Marina Belia1,
Asimina Papanikolaou2, Panagiotis Skendros3
and Theodoros P. Vassilakopoulos1.
1 Department
of Haematology and Bone Marrow Transplantation, Laikon General
Hospital, National and Kapodistrian University of Athens, Athens,
Greece.
2 Department of Pathology, Evangelismos
General Hospital, Athens, Greece.
3 First Department of Internal Medicine and
Laboratory of Molecular Haematology, University Hospital of
Alexandroupolis, Democritus University of Thrace, Alexandroupolis,
Greece.
Correspondence to:
Theodoros P. Vassilakopoulos, Professor in Haematology, Department of
Haematology, National and Kapodistrian University of Athens, School of
Medicine, Laikon General Hospital, Athens 11527, Greece, Tel: 0030213-
2061702. E-mail:
tvassilak@med.uoa.gr
or
theopvass@hotmail.com
Published: July 1, 2022
Received: April 14, 2022
Accepted: June 18, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022059 DOI
10.4084/MJHID.2022.059
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Regarding the paper on clinical and treatment aspects of Familial Mediterranean fever (FMF) published by Manna & Rigante,[1]
we would like to add our experience on a unique case of a young FMF
male who developed nodular lymphocyte-predominant Hodgkin lymphoma
(NLPHL) under treatment with canakinumab, and interleukin-1β (IL-1β)
inhibitor.
A 21-year-old male patient presented in December 2019
with asymptomatic right axillary and left inguinal lymphadenopathy over
the preceding six months. Lymph node biopsy revealed NLPHL with a
diffuse architectural pattern mimicking T-cell/histiocyte-rich large
B-cell lymphoma (TCRBCL or DLBCL-like, Fan pattern E)( Figure
1).
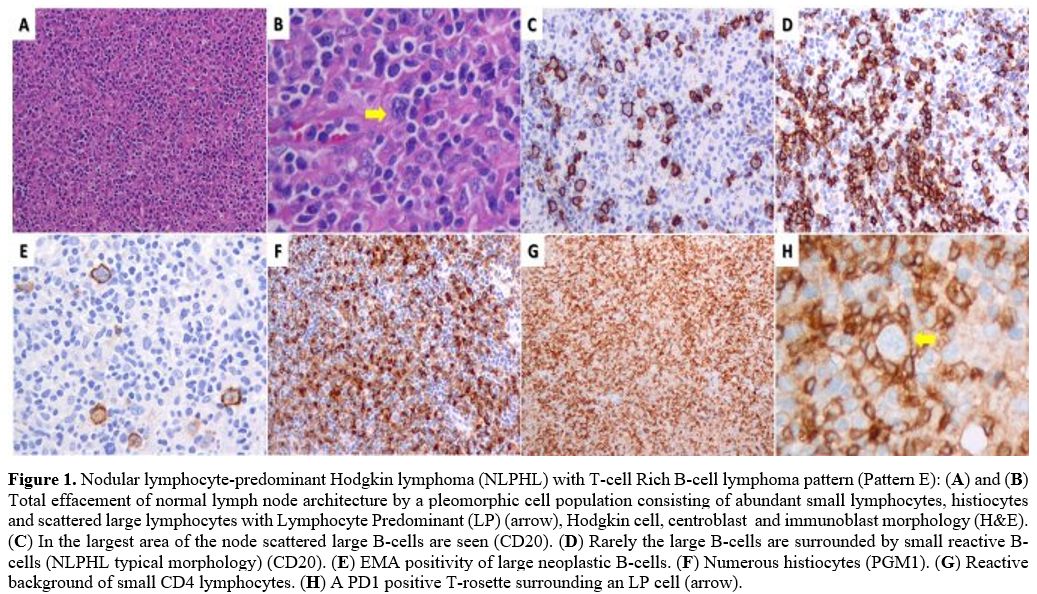 |
Figure
1. Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) with T-cell Rich B-cell lymphoma pattern (Pattern E): (A) and (B)
Total effacement of normal lymph node architecture by a pleomorphic
cell population consisting of abundant small lymphocytes, histiocytes
and scattered large lymphocytes with Lymphocyte Predominant (LP)
(arrow), Hodgkin cell, centroblast and immunoblast morphology
(H&E). (C) In the largest area of the node scattered large B-cells are seen (CD20). (D) Rarely the large B-cells are surrounded by small reactive B-cells (NLPHL typical morphology) (CD20). (E) EMA positivity of large neoplastic B-cells. (F) Numerous histiocytes (PGM1). (G) Reactive background of small CD4 lymphocytes. (H) A PD1 positive T-rosette surrounding an LP cell (arrow). |
The
clinical stage, confirmed by PET/CT, was IIIA with right axillary,
mesenteric, and paraaortic lymphadenopathy and a 5.5 cm left
iliac/inguinal mass (left inguinal SUVmax 35.8; Figure 2A).
FMF was diagnosed at age 4 with homozygosity of pyrin gene mutation
M694V. Since childhood, he experienced colchicine-refractory episodes
of fever, abdominal and chest pain, and arthritis of the knees and
ankles. He was placed on the anti-IL-1β canakinumab from age 13. No
other symptoms such as aphthous stomatitis, pharyngotonsillitis, or
lymphadenopathy were present, and the patient had not undergone
tonsillectomy in childhood. Upon NLPHL diagnosis, canakinumab was
discontinued due to the concern of adverse interaction with
chemotherapy. ABVD chemotherapy was initiated. Interim
PET/CT after ABVDx2 revealed progressive disease with
new nodal lesions and spleen infiltration [SUVmax 33, Deauville 5-point
scale score 5 (D5PSS 5)] (Figure 2B).
Treatment was switched to R-CHOP. PET/CT after R-CHOPx3 revealed
complete metabolic response (left inguinal SUVmax 2.2; D5PSS 3) (Figure 2C).
End-of-treatment restaging after R-CHOPx6 in August 2020 confirmed
complete remission (D5PSS 3, left iliac/inguinal SUVmax 1.8, dmax 2.8 cm) (Figure 2D).
The patient relapsed six months later with growing inguinal/iliac and
abdominal lymphadenopathy. Salvage chemotherapy and autologous stem
cell transplantation were scheduled, but he was admitted to another
Center closer to his residency.
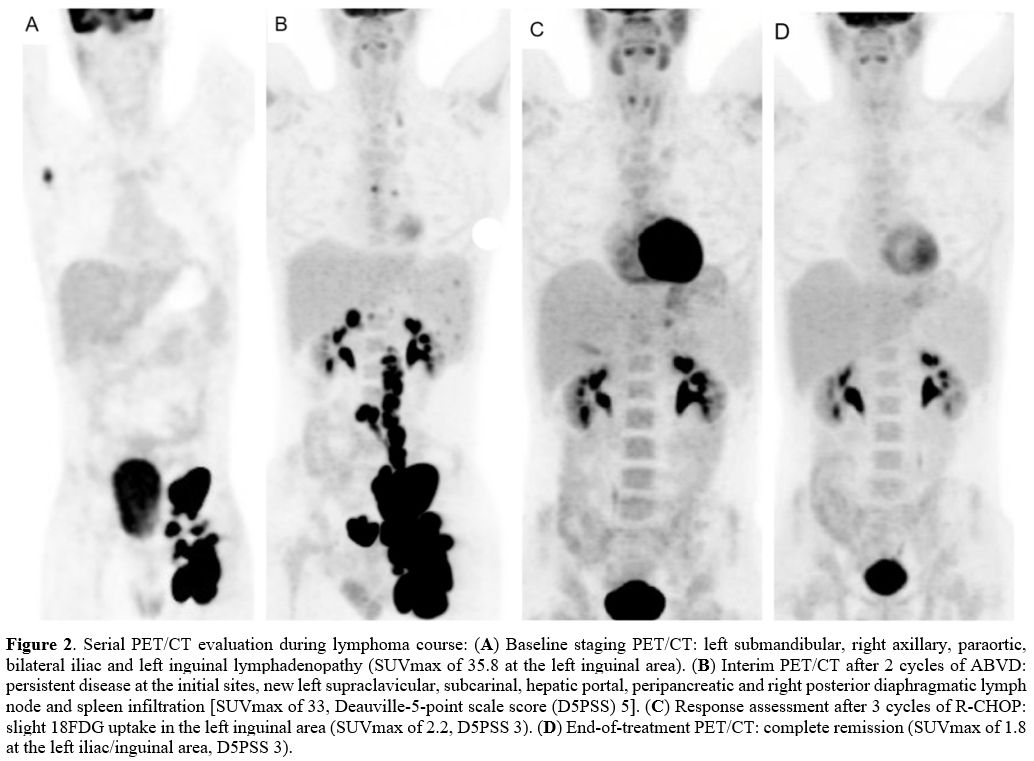 |
Figure
2.
Serial PET/CT evaluation during lymphoma course: (A) Baseline staging
PET/CT: left submandibular, right axillary, paraortic, bilateral iliac
and left inguinal lymphadenopathy (SUVmax of 35.8 at the left inguinal
area). (B)
Interim PET/CT after 2 cycles of ABVD: persistent disease at the
initial sites, new left supraclavicular, subcarinal, hepatic portal,
peripancreatic and right posterior diaphragmatic lymph node and spleen
infiltration [SUVmax of 33, Deauville-5-point scale score (D5PSS) 5]. (C) Response
assessment after 3 cycles of R-CHOP: slight 18FDG uptake in the left
inguinal area (SUVmax of 2.2, D5PSS 3). (D) End-of-treatment
PET/CT: complete remission (SUVmax of 1.8 at the left iliac/inguinal
area, D5PSS 3). |
During
ABVD and given the cessation of FMF-specific therapy, the patient had
several minor FMF flares that required no intervention. However,
following R-CHOP initiation, he suffered from 3 major episodes of fever
and severe abdominal pain during a short period of ~ three weeks. The
initial two events were treated with a single dose of betamethasone and
anakinra, another anti-IL-1 (α and β) agent to which the patient had
never been exposed. However, a single dose of canakinumab was
eventually administered to control the severity of the third episode (Figure 3).
After that, chemotherapy was completed uneventfully. Following
rheumatology consultation, the patient occasionally received
betamethasone for FMF after lymphoma presentation and remained on this
until the last follow-up.
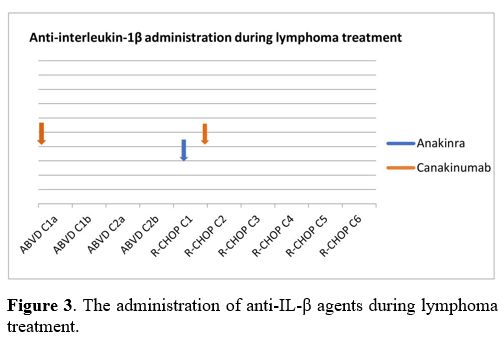 |
Figure
3. The
administration of anti-IL-β agents during lymphoma treatment. |
The
coexistence or overlap of periodic fever, aphthous stomatitis,
pharyngitis, and cervical adenitis (PFAPA) with FMF has been described
in the literature.[2-4] However, our case is a
typical, refractory to colchicine, FMF patient without any
PFAPA-related symptoms or tonsillectomy in childhood. This diagnosis is
also supported by the requirement of anti-IL-1 inhibitors to maintain
full remission and the relapse of inflammatory attacks after the
administration of R-CHOP, a therapeutic regimen containing
corticosteroids, the mainstay treatment of PFAPA.
In contrast to
cHL, very little data on the pathogenesis of NLPHL is available. In
2010 Celik et al. published a small study evaluating the frequency of
MEFV gene mutations in patients with hematolymphoid neoplasms without
FMF history. Gene carrier rate in patients with chronic lymphocytic
leukemia, NHL, and HL was lower than in the general population; a
higher frequency of MEFV gene mutations was observed in MM and acute
lymphoblastic leukemia.[5] Abnormal regulation of
apoptosis and NF-κΒ pathway enhancement via pyrin were presumably
implicated in the susceptibility to hematologic malignancy. However,
data on the correlation between MEFV mutations and hematologic
neoplasms remains limited, and the involvement of FMF in cancer remains
speculative. Several studies have linked chronic inflammatory and
autoimmune diseases with increased cancer incidence, including
lymphomas. Still, a large cohort of 8,435 Israeli FMF patients included
only 18 (5 males) to suffer from lymphomas (0.21% in total, 0.06% in
males).[6-8]
The appearance of malignancy in
patients treated with immunomodulators for various rheumatologic
conditions has been widely discussed. Specifically, cancer incidence in
rheumatoid arthritis patients under anti-IL-1 therapy with anakinra may
not be different compared to the general population. However, lymphoma
incidence in these patients is consistent with that reported in
patients with rheumatoid arthritis and other autoimmune disorders,
which may reflect that lymphomagenesis may be related to uncontrolled
chronic inflammation and autoimmunity rather than biologic agents.[9]
Notably, FMF patients are characterized by chronic subclinical
inflammation, even during attack-free periods, especially those
carrying the homozygous M694V mutation.[10] In
addition, recent data on the long-term safety of canakinumab in
pediatric FMF patients reveal an extremely low incidence of serious
adverse events, including malignancy.[11-12] However,
most data come from retrospective studies in children with relatively
short follow-up after initiating IL-1 inhibition. In our case, NLPHL
appeared almost eight years after the first administration of
canakinumab. Therefore, a causative relation between the canakinumab
and the development of lymphoma, especially in adult patients under
long-standing anti-IL-1 treatment, must be further elucidated.
In the context of preexisting FMF, only two cases of nodular sclerosing and mixed cellularity cHL have been reported worldwide,[13-14]
but no NLPHL case has been published so far. Our patient had
advanced-stage NLPHL appearing under long-term anti-IL-1β therapy.
Canakinumab was ceased before chemotherapy initiation, and managing FMF
during concomitant (immuno)chemotherapy appeared to be fairly
challenging. Soon after the initiation of R-CHOP, the presentation of
severe, serial FMF episodes made IL-1 inhibitors anakinra and
canakinumab inevitable. No early-onset side effects were observed with
the concurrent administration of immunochemotherapy and anti-IL-1
agents, and hematologic toxicity did not differ from that typically
expected, with only limited filgrastim use. There are published data
supporting the successful prevention of the progression of prodrome to
full-blown attacks by on-demand use of anakinra in selected patients.[15]
In difficult cases like the present one, on-demand anakinra, with its
short half-life (4-6 hours), might be a beneficial and safer approach
compared to the much longer half-life (23-26 days) canakinumab.
Following remission, further administration of canakinumab was
considered unsafe for the underlying malignancy, and the patient has
been occasionally treated with betamethasone thereafter.
The
novelty of this case report is dual: the description for the first time
of NLPHL in FMF in a canakinumab-dependent case, pointing to the
potential implication of autoinflammatory syndromes / biologic
immunosuppressing agents in the pathogenesis of NLPHL. Secondly, this
case highlights the query that IL-1 inhibitors and concurrent
chemotherapy may be critical for successfully completing lymphoma
treatment and preserving the quality of life.
References
- Manna R, Rigante D.
Familial Mediterranean Fever: Assessing the Overall Clinical Impact and
Formulating Treatment Plans. Mediterr J Hematol Infect Dis. 2019 May
1;11(1):e2019027. https://doi.org/10.4084/mjhid.2019.027
PMid:31205631 PMCid:PMC6548206
- Butbul Aviel Y, Harel L,
Abu Rumi M, Brik R, Hezkelo N, Ohana O, Amarilyo G. Familial
Mediterranean Fever Is Commonly Diagnosed in Children in Israel with
Periodic Fever Aphthous Stomatitis, Pharyngitis, and Adenitis Syndrome.
J Pediatr. 2019 Jan;204:270-274. https://doi.org/10.1016/j.jpeds.2018.08.080
PMid:30361059
- Gattorno M, Caorsi R,
Meini A, Cattalini M, Federici S, Zulian F, Cortis E, Calcagno G,
Tommasini A, Consolini R, Simonini G, Pelagatti MA, Baldi M, Ceccherini
I, Plebani A, Frenkel J, Sormani MP, Martini A. Differentiating PFAPA
syndrome from monogenic periodic fevers. Pediatrics. 2009
Oct;124(4):e721-8. https://doi.org/10.1542/peds.2009-0088
PMid:19786432
- Deneau M, Wallentine J,
Guthery S, O'Gorman M, Bohnsack J, Fluchel M, Bezzant J, Pohl JF.
Natural killer cell lymphoma in a pediatric patient with inflammatory
bowel disease. Pediatrics. 2010 Oct;126(4):e977-81. https://doi.org/10.1542/peds.2010-0486
PMid:20837584
- Celik S, Erikci AA,
Tunca Y, Sayan O, Terekeci HM, Umur EE, Torun D, Tangi F, Top C,
Oktenli C. The rate of MEFV gene mutations in hematolymphoid neoplasms.
Int J Immunogenet. 2010 Oct;37(5):387-91. https://doi.org/10.1111/j.1744-313X.2010.00938.x
PMid:20518828
- Theander E, Henriksson
G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other
malignancies in primary Sjögren's syndrome: a cohort study on cancer
incidence and lymphoma predictors. Ann Rheum Dis. 2006
Jun;65(6):796-803. https://doi.org/10.1136/ard.2005.041186
PMid:16284097 PMCid:PMC1798187
- Ladouceur A, Clarke AE,
Ramsey-Goldman R, Bernatsky S. Malignancies in systemic lupus
erythematosus: an update. Curr Opin Rheumatol. 2019 Nov;31(6):678-681. https://doi.org/10.1097/BOR.0000000000000648
PMid:31403485
- Brenner R, Ben-Zvi I,
Shinar Y, Liphshitz I, Silverman B, Peled N, Levy C, Ben-Chetrit E,
Livneh A, Kivity S. Familial Mediterranean Fever and Incidence of
Cancer: An Analysis of 8,534 Israeli Patients With 258,803
Person-Years. Arthritis Rheumatol. 2018 Jan;70(1):127-133. https://doi.org/10.1002/art.40344
PMid:28992365
- Rubbert-Roth A.
Assessing the safety of biologic agents in patients with rheumatoid
arthritis. Rheumatology (Oxford). 2012 Jul;51 Suppl 5:v38-47. https://doi.org/10.1093/rheumatology/kes114
PMid:22718926
- Kelesoglu FM, Aygun E,
Okumus NK, Ersoy A, Karapınar E, Saglam N, Aydın NG, Senay BB, Gonultas
S, Sarisik E, Can MZ, Atay S, Basbug D, Tiryaki FK, Ozer S, Durmus RB,
Orem F, Atay T, Acar A, Yilmaz Y, Kaya S, Ciftkaya A, Sarac Z, Makar
CC, Saracoglu B, Dogdu G, Omeroglu RE. Evaluation of
subclinical inflammation in familial Mediterranean fever patients:
relations with mutation types and attack status: a retrospective study.
Clin Rheumatol. 2016 Nov;35(11):2757-2763. https://doi.org/10.1007/s10067-016-3275-0
PMid:27106545
- Gülez N, Makay B,
Sözeri B. Long-term effectiveness and safety of canakinumab in
pediatric familial Mediterranean fever patients. Mod Rheumatol. 2020
Jan;30(1):166-171. https://doi.org/10.1080/14397595.2018.1559488
PMid:30556769
- Sag E, Akal F, Atalay
E, Akca UK, Demir S, Demirel D, Batu ED, Bilginer Y, Ozen S. Anti-IL1
treatment in colchicine-resistant paediatric FMF patients: real life
data from the HELIOS registry. Rheumatology (Oxford). 2020 Nov
1;59(11):3324-3329. https://doi.org/10.1093/rheumatology/keaa121
PMid:32306038
- Langenberg-Ververgaert
KPS, Laxer RM, Punnett AS, Dupuis LL, Finkelstein Y, Abla O.
Chemotherapy-Colchicine Interaction in a Child with Familial
Mediterranean Fever and Hodgkin Lymphoma. Mediterr J Hematol Infect
Dis. 2018 Mar 1;10(1):e2018019. https://doi.org/10.4084/mjhid.2018.019
PMid:29531656 PMCid:PMC5841933
- Demir F, Bahadir A,
Mungan S, Çobanoğlu Ü, Kalyoncu M. Systemic Amyloidosis in a Patient
With Familial Mediterranean Fever and Hodgkin Lymphoma: A Case Report.
J Pediatr Hematol Oncol. 2020 Apr;42(3):234-237. https://doi.org/10.1097/MPH.0000000000001504
PMid:31094904
- Babaoglu H, Varan O,
Kucuk H, Atas N, Satis H, Salman R, Ozturk MA, Haznedaroglu S, Goker B,
Tufan A. On demand use of anakinra for attacks of familial
Mediterranean fever (FMF). Clin Rheumatol. 2019 Feb;38(2):577-581. https://doi.org/10.1007/s10067-018-4230-z
PMid:30062447
[TOP]


