that white blood cell count at the
time of hospitalization can affect the surgical intervention in
patients with hematological malignancy (in the majority with acute
leukemia) affected by anal pathologies occurring in the neutropenic
period.
|
Introduction
Infections are the most common cause of anal and perianal pathologies in patients with hematological malignancies.[1] The incidence of perianal infections is 8%-9% in patients with acute leukemia.[2]
Immunosuppression due to chemotherapies or underlying disease causes
the onset of perianal infections due to opportunistic organisms.[3] When blood neutrophil count falls below 500/mm3
and the patient becomes thrombocytopenic, delayed healing and
opportunistic infections become imminent. Furthermore, mucosal and
mechanical barrier damage provide additional entry of pathogens in the
neutropenic stage.[4]
Anorectal infections in
patients with cancer are diagnosed for the presence of redness or
tenderness, which are infection findings during a physical examination,
abscess by incision or drainage, or pathognomonic findings revealed by
imaging methods.[5] The clinical evaluation of
anorectal infections in patients with cancer differs from healthy
individuals. These evaluations include immunosuppression status,
steroid use, chemo, and radio treatment-related toxicities.[6]
Perianal infection diagnosis in this group of patients is difficult;
thus, a careful anorectal examination is necessary with imaging
modalities.[4] These infections have a large spectrum
ranging from local cellulitis to severe sepsis and have a mortality
rate of 11%-57% in patients with neutropenia; thus, prompt treatment
and the type of intervention (surgical or medical) becomes a challenge.[7]
Non-septic conditions, such as hemorrhoids and fissures, should be
conservatively approached, whereas septic conditions should be more
carefully approached, and surgery should be performed as necessary.[8]
A literature review on the treatment of perianal pathologies in
patients with neutropenic hematologic malignancy recommended surgical
intervention only in patients with frank abscess formation and those
without non-surgical treatment response.[7]
Therapy
decisions must consider the risks and benefits of treatment and the
clinical course related to chemotherapy and oncologic prognosis.[6]
The literature revealed a knowledge gap in the approach to anal
pathologies in patients with hematologic neutropenia during diagnosis
or chemotherapy. Demonstrating a correlation between grade and duration
of neutropenia with complications of surgical intervention could be
useful to improve the management of these challenging cases. Therefore,
this study aimed to examine our institutional data on perianal
pathologies during the neutropenic period and investigate the
relationship among the total white blood cell (WBC; neutrophils,
monocytes, eosinophils, basophils, lymphocytes, and blasts)-neutrophil
count, perianal lesion, and the type of treatment in patients with
hematologic malignancies.
Material and Methods
The
demographic and clinical characteristics of 480 adult patients with a
hematologic malignancy and hospitalized for cytotoxic chemotherapy at
the Bursa Uludag University Hospital Department of Hematology between
January 2010 and May 2021 were retrieved from the medical files and
then retrospectively analyzed. Patients who are complicated by perianal
pathology and with at least one imaging method (computerized
tomography, magnetic resonance imaging, and ultrasonography) were
included in the study. The median age, gender, diagnosis, complete
blood count analysis at the time of hospitalization for hematologic
malignancy, biochemical parameters, duration of neutropenia, active
disease at hospitalization, perianal pathology types, anorectal disease
history, and surgical history were evaluated. Our study was conducted
under the institutional research committee's ethical standards and
according to the 1964 Helsinki Declaration. This study was approved by
the clinical research ethics committee of Bursa Uludag University
Faculty of Medicine (Decision No: 2021-15/6).
Continuous variables
were expressed as median (range) values and categorical variables as
frequency and corresponding percentage values. Statistical analyses
were done using the Statistical Package for the Social Sciences version
23 package program. The Mann-Whitney U test was used for group
comparison. Categorical data were evaluated using the Chi-square test.
Statistical significance was accepted as p < 0.05. Correlations were
determined using the Spearman correlation coefficient.
Results
The study included a total of 42 patients. The demographic data and treatment characteristics of patients are shown in Table 1.
The incidence of perianal pathology was 8.7% (42/480). The median age
of patients during the diagnosis was 41 (18–70) years. Of the patients,
29 were males, 13 were females, 31 had acute myeloid leukemia (AML), 7
had acute lymphoblastic leukemia (ALL), 3 had Burkitt's lymphoma, and 1
had multiple myeloma. Patients with a history of anorectal disease
before diagnosis or treatment accounted for 18, and those without were
24. Perianal complications that developed after cytotoxic therapy
included anal abscess in 30, anal fistula in 7, anal fissure in 4, and
external hemorrhoid in 1 patient.
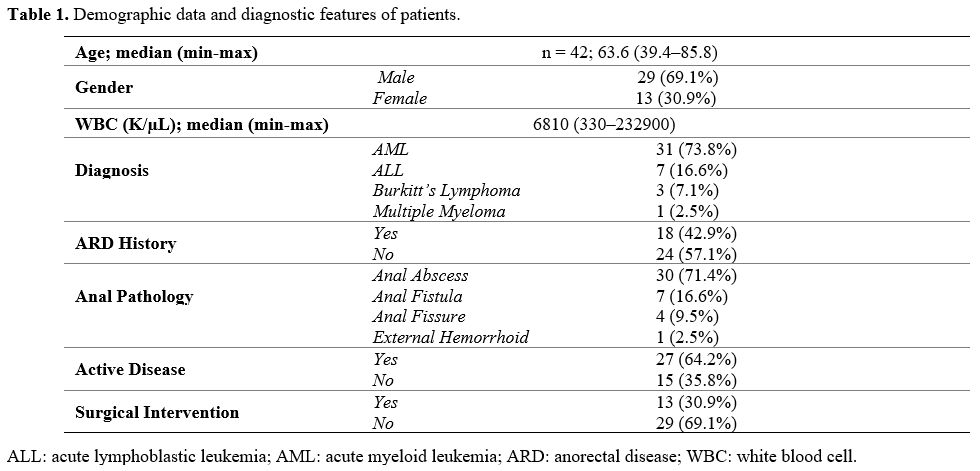 |
Table 1. Demographic data and diagnostic features of patients. |
Additionally,
13 were operated on, and 29 did not require surgical intervention.
While 27 patients had active disease, 15 received consolidation
therapy. The comparison between the groups revealed no statistical
significance between the anal abscess formation and neutrophil count (Figure 1;
p = 0.091). No correlation could be found between the neutrophil count
at the time of hospitalization for hematological disease and the
development of an anal abscess. However, a statistically significant
association was found between AML diagnosis and anal abscess
development (Table 2; p = 0.002, X2
= 11.638). The incidence of the anal abscess was found to be higher in
AML patients than in ALL patients. When the patients were compared
according to their white blood cell counts at the time of
hospitalization for hematological malignancy, the surgical intervention
for the anal abscess was found to be low in patients with high white
blood cell counts, and this relationship is statistically significant (Figure 2A;
p = 0.0209). There was no correlation between neutrophil counts at
hospitalization for hematological malignancy and surgical intervention
for anal abscess (Figure 2B; p
= 0.3486). Although there was no statistical significance between the
duration of neutropenia and the development of anal abscess (Figure 2D;
p = 0.1023), a positive correlation statistically significant was
observed between the duration of the neutropenia and the outcome of the
surgical intervention requested for the anal abscess (Figure 2C;
p = 0.0297). No statistical significance was found between the anal
abscess formation (p = 0.924) or surgical intervention (p = 0.520) and
the history of perianal pathology (Table 3).
The median overall survival (OS) of patients was 30.1 months (95%
confidence interval [CI]: 21.2–38.9), and the overall mortality rate
was 52.3%. The comparison of patient survival to their anal abscess
status revealed no significant difference between the two groups (Figure 3; p = 0.3587). Only one patient with an anal abscess died during the first month due to perianal sepsis.
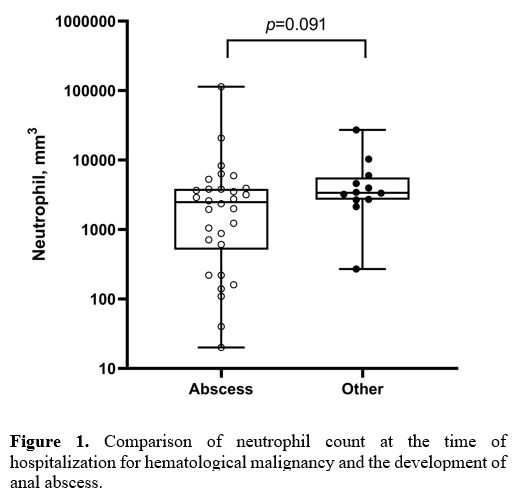 |
Figure
1. Comparison of neutrophil count at the time of hospitalization for hematological malignancy and the development of anal abscess. |
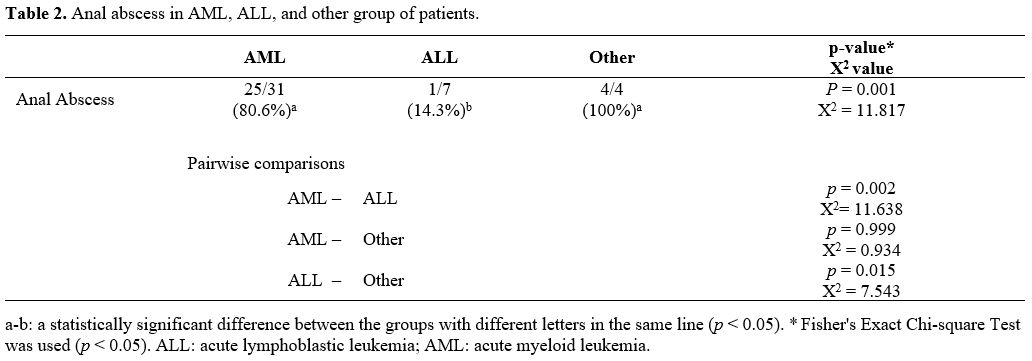 |
Table 2. Anal abscess in AML, ALL, and other group of patients. |
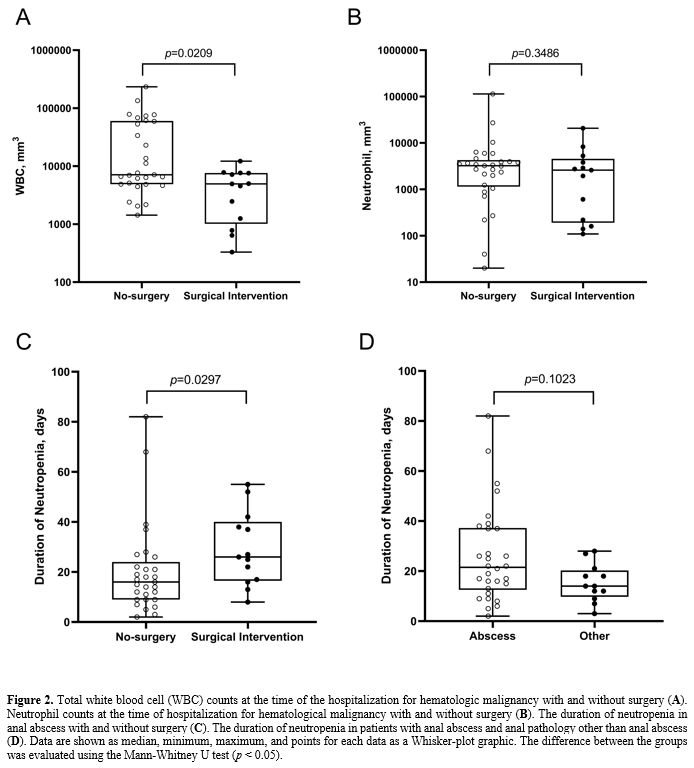 |
Figure 2. Total white
blood cell (WBC) counts at the time of the hospitalization for
hematologic malignancy with and without surgery (A). Neutrophil counts at the time of hospitalization for hematological malignancy with and without surgery (B). The duration of neutropenia in anal abscess with and without surgery (C). The duration of neutropenia in patients with anal abscess and anal pathology other than anal abscess (D).
Data are shown as median, minimum, maximum, and points for each data as
a Whisker-plot graphic. The difference between the groups was evaluated
using the Mann-Whitney U test (p < 0.05). |
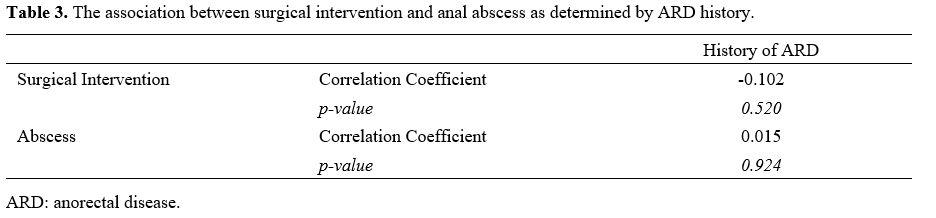 |
Table 3. The association between surgical intervention and anal abscess as determined by ARD history. |
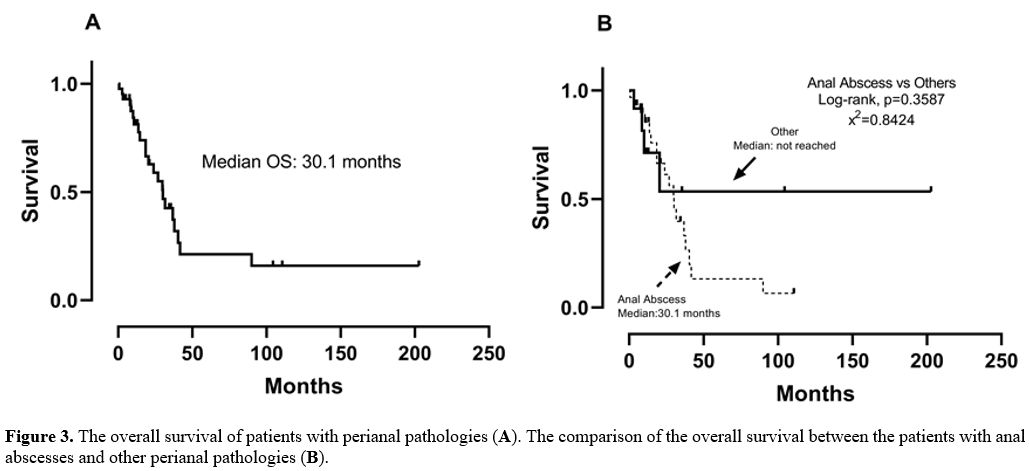 |
Figure 3. The overall survival of patients with perianal pathologies (A). The comparison of the overall survival between the patients with anal abscesses and other perianal pathologies (B). |
Abscess
material cultures of 14 (14/30) patients with anal abscesses were
positive. Isolated species under which antibiotic coverage cultures
were positive are shown in Table 4. The most commonly isolated species are Escherichia coli (E. coli), Enterococcus faecium, and Klebsiella pneumoniae.
Since the patients in the study were diagnosed with a hematological
malignancy in the neutropenic period, the patients were under empirical
antibiotherapy at the time of anal abscess development. Patients were
usually given carbapenems (13/14) for gram-negative bacteria and
glycopeptides (8/14) for gram-positive bacteria when their cultures
were positive. All 13 patients were put on piperacillin/tazobactam
after the initial febrile neutropenic episode. The switch to Carbapenem
was done when the surgery material cultures were taken.
 |
Table 4. Isolated species and empirical antibiotic use in patients with anal abscess. |
Discussion
Patients
with neutropenia are susceptible to anorectal complications, and
symptomatic anorectal pathology affects 2%-32% of patients with
hematology-oncology.[9] The absence of inflammatory
cells masks the clinical manifestations of anorectal infections in
patients with neutropenia; thus, detecting the symptoms in these
patients is difficult. If left untreated, anorectal infections can
progress between tissue layers and cause an abscess or necrotizing
fasciitis.[10]
Our study's rate of perianal
pathology was 8.7%, and 29 of the patients were male, and 14 were
female. Previous studies revealed a male-to-female ratio between 3-3.5,
with AML predominance in patients with perianal infections. Male and
AML predominance was similar to previous studies.[1,2]
Male predominance is explained by the higher incidence of patients with
AML in our series and the higher incidence of AML in males.[11]
A previous history of anorectal disease did not favor, in a way
statistically significant, anal abscess development and surgical
intervention in our study, according to Badgwell et al., who compared
the presence or no of anorectal history in patients submitted to
surgical intervention for anal pathologies in the neutropenic period.[6]
On the contrary, another study comparing the patients with anorectal
history with anal abscesses or not revealed a statistical significance.[9]
Multicenter studies with a large number of patients are needed to
evaluate better the anorectal disease history and perianal infections
in the neutropenic period.
The incidence of anal abscess varies in
retrospective studies on perianal complications in patients with
hematological malignancies.[1,2] In our research, anal
abscess constituted the majority of perianal complications.
Additionally, the incidence of anal abscesses was higher in AML
patients than in ALL patients with statistical significance(p = 0.002).
Some similar studies revealed no relationship between the disease type
and anal abscess development.[1,2,9] However, other studies showed that recurrent perianal infections are more common in patients with AML than in ALL.[1,12]
The small number of patients may explain the higher incidence of
perianal complications in patients with AML than ALL included in this
study and myeloid leukemias, the use of high-dose cytarabine-based
regimens with more mucositis side effects.[13]
In our study, E.coli, Enterococcus faecium, and Klebsiella pneumoniae were the most common agents in cultures from anal abscess surgery material. Similarly, E. coli, Enterococcus spp., and Non-Bacteroides fragilis were the most prevalent agents recovered from cultures sent from anal abscess surgery material in a study of cancer patients.[10] The most frequently isolated pathogens in another investigation of anal infections in individuals with acute leukemia were Enterococcus spp and E.coli, respectively, and carbapenems, piperacillin/tazobactam, cefepime, and vancomycin were the most often utilized antibiotics.[1]
It was highlighted again that, in accordance with the relevant
literature, the antibiotics used empirically should be active versus
enteric agents such as Enterococcus spp. and E. coli,
as well as isolated agents and medications employed in this study.
Another noteworthy finding in this study is that a bacterium known to
cause complications in cancer centers, Pseudomonas aeruginosa,
was not found in the cultures. This could be because of the
prophylactic use of ciprofloxacin in acute leukemia remission/induction
regimens.[14]
Buyukasik et al. revealed that the
neutrophil count during diagnosis affected the surgical intervention
due to anal abscess and possible cure after the surgical intervention.
Still, the authors did not mention the number of leukocytes.[2]
Our study revealed that the neutrophil count did not statistically
affect the anal abscess development or the surgical intervention;
however, statistical significance was found between the total WBC
counts during the diagnosis of hematologic malignancy and the surgical
intervention. Furthermore, the frequency of surgery in patients with
high WBC count (most of them with AML) was lower than in those
with low WBC count. This relationship has been demonstrated for the
first time in the literature. However, it needs to be further discussed
and validated, which may be explained by the worse clinical course of
patients with high WBC count during diagnosis and the early use of
antibiotics.
In our study, the comparison of the duration of
neutropenia with the anal abscess development revealed no statistical
significance, and more neutropenic days increased the incidence of
undergoing anal abscess surgery. This relationship was statistically
significant. However, a study showed no statistical significance in the
duration of neutropenia compared with the perianal complication
development.[9] Therefore, it is difficult to say that
there is a relationship between the time of neutropenia and the
surgical intervention for anal abscess.
Mortality rates and
survival times differ in studies that evaluated anal pathologies of
patients with cancer. A study that assessed the anorectal complications
of patients with hematological cancer in the neutropenic period
revealed a 41.2% overall mortality rate.[9] A similar study showed a 2.4% mortality rate within one month.[4] A cohort study of most patients with leukemia revealed that the median survival was 14.4 months (95% CI: 7.9–19.5).[6]
The OS in our study was 30.1 months, and the overall mortality rate was
52.3%. Only one patient died in the first month (mortality rate: 2.3%).
The survival of patients with anal abscesses compared with other anal
pathologies revealed no statistical significance in our study.
Therefore, the OS seems to be better, and the mortality rates were
compatible with other studies.
Study Limitations
Limitations
of this study are its retrospective nature, its small sample size, and
the inclusion of patients having both induction and consolidation
chemotherapy. The study, even if includes a small proportion of
nonleukemic patients, reflects the behavior of leukemic patients,
considering that also the Burkit lymphoma assumes a leukemic character.
Conclusions
In
conclusion, this article has shown that white blood cell count at the
time of hospitalization in patients with hematological malignancy can
affect the surgical intervention due to anal pathologies that may occur
in the neutropenic period. Therefore, large-scale randomized studies
with more patients are needed to predict the course of anal pathology,
the need for surgery, and appropriate treatment in those groups of
patients.
References
- Chen CY, Cheng A, Huang SY, Sheng WH, Liu JH, Ko
BS, Yao M, Chou WC, Lin HC, Chen YC, Tsay W, Tang JL, Chang SC, Tien
HF. Clinical and microbiological characteristics of perianal infections
in adult patients with acute leukemia. PLoS One. 2013;8(4):e60624. https://doi.org/10.1371/journal.pone.0060624 PMid:23577135 PMCid:PMC3618431
- Büyükaşik
Y, Ozcebe OI, Sayinalp N, Haznedaroğlu IC, Altundağ OO, Ozdemir O,
Dündar S. Perianal infections in patients with leukemia: importance of
the course of neutrophil count. Dis Colon Rectum. 1998;41(1):81-5. https://doi.org/10.1007/BF02236900 PMid:9510315
- Haliloglu
N, Gulpinar B, Ozkavukcu E, Erden A. Typical MR imaging findings of
perianal infections in patients with hematologic malignancies. Eur J
Radiol. 2017;93:284-8. https://doi.org/10.1016/j.ejrad.2017.05.046 PMid:28668427
- Loureiro
RV, Borges VP, Tomé AL, Bernardes CF, Silva MJ, Bettencourt MJ.
Anorectal complications in patients with haematological malignancies.
Eur J Gastroenterol Hepatol. 2018;30(7):722-6.
https://doi.org/10.1097/MEG.0000000000001133 PMid:29659377
- Schimpff SC, Wiernik PH, Block JB. Rectal abscesses in cancer patients. Lancet. 1972;2(7782):844-7. https://doi.org/10.1016/S0140-6736(72)92210-6
- Badgwell
BD, Chang GJ, Rodriguez-Bigas MA, Smith K, Lupo PJ, Frankowski RF,
Delclos G, Du XL, Cormier J. Management and outcomes of anorectal
infection in the cancer patient. Ann Surg Oncol. 2009;16(10):2752-8. https://doi.org/10.1245/s10434-009-0626-y PMid:19649556
- Baker
B, Al-Salman M, Daoud F. Management of acute perianal sepsis in
neutropenic patients with hematological malignancy. Tech Coloproctol.
2014;18(4):327-33. https://doi.org/10.1007/s10151-013-1082-z PMid:24276114
- Morcos
B, Amarin R, Abu Sba A, Al-Ramahi R, Abu Alrub Z, Salhab M.
Contemporary management of perianal conditions in febrile neutropenic
patients. Eur J Surg Oncol. 2013;39(4):404-7. https://doi.org/10.1016/j.ejso.2013.01.001 PMid:23347777
- Solmaz
S, Korur A, Gereklioğlu Ç, Asma S, Büyükkurt N, Kasar M, Yeral M,
Kozanoğlu İ, Boğa C, Ozdoğu H. Anorectal Complications During
Neutropenic Period in Patients with Hematologic Diseases. Mediterr J
Hematol Infect Dis. 2016;8(1):e2016019. https://doi.org/10.4084/mjhid.2016.019 PMid:26977278 PMCid:PMC4771136
- Lehrnbecher
T, Marshall D, Gao C, Chanock SJ. A second look at anorectal infections
in cancer patients in a large cancer institute: the success of early
intervention with antibiotics and surgery. Infection. 2002;30(5):272-6.
https://doi.org/10.1007/s15010-002-2197-8 PMid:12382085
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10-29. https://doi.org/10.3322/caac.20138 PMid:22237781
- Chang
H, Kuo MC, Tang TC, Lin TL, Wu JH, Hung YS, Wang PN. Clinical Features
and Recurrence Pattern of Perianal Abscess in Patients with Acute
Myeloid Leukemia. Acta Haematol. 2017;138(1):10-3. https://doi.org/10.1159/000475589 PMid:28586772
- Camera
A, Andretta C, Villa MR, Volpicelli M, Picardi M, Rossi M, Rinaldi CR,
Della Cioppa P, Ciancia R, Selleri C, Rotoli B. Intestinal toxicity
during induction chemotherapy with cytarabine-based regimens in adult
acute myeloid leukemia. Hematol J. 2003;4(5):346-50. https://doi.org/10.1038/sj.thj.6200304 PMid:14502260
- Rozenberg-Arska
M, Dekker AW, Verhoef J. Ciprofloxacin for selective decontamination of
the alimentary tract in patients with acute leukemia during remission
induction treatment: the effect on fecal flora. J Infect Dis.
1985;152(1):104-7. https://doi.org/10.1093/infdis/152.1.104 PMid:3159811





