Guang-Liang Chen1,2†, Doudou Li2†, Sufen Cao1,3†, Shiyu Jiang1,2, Qunling Zhang1,2, Jia Jin1,2, Zuguang Xia1,2, Yizhen Liu1,2, Xiaojian Liu1,2, Yanzhe Zhu4, Yu Chen5, Lingli Gu1,3, Xiaonan Hong1,2, Junning Cao1,2, Rong Tao1,2 and Fangfang Lv1,2.
1 Department of Lymphoma, Fudan University Shanghai Cancer Center, Shanghai, 200032, China.
2 Department of Oncology, Shanghai Medical College Fudan University, Shanghai, 200032, China.
3 Department of Nursing, Fudan University Shanghai Cancer Center, Shanghai, 200032, China.
4 Department of Oncology, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, China.
5 Department of Oncology, The Fourth Affiliated Hospital of Anhui Medical University, Hefei, 230061, China.
†These authors have contributed equally to this work and share the first authorship.
Correspondence to: Dr.
Fangfang Lv. Department of Lymphoma, Fudan University Shanghai Cancer
Center, Shanghai, 200032, China and Department of Oncology, Shanghai
Medical College Fudan University, Shanghai, 200032, China. E-mail:
lvff80@163.com
Published: September 1, 2022
Received: May 7, 2022
Accepted: August 12, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022066 DOI
10.4084/MJHID.2022.066
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Primary breast diffuse large B-cell lymphoma (PB-DLBCL) is a rare
subtype of non-Hodgkin lymphoma (NHL) with limited data on the clinical
features and prognostic factors.
Patients and Methods:
A consecutive cohort of patients with PB-DLBCL was retrospectively
analyzed in our hospital from February 1997 through July 2018. The
primary endpoint is overall survival (OS) contributing to any cause.
Results:
A total of 76 patients were diagnosed with PB-DLBCL. The median age at
diagnosis was 51 years (range: 25-80 years), with female prevalence
(98.7%). Forty (52.6%) patients had right-sided breast involvement but
no bilateral breast involvement at diagnosis. Overall, disease stages
IE and IIE were seen in 55 (72.4%) and 21 (27.6%) patients,
respectively. According to the stage-modified International Prognostic
Index (IPI), 37 (48.7%) patients were classified in the very good risk
group (IPI 0). Of the 72 patients available, the non-germinal center
B-cell (non-GCB) subtype of DLBCL was observed in 66 (91.6%) patients.
All patients received anthracycline-based chemotherapy, 56 (73.7%) with
rituximab, 31 (40.8%) also with additional radiation therapy, and 14
(18.4%) patients received a prophylactic intrathecal injection. Seven
(9.2%) patients had refractory disease. With a median follow-up of 6.8
years (range 0.4-25.0 years), 10 (13.2%) patients had a relapse in the
central nervous system (CNS) site. The 5-year and 10-year OS of all the
patients was 97.2% (95% CI: 99.3-89.5) and 84.8% (95% CI: 70.0-93.5),
respectively. The median OS was not reached. The median
progression-free survival (PFS) was 10.3 years for patients with
PB-DLBCL. The 5-year PFS of all the patients was 76.3% (95% CI:
64.6-84.6). Univariate analysis revealed several prognostic factors,
including stage-modified IPI, breast surgery, refractory disease, and
CNS relapse. Multivariate analyses produced two independent prognostic
factors for patients with PB-DLBCL, including stage-modified IPI score
(2-3 versus 0) (hazard ratio: 19.114, 95% CI 1.841 to 198.451, p=0.013)
and CNS relapse (hazard ratio: 5.522, 95% CI 1.059 to 28.788, p=0.043).
Conclusion:
In our cohort, PB-DLBCL clinical features are similar to prior
literature reports. Stage-modified IPI score and CNS relapse were
associated with overall survival.
|
Introduction
Primary
breast diffuse large B-cell lymphoma (PB-DLBCL) is an aggressive
non-Hodgkin lymphoma (NHL) that affects the breast with or without
regional lymph node involvement.[1,2,3,4] PB-DLBCL is primarily presented as a painless unilateral breast mass in women,[3,4,5] often misdiagnosed with breast carcinoma.[6] The increase in pathological data revealed a predominance of a non-germinal center B-cell (non-GCB) phenotype in PB-DLBCL.[3,7,8,9,10,11,12]
However, most studies included a small sample for analysis, and the
optimal treatment approach is not yet clear. Indeed, a rapid rise in
the incidence of PB-DLBCL has been identified.[13,14] Additionally, younger or breast cancer patients with hormone therapy have an increased risk of developing NHL.[15]
To date, significant advances have been made in the genetic subtypes of DLBCL with different prognoses,[16,17] as well as in the molecular features of PB-DLBCL.[18,19,20]
The revolution of the therapeutic strategies of PB-DLBCL with new
emerging targeted therapies requires more knowledge of PB-DLBCL.[19,20,21]
Furthermore, the incidence rate of central nervous system (CNS) relapse
is higher in primary breast DLBCL than those in nodal DLBCL,[2,22] despite the widespread use of prophylactic intrathecal injection.[23] Nevertheless, the Asian population of PB-DLBCL was analyzed in limited research and a lack of a long-term follow-up.[24,25,26,27,28] Consequently, further understanding of the clinical features and prognostic factors of PB-DLBCL remains of interest.
We,
therefore, conducted this retrospective study to investigate the
clinical features, treatment outcomes, and prognosis in patients with
PB-DLBCL. The primary endpoint of this study was the overall survival
(OS).
Patients and Methods
Participants and Study design.
We retrospectively reviewed data from the department of cancer
prevention at Fudan University Shanghai Cancer Center (FUSCC) of
patients who received a diagnosis of PB-DLBCL[29] between February 1997 and July 2018 (Figure 1).
This study was approved by the Institutional Review Board of the FUSCC
(ZRB1612167-18). Eligibility criteria required a confirmed pathological
diagnosis of DLBCL according to the 2017 WHO classification of lymphoid
neoplasms and localized disease (involvement of breast and localized
lymph nodes). Patients with transformed DLBCL from low-grade lymphoma
or other types of lymphoma and patients with incomplete data after
diagnosis were excluded. Electronic medical records were used to obtain
demographic and clinical variables, laboratory values, and medications.
The primary outcome of interest was OS, measured as the time from the
diagnosis of PB-DLBCL to death attributed to any cause; the last
follow-up date was October 01, 2021. Progression-free survival (PFS)
was calculated as the time from the date of diagnosis to disease
progression or death from any cause. Mortality data and the timing of
death were obtained from the department of cancer prevention, FUSCC.
Five patients (6.6%) were considered lost to follow-up if the last
visit was >12 months before the end of the study.
The stage-modified International Prognostic Index (IPI) score was assessed as previously described.[2,22,30] The GCB and non-GCB subtypes of DLBCL were determined using all the available information by Hans criteria.[31] Response assessment was carried out as in previous reports, according to the International Working Group response criteria.[2,32]
Relapse refers to lymphoma, which recurs or develops after a period of
complete remission. When the lymphoma does not or only partially
responds to first-line chemotherapy is called refractory. Body Mass
Index (BMI) is measured by a person's weight in kilograms divided by
the square of height in meters.
Statistical analysis.
All statistical analyses were two-sided and conducted with the IBM SPSS
Statistics 26.0 or GraphPad Prism software 9.0. The association between
demographic, clinical, or laboratory variables and the primary outcome
(OS) were assessed using univariate Kaplan-Meier (KM) analysis (a
stratified log-rank test) and multivariate Cox regression models as
previously reported.[33] Briefly, the prognostic effect of each factor was analyzed by a log-rank test. Clinically relevant covariates (p<0.15)
identified in univariate analyses were included in multivariate models.
In addition, Cox's proportional hazards (Cox's PH) were assessed, and
time-varying Cox regression analysis (Forward: LR) (entry significance
level=0.05, exit significance level=0.1) was used to evaluate
independent factors for survival. A two-sided P-value < 0.05 was
considered statistically significant.
Results
Between February 1997 and July 2018, a consecutive cohort of 76 patients with PB-DLBCL was included in this study (Figure 1). The clinical-related characteristics and univariate analysis of patients with PB-DLBCL are shown in Table 1.
The median age at diagnosis was 51 years (range: 25-80), and 98.7% of
the patients were female. At presentation, only one (1.3%) patient
presented with B-symptoms or poor performance status (Eastern
Cooperative Oncology Group (ECOG) ≥2). Forty (52.6%) patients had a
right breast lesion at diagnosis. No bilateral breast involvement at
diagnosis was observed. Among all patients, 55 (72.4%) had disease
stage IE, and 21 (27.6%) had IIE. The stage-modified IPI was 0 or 1 in
68 patients (89.5%). Of the 72 patients with available
immunohistochemistry, the subtypes of DLBCL were non-GCB in 66 (91.6%)
patients. Nine (11.8%) patients were positive for hepatitis B surface
antigen upon diagnosis.
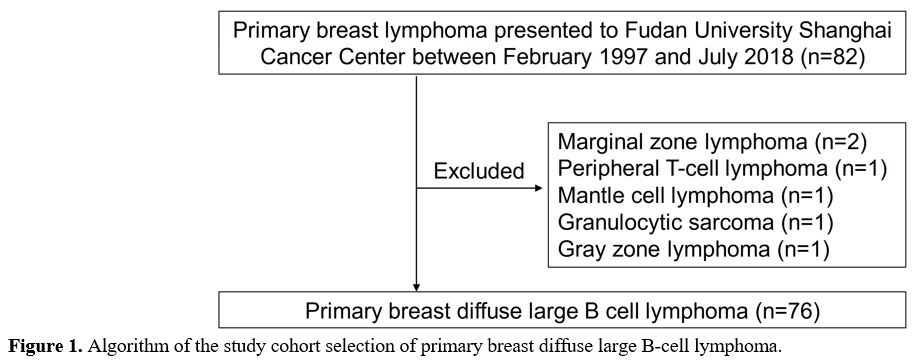 |
Figure
1. Algorithm of the study cohort selection of primary breast diffuse large B-cell lymphoma. |
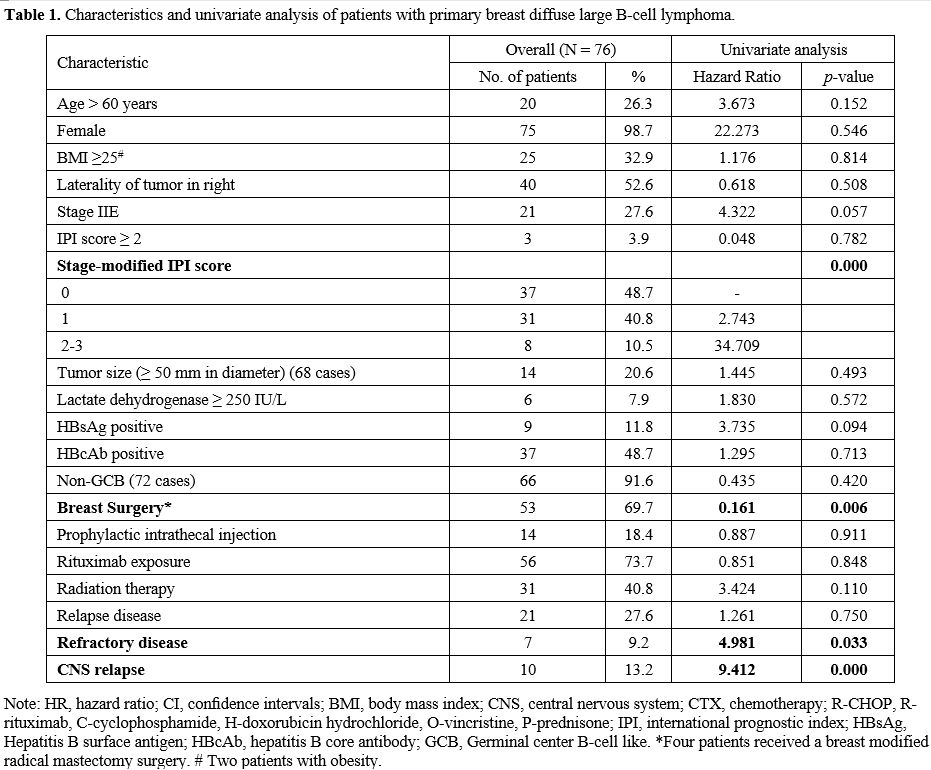 |
Table
1. Characteristics and univariate analysis of patients with primary breast diffuse large B-cell lymphoma. |
In
our cohort, all patients were treated with anthracycline-based
chemotherapy following the diagnosis of PB-DLBCL; 14 (18.4%) patients
received a prophylactic intrathecal injection for CNS relapse (Table 1).
Fifty-three (69.7%) patients had breast surgery prior to frontline
chemotherapy. Fifty-six (73.7%) patients were treated with chemotherapy
plus rituximab regimens. Radiation therapy (RT) after the frontline
chemotherapy was administered in 31 (40.8%) patients. After a median
follow-up of 6.8 years (range 0.4-25.0 years), eight (10.5%) patients
died. The median OS was not reached (Figure 2A).
The 5-year and 10-year OS of all the patients was 97.2% (95% CI:
99.3-89.5) and 84.8% (95% CI: 70.0-93.5), respectively. The median PFS
was 10.3 years for patients with PB-DLBCL (Figure 2B).
The 5-year PFS of all the patients was 76.3% (95% CI: 64.6-84.6). At
the end of the frontline chemotherapy, five (6.6%) patients achieved a
partial response (PR), and two (2.6%) progressed during treatment. A
total of 28 (36.8%) patients had a relapse or refractory disease, with
7 (9.2%) classified as a refractory disease. CNS relapse occurred in 10
(13.2%) patients. The median time from the initial diagnosis to CNS
relapse was 3.8 years (range: 1.0-10.3 years), and survival after CNS
relapse in patients with PB-DLBCL was 34.4 months.
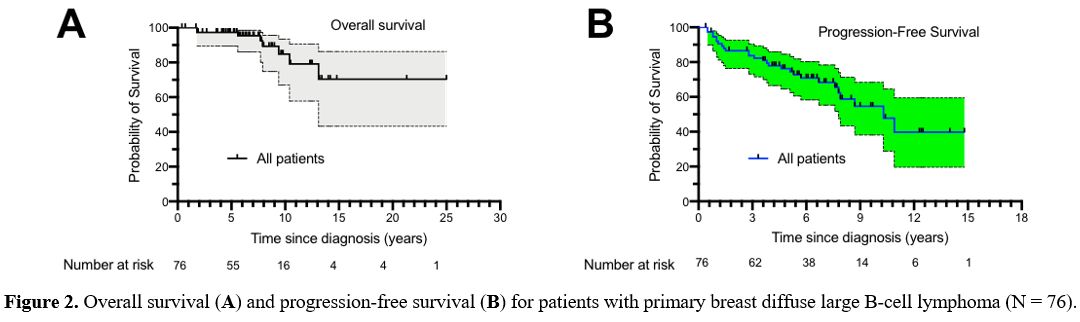 |
Figure
2. Overall survival (A) and progression-free survival (B) for patients with primary breast diffuse large B-cell lymphoma (N = 76).
|
In univariate analysis (Table1), prognostic factors that retained statistical significance for OS were stage-modified IPI score (p=0.000) (Figure 3A), breast surgery (p=0.006) (Figure 3B), refractory disease (p=0.033) (Figure 3C), and CNS relapse (p=0.000) (Figure 3D).
However, the two factors that have independent prognostic significance
in multivariate analysis are stage-modified IPI score and CNS relapse (Table 2).
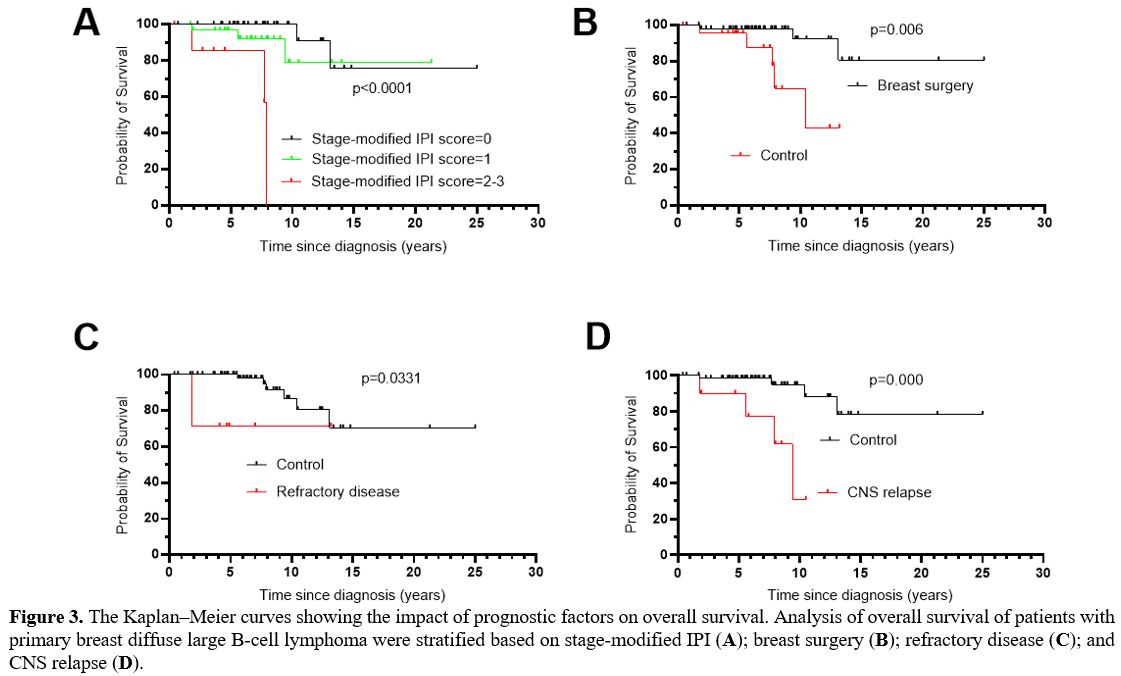 |
Figure 3. The Kaplan–Meier
curves showing the impact of prognostic factors on overall survival.
Analysis of overall survival of patients with primary breast diffuse
large B-cell lymphoma were stratified based on stage-modified IPI (A); breast surgery (B); refractory disease (C); and CNS relapse (D). |
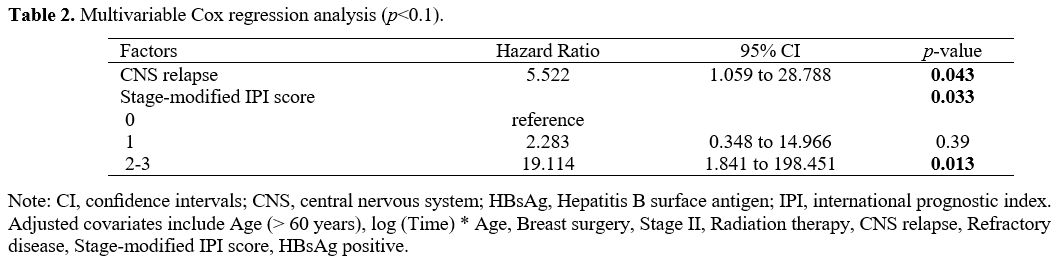 |
Table 2. Multivariable Cox regression analysis (p<0.1). |
Discussion
According
to the current literature in this study, the PB-DLBC was the most
frequent histologic subtype of primary breast lymphoma, a well-defined
subtype of non-Hodgkin lymphoma (NHL);[1,2,11,13,22,34,35] the initial clinical presentation of PB-DLBCL was consistent with the published literature.[2,6,34,36]
Most patients with PB-DLBCL were female aged less than 60 years, were
unilaterally breast lumpy, and slightly more frequent on the right
side. In addition, our data confirmed that the stage-modified IPI score
is a major independent prognostic factor for PB-DLBCL.[2,6,30,36]
Furthermore, a high rate of CNS relapse in our cohort was observed over
a long follow-up and was found to be another important independent
prognostic factor for OS. For considerable patients who have already
received immunochemotherapy, RT, and prophylactic intrathecal
injection,[23] other approaches to reducing CNS relapse still require exploration.
In
the present study, the 10-year OS of the PB-DLBCL patients was 84.8%
(95% CI: 70.0-93.5). The superior outcome is probably due to early
screening, diagnosis, and multiple management approaches.[37]
In our cohort, less than one-fourth of patients have a tumor diameter
of more than 50 mm, which may preclude the adverse effect of bulky
disease on the clinical outcome of PB-DLBCL.[38]
Nearly half of our PB-DLBCL cohort was a younger population, which may
partially explain the good prognosis. In addition, increased BMI does
not seem to impact on survival of PB-DLBCL. Indeed, the risk and death
of DLBCL patients are largely affected by chronic infection,[39,40,41,42,43,44] including hepatitis B virus infection.[45,46,47]
However, our data found that HBsAg-positive status is not associated
with OS (p=0.094). In the era of precision medicine, HBsAg-positive
influence on OS in patients with PB-DLBCL should be evaluated in
prospective studies.
The optimal therapeutic strategies for
PB-DLBCL remain largely unrevealed. No benefit from breast mastectomy
seems to have reached a consensus in patients with primary breast
lymphoma.[5,6,34,48] In the present study, breast surgery, which consists primarily of lumpectomy (Table 1), is associated with an improved OS in univariate analysis but lost in the multivariate analysis (Table 2). Indeed, a study based on Surveillance, Epidemiology, and End Results (SEER) database analysis supports our findings.[37] In our opinion, breast surgery can provide perfect local control and sufficient samples for a precise diagnosis of disease.[35]
However, a prospective investigation needs to establish whether the
prognosis of PB-DLBCL can benefit from a rapid and accurate diagnosis
and molecular identification.
In contrast to previous reports,[49,50]
RT is not associated with improved OS (p=0.110). One possible
explanation is the unfavorable characteristics of PB-DLBCL patients
receiving an additional RT in our cohort. However, this raised a
concern about the late complications of RT on PB-DLBCL, as the
tumor-involved field of PB-DLBCL included several key organs of the
heart and lungs. Therefore, the precise selection of PB-DLBCL patients
for additional RT may be the subject of further research. Furthermore,
in our study, rituximab use was not associated with a survival benefit,[2] although there is some controversial data.[51]
To date, a non-GCB phenotype of PB-DLBCL is associated with a poor prognosis.[52,53,54,55]
However, the non-GCB phenotype of PB-DLBCL was not associated with the
OS in the KM analysis of our cohort. An elevated incidence of CNS
relapse was identified as an independent unfavorable OS factor. It
could be associated with the intrinsic biological characteristics of
the non-GC phenotype,[22] mutation of myeloid differentiation 88 (MYD88), and a cluster of differentiation 79b (CD79b).[18,19,56,57,58]
However, due to the limited data available, our cohort did not analyze
the role of the genomic mutation of MYD88 and CD79b in the prognosis of
the OS. Indeed, the activated B cell-like (ABC) subtype of DLBCL with B
cell receptor (BCR) and MYD88 mutations potentially respond to Bruton's
tyrosine kinase (BTK) inhibitor.[21,59]
In the future, the impact of the BTK inhibitor on the incidence of CNS
relapse and the prognosis in PB-DLBCL need to be assessed.
The
small sample size and the inherent nature of observational and
retrospective studies limited the current research. Therefore, we
adjusted some known prognostic factors on DLBCL. Still, did not examine
the role of Chinese traditional medicine, Epstein-Barr virus (EBV)
infection, oncogenic gene mutation, or other confounding factors
associated with treatment and DLBCL. Furthermore, assessment of CNS
involvement was not carried out in all patients at diagnosis. However,
PB-DLBCL as a rare disease is far from being fully recognized.
Therefore, the clinical features identified and several independent
prognostic factors can help improve daily practice and guide the design
of future clinical trials in this disease.
In summary,
stage-modified IPI score and CNS relapse are valuable predictors for
the prognosis of PB-DLBCL. However, additional efforts are required to
decrease the rate of CNS relapse.
References
- C. Wiseman, and K.T. Liao, Primary lymphoma of the breast. Cancer 29 (1972) 1705-12. https://doi.org/10.1002/1097-0142(197206)29:6<1705::AID-CNCR2820290640>3.0.CO;2-I
- P.J.
Hosein, J.C. Maragulia, M.P. Salzberg, O.W. Press, T.M. Habermann, J.M.
Vose, M. Bast, R.H. Advani, R. Tibshirani, A.M. Evens, N. Islam, J.P.
Leonard, P. Martin, A.D. Zelenetz, and I.S. Lossos, A multicentre study
of primary breast diffuse large B-cell lymphoma in the rituximab era.
Br J Haematol 165 (2014)
358-63. https://doi.org/10.1111/bjh.12753 PMid:24467658 PMCid:PMC3990235
- W.
Jeanneret-Sozzi, A. Taghian, R. Epelbaum, P. Poortmans, D. Zwahlen, B.
Amsler, S. Villette, Y. Belkacémi, T. Nguyen, P. Scalliet, P. Maingon,
C. Gutiérrez, P. Gastelblum, M. Krengli, R.A. Raad, M. Ozsahin, and
R.-O. Mirimanoff, Primary breast lymphoma: Patient profile, outcome and
prognostic factors. A multicentre Rare Cancer Network study. BMC Cancer
8 (2008) https://doi.org/10.1186/1471-2407-8-86 PMid:18380889 PMCid:PMC2330152
- G.
Ryan, G. Martinelli, M. Kuper-Hommel, R. Tsang, G. Pruneri, K. Yuen, D.
Roos, A. Lennard, L. Devizzi, S. Crabb, D. Hossfeld, G. Pratt, M.
Dell'Olio, S.P. Choo, R.G. Bociek, J. Radford, S. Lade, A.M. Gianni, E.
Zucca, F. Cavalli, and J.F. Seymour, Primary diffuse large B-cell
lymphoma of the breast: prognostic factors and outcomes of a study by
the International Extranodal Lymphoma Study Group. Annals of Oncology
19 (2008) 233-241 https://doi.org/10.1093/annonc/mdm471 PMid:17932394
- W.C.
Jennings, R.S. Baker, S.S. Murray, C.A. Howard, D.E. Parker, L.F.
Peabody, H.M. Vice, W.W. Sheehan, and T.A. Broughan, Primary breast
lymphoma: the role of mastectomy and the importance of lymph node
status. Ann Surg 245 (2007) 784-9. https://doi.org/10.1097/01.sla.0000254418.90192.59 PMid:17457172 PMCid:PMC1877073
- G.
Ryan, G. Martinelli, M. Kuper-Hommel, R. Tsang, G. Pruneri, K. Yuen, D.
Roos, A. Lennard, L. Devizzi, S. Crabb, D. Hossfeld, G. Pratt, M.
Dell'Olio, S.P. Choo, R.G. Bociek, J. Radford, S. Lade, A.M. Gianni, E.
Zucca, F. Cavalli, J.F. Seymour, and G. International Extranodal
Lymphoma Study, Primary diffuse large B-cell lymphoma of the breast:
prognostic factors and outcomes of a study by the International
Extranodal Lymphoma Study Group. Ann Oncol 19 (2008) 233-41. https://doi.org/10.1093/annonc/mdm471 PMid:17932394
- P.
Validire, M. Capovilla, B. Asselain, Y. Kirova, R. Goudefroye, C.
Plancher, A. Fourquet, M. Zanni, P. Gaulard, A. Vincent-Salomon, and D.
Decaudin, Primary breast non-Hodgkin's lymphoma: a large single center
study of initial characteristics, natural history, and prognostic
factors. Am J Hematol 84 (2009) 133-9. https://doi.org/10.1002/ajh.21353 PMid:19199367
- H.
Shen, Z. Wei, D. Zhou, Y. Zhang, X. Han, W. Wang, L. Zhang, C. Yang,
and J. Feng, Primary extra-nodal diffuse large B-cell lymphoma: A
prognostic analysis of 141 patients. Oncol Lett 16 (2018) 1602-1614. https://doi.org/10.3892/ol.2018.8803
- J.
Caon, E.S. Wai, J. Hart, C. Alexander, P.T. Truong, L.H. Sehn, and J.M.
Connors, Treatment and Outcomes of Primary Breast Lymphoma. Clinical
Breast Cancer 12 (2012) 412-419 https://doi.org/10.1016/j.clbc.2012.07.006 PMid:23018097
- D.
Li, J. Deng, H. He, Y. Bu, F. Peng, X. Tang, B. Wang, Y. Lei, H. Zhang,
and P. Xie, Primary breast diffuse large B-cell lymphoma shows an
activated B-cell-like phenotype. Annals of Diagnostic Pathology 16
(2012) 335-343.https://doi.org/10.1016/j.anndiagpath.2012.01.004 PMid:22569408
- S.
Yoshida, N. Nakamura, Y. Sasaki, S. Yoshida, M. Yasuda, H. Sagara, T.
Ohtake, S. Takenoshita, and M. Abe, Primary breast diffuse large B-cell
lymphoma shows a non-germinal center B-cell phenotype. Modern Pathology
18 (2004) 398-405
https://doi.org/10.1038/modpathol.3800266 PMid:15492762 - H.-Y.
Yhim, H.J. Kang, Y.H. Choi, S.J. Kim, W.S. Kim, Y.S. Chae, J.S. Kim,
C.W. Choi, S.Y. Oh, H.S. Eom, J.-A. Kim, J.H. Lee, J.-H. Won, H. Shim,
J.-J. Lee, H.J. Sung, H.J. Kim, D.H. Lee, C. Suh, and J.-Y. Kwak,
Clinical outcomes and prognostic factors in patients with breast
diffuse large B cell lymphoma; Consortium for Improving Survival of
Lymphoma (CISL) study. BMC Cancer 10 (2010) https://doi.org/10.1186/1471-2407-10-321 PMid:20569446 PMCid:PMC2927999
- A.
Thomas, B.K. Link, S. Altekruse, P.A. Romitti, and M.C. Schroeder,
Primary Breast Lymphoma in the United States: 1975-2013. JNCI: Journal
of the National Cancer Institute 109 (2017) https://doi.org/10.1093/jnci/djw294
- Y.
Jia, C. Sun, Z. Liu, W. Wang, and X. Zhou, Primary breast diffuse large
B-cell lymphoma: a population-based study from 1975 to 2014. Oncotarget
9 (2017) 3956-3967 https://doi.org/10.18632/oncotarget.23285 PMid:29423097 PMCid:PMC5790514
- D.
Kang, S.E. Yoon, D. Shin, J. Lee, Y.S. Hong, S.K. Lee, J.E. Lee, Y.H.
Park, J.S. Ahn, E. Guallar, W.S. Kim, J. Lee, S.J. Kim, and J. Cho,
Risk of non-Hodgkin lymphoma in breast cancer survivors: a nationwide
cohort study. Blood Cancer Journal 11 (2021) https://doi.org/10.1038/s41408-021-00595-0 PMid:34907177 PMCid:PMC8671407
- R.
Schmitz, G.W. Wright, D.W. Huang, C.A. Johnson, J.D. Phelan, J.Q. Wang,
S. Roulland, M. Kasbekar, R.M. Young, A.L. Shaffer, D.J. Hodson, W.
Xiao, X. Yu, Y. Yang, H. Zhao, W. Xu, X. Liu, B. Zhou, W. Du, W.C.
Chan, E.S. Jaffe, R.D. Gascoyne, J.M. Connors, E. Campo, A.
Lopez-Guillermo, A. Rosenwald, G. Ott, J. Delabie, L.M. Rimsza, K. Tay
Kuang Wei, A.D. Zelenetz, J.P. Leonard, N.L. Bartlett, B. Tran, J.
Shetty, Y. Zhao, D.R. Soppet, S. Pittaluga, W.H. Wilson, and L.M.
Staudt, Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. New
England Journal of Medicine 378 (2018) 1396-1407 https://doi.org/10.1056/NEJMoa1801445 PMid:29641966 PMCid:PMC6010183
- G.W.
Wright, D.W. Huang, J.D. Phelan, Z.A. Coulibaly, S. Roulland, R.M.
Young, J.Q. Wang, R. Schmitz, R.D. Morin, J. Tang, A. Jiang, A. Bagaev,
O. Plotnikova, N. Kotlov, C.A. Johnson, W.H. Wilson, D.W. Scott, and
L.M. Staudt, A Probabilistic Classification Tool for Genetic Subtypes
of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer
Cell 37 (2020) 551-568.e14 https://doi.org/10.1016/j.ccell.2020.03.015 PMid:32289277 PMCid:PMC8459709
- K.
Taniguchi, K. Takata, S.S. Chuang, T. Miyata-Takata, Y. Sato, A. Satou,
Y. Hashimoto, M. Tamura, K. Nagakita, N. Ohnishi, M. Noujima-Harada, T.
Tabata, Y.Y. Kikuti, Y. Maeda, N. Nakamura, M. Tanimoto, and T.
Yoshino, Frequent MYD88 L265P and CD79B Mutations in Primary Breast
Diffuse Large B-Cell Lymphoma. Am J Surg Pathol 40 (2016)
324-34.https://www.ncbi.nlm.nih.gov/pubmed/26752547 https://doi.org/10.1097/PAS.0000000000000592 PMid:26752547
- K.
Taniguchi, K. Takata, S.-S. Chuang, T. Miyata-Takata, Y. Sato, A.
Satou, Y. Hashimoto, M. Tamura, K. Nagakita, N. Ohnishi, M.
Noujima-Harada, T. Tabata, Y.Y. Kikuti, Y. Maeda, N. Nakamura, M.
Tanimoto, and T. Yoshino, Frequent MYD88 L265P and CD79B Mutations in
Primary Breast Diffuse Large B-Cell Lymphoma. American Journal of
Surgical Pathology 40 (2016) 324-334 https://doi.org/10.1097/PAS.0000000000000592 PMid:26752547
- R.
Chen, D. Zhou, L. Wang, L. Zhu, and X. Ye, MYD88L265P and CD79B double
mutations type (MCD type) of diffuse large B-cell lymphoma: mechanism,
clinical characteristics, and targeted therapy. Therapeutic Advances in
Hematology 13 (2022) 204062072110728 https://doi.org/10.1177/20406207211072839 PMid:35126963 PMCid:PMC8808040
- W.H.
Wilson, R.M. Young, R. Schmitz, Y. Yang, S. Pittaluga, G. Wright, C.-J.
Lih, P.M. Williams, A.L. Shaffer, J. Gerecitano, S. de Vos, A. Goy,
V.P. Kenkre, P.M. Barr, K.A. Blum, A. Shustov, R. Advani, N.H. Fowler,
J.M. Vose, R.L. Elstrom, T.M. Habermann, J.C. Barrientos, J. McGreivy,
M. Fardis, B.Y. Chang, F. Clow, B. Munneke, D. Moussa, D.M. Beaupre,
and L.M. Staudt, Targeting B cell receptor signaling with ibrutinib in
diffuse large B cell lymphoma. Nature Medicine 21 (2015) 922-926 https://doi.org/10.1038/nm.3884 PMid:26193343 PMCid:PMC8372245
- H.-Y.
Yhim, J.S. Kim, H.J. Kang, S.J. Kim, W.S. Kim, C.W. Choi, H.S. Eom,
J.-A. Kim, J.H. Lee, J.H. Won, H. Shim, J. Huh, D.-H. Lee, C. Suh, and
J.-Y. Kwak, Matched-pair analysis comparing the outcomes of primary
breast and nodal diffuse large B-cell lymphoma in patients treated with
rituximab plus chemotherapy. International Journal of Cancer 131 (2012)
235-243 https://doi.org/10.1002/ijc.26352 PMid:21823120
- H.Y.
Yhim, D.H. Yoon, S.J. Kim, D.H. Yang, H.S. Eom, K.H. Kim, Y. Park, J.S.
Kim, H.J. Kim, C. Suh, W.S. Kim, and J.Y. Kwak, First-Line Treatment
for Primary Breast Diffuse Large B-Cell Lymphoma Using
Immunochemotherapy and Central Nervous System Prophylaxis: A
Multicenter Phase 2 Trial. Cancers (Basel) 12
(2020). https://doi.org/10.3390/cancers12082192 PMid:32781541 PMCid:PMC7463683
- H.
Shen, Z. Wei, D. Zhou, Y. Zhang, X. Han, W. Wang, L. Zhang, C. Yang,
and J. Feng, Primary extra‑nodal diffuse large B‑cell lymphoma: A
prognostic analysis of 141 patients. Oncology Letters (2018) https://doi.org/10.3892/ol.2018.8803
- Y.
Sun, L.-M. Xu, X. Chen, D. Qian, J.-Q. You, Z. Yuan, and M. Joks,
Diffuse large B-cell lymphoma of the breast: prognostic factors and
treatment outcomes. OncoTargets and Therapy (2016) 2069 https://doi.org/10.2147/OTT.S98566 PMid:27103833 PMCid:PMC4827925
- S.
Hu, Y. Song, X. Sun, L. Su, W. Zhang, J. Jia, O. Bai, S. Yang, R.
Liang, X. Li, H. Zhang, Y. Gao, W. Zhang, X. Xiao, H. Bao, N. Wang, H.
Ren, X. Cen, S.e. Yang, Y. Zhao, Y. Wang, Y. Wang, A. Liu, J. Wang, Y.
Shi, M. Yuan, Y. Li, and X. He, Primary breast diffuse large B‐cell
lymphoma in the rituximab era: Therapeutic strategies and patterns of
failure. Cancer Science 109 (2018) 3943-3952 https://doi.org/10.1111/cas.13828 PMid:30302857 PMCid:PMC6272095
- X.
Sun, B. Xu, Y. Li, J. Du, L. Dong, X. Gao, G. Li, X. Wei, and Y. Song,
Primary breast diffuse large B-cell lymphoma-report of 21 cases from
China with literatures review. Zhonghua Xue Ye Xue Za Zhi 36 (2015)
853-7. https://www.ncbi.nlm.nih.gov/pubmed/26477765
- Y.H.
Zhu, W.J. Meng, L.H. He, Y.S. Jia, and Z.S. Tong, Prognosis analysis of
primary breast diffuse large B cell lymphoma. Zhonghua Zhong Liu Za Zhi
41 (2019) 235-240 https://www.ncbi.nlm.nih.gov/pubmed/30917462
- A.
Aviv, T. Tadmor, and A. Polliack, Primary diffuse large B-cell lymphoma
of the breast: looking at pathogenesis, clinical issues and therapeutic
options. Ann Oncol 24 (2013) 2236-44. https://doi.org/10.1093/annonc/mdt192 PMid:23712546
- T.P.
Miller, S. Dahlberg, J.R. Cassady, D.J. Adelstein, C.M. Spier, T.M.
Grogan, M. LeBlanc, S. Carlin, E. Chase, and R.I. Fisher, Chemotherapy
alone compared with chemotherapy plus radiotherapy for localized
intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med 339
(1998) 21-6. https://doi.org/10.1056/NEJM199807023390104 PMid:9647875
- C.P.
Hans, Confirmation of the molecular classification of diffuse large
B-cell lymphoma by immunohistochemistry using a tissue microarray.
Blood 103 (2004) 275-282 https://doi.org/10.1182/blood-2003-05-1545 PMid:14504078
- B.D.
Cheson, S.J. Horning, B. Coiffier, M.A. Shipp, R.I. Fisher, J.M.
Connors, T.A. Lister, J. Vose, A. Grillo-López, A. Hagenbeek, F.
Cabanillas, D. Klippensten, W. Hiddemann, R. Castellino, N.L. Harris,
J.O. Armitage, W. Carter, R. Hoppe, and G.P. Canellos, Report of an
International Workshop to Standardize Response Criteria for
Non-Hodgkin's Lymphomas. Journal of Clinical Oncology 17 (1999)
1244-1244 https://doi.org/10.1200/JCO.1999.17.4.1244 PMid:10561185
- G.L.
Chen, Y. Huang, W. Zhang, X. Pan, W.J. Feng, X.Y. Zhao, X.D. Zhu, W.H.
Li, M. Huang, Z.Y. Chen, and W.J. Guo, Three-Tier Prognostic Index in
Young Adults With Advanced Gastric Cancer. Front Oncol 11 (2021)
667655. https://doi.org/10.3389/fonc.2021.667655 PMid:34568007 PMCid:PMC8462089
- F.
Franco Perez, J. Lavernia, D. Aguiar-Bujanda, J. Miramon, J. Guma, R.
Alvarez, J. Gomez-Codina, F.G. Arroyo, M. Llanos, M. Marin, J. Alfaro,
C. Quero, M. Delgado, E. Nogales, F. Menarguez, N. Martinez, M.
Torrente, A. Royuela, D. Abreu, and M. Provencio, Primary Breast
Lymphoma: Analysis of 55 Cases of the Spanish Lymphoma Oncology Group.
Clin Lymphoma Myeloma Leuk 17 (2017) 186-191. https://doi.org/10.1016/j.clml.2016.09.004 PMid:27847267
- P.
Radkani, D. Joshi, J.C. Paramo, and T.W. Mesko, Primary breast
lymphoma: 30 years of experience with diagnosis and treatment at a
single medical center. JAMA Surg 149 (2014) 91-3. https://doi.org/10.1001/jamasurg.2013.2283 PMid:24257833
- H.Y.
Yhim, H.J. Kang, Y.H. Choi, S.J. Kim, W.S. Kim, Y.S. Chae, J.S. Kim,
C.W. Choi, S.Y. Oh, H.S. Eom, J.A. Kim, J.H. Lee, J.H. Won, H. Shim,
J.J. Lee, H.J. Sung, H.J. Kim, D.H. Lee, C. Suh, and J.Y. Kwak,
Clinical outcomes and prognostic factors in patients with breast
diffuse large B cell lymphoma; Consortium for Improving Survival of
Lymphoma (CISL) study. BMC Cancer 10 (2010) 321. https://doi.org/10.1186/1471-2407-10-321 PMid:20569446 PMCid:PMC2927999
- Y.
Jia, C. Sun, Z. Liu, W. Wang, and X. Zhou, Primary breast diffuse large
B-cell lymphoma: a population-based study from 1975 to 2014. Oncotarget
9 (2018) 3956-3967. https://doi.org/10.18632/oncotarget.23285 PMid:29423097 PMCid:PMC5790514
- S.
Fukuhara, T. Watanabe, W. Munakata, M. Mori, D. Maruyama, S.W. Kim, Y.
Kobayashi, H. Taniguchi, A.M. Maeshima, R. Tanosaki, Y. Matsuno, and K.
Tobinai, Bulky disease has an impact on outcomes in primary diffuse
large B-cell lymphoma of the breast: a retrospective analysis at a
single institution. Eur J Haematol 87 (2011) 434-40. https://doi.org/10.1111/j.1600-0609.2011.01679.x PMid:21740461
- G.L. Chen, L. Guo, S. Yang, and D.M. Ji, Cancer risk in tuberculosis patients in a high endemic area. BMC Cancer 21 (2021) 679. https://doi.org/10.1186/s12885-021-08391-6 PMid:34107921 PMCid:PMC8190842
- G.
Li, G.L. Chen, Y. Zhou, G.Q. Yao, S. Yang, and D.M. Ji, Increased Risk
of Lymphoma in Men or the Elderly Infected with Tuberculosis. Mediterr
J Hematol Infect Dis 13 (2021) e2021053. https://doi.org/10.4084/MJHID.2021.053 PMid:34527205 PMCid:PMC8425346
- G.L.
Chen, Z.G. Xia, J. Jin, B.H. Yu, and J. Cao, Characterization of
Artificial Pneumothorax-Unrelated Pyothorax-Associated Lymphoma. J
Oncol 2021 (2021) 3869438. https://doi.org/10.1155/2021/3869438 PMid:33564306 PMCid:PMC7850845
- C.
Dendle, M. Gilbertson, T. Spelman, R.L. Stuart, T.M. Korman, K.
Thursky, S. Opat, and Z. McQuilten, Infection is an Independent
Predictor of Death in Diffuse Large B Cell Lymphoma. Sci Rep 7 (2017)
4395. https://doi.org/10.1038/s41598-017-04495-x PMid:28667319 PMCid:PMC5493675
- S.
Lanini, A.C. Molloy, P.E. Fine, A.G. Prentice, G. Ippolito, and C.C.
Kibbler, Risk of infection in patients with lymphoma receiving
rituximab: systematic review and meta-analysis. BMC Med 9 (2011) 36. https://doi.org/10.1186/1741-7015-9-36 PMid:21481281 PMCid:PMC3094236
- T.A.
Eyre, W. Wilson, A.A. Kirkwood, J. Wolf, C. Hildyard, H. Plaschkes, J.
Griffith, P. Fields, A. Gunawan, R. Oliver, S. Booth, J. Kothari, C.P.
Fox, N. Martinez-Calle, A. McMillan, M. Bishton, G.P. Collins, and
C.S.R. Hatton, Infection-related morbidity and mortality among older
patients with DLBCL treated with full- or attenuated-dose R-CHOP. Blood
Adv 5 (2021) 2229-2236. https://doi.org/10.1182/bloodadvances.2021004286 PMid:33890978 PMCid:PMC8095135
- X.
Rong, H. Wang, J. Ma, S. Pan, H. Wang, S. Jing, Y. Su, L. Wang, and C.
Zhao, Chronic hepatitis B virus infection is associated with a poorer
prognosis in diffuse large B-cell lymphoma: a meta-analysis and
systemic review. J Cancer 10 (2019) 3450-3458. https://doi.org/10.7150/jca.31033 PMid:31293649 PMCid:PMC6603406
- M.M.
Al-Mansour, S.A. Alghamdi, M.A. Alsubaie, A.A. Alesa, and M.A. Khan,
Negative effect of hepatitis in overall and progression-free survival
among patients with diffuse large B-cell lymphoma. Infect Agent Cancer
13 (2018) 18. https://doi.org/10.1186/s13027-018-0190-9 PMid:29977329 PMCid:PMC5992760
- H.H.
Huang, F.Y. Hsiao, H.M. Chen, C.Y. Wang, and B.S. Ko, Antiviral
prophylaxis for hepatitis B carriers improves the prognosis of diffuse
large B-cell lymphoma in Taiwan - a population-based study. Br J
Haematol 192 (2021) 110-118. https://doi.org/10.1111/bjh.17142 PMid:33131074
- R.N.
Miranda, T.N. Aladily, H.M. Prince, R. Kanagal-Shamanna, D. de Jong,
L.E. Fayad, M.B. Amin, N. Haideri, G. Bhagat, G.S. Brooks, D.A.
Shifrin, D.P. O'Malley, C.Y. Cheah, C.E. Bacchi, G. Gualco, S. Li, J.A.
Keech, Jr., E.P. Hochberg, M.J. Carty, S.E. Hanson, E. Mustafa, S.
Sanchez, J.T. Manning, Jr., Z.Y. Xu-Monette, A.R. Miranda, P. Fox, R.L.
Bassett, J.J. Castillo, B.E. Beltran, J.P. de Boer, Z. Chakhachiro, D.
Ye, D. Clark, K.H. Young, and L.J. Medeiros, Breast implant-associated
anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J
Clin Oncol 32 (2014) 114-20. https://doi.org/10.1200/JCO.2013.52.7911 PMid:24323027 PMCid:PMC4062709
- P.P.
Liu, K.F. Wang, J.T. Jin, X.W. Bi, P. Sun, Y. Wang, H. Yang, Z.M. Li,
W.Q. Jiang, and Y. Xia, Role of radiation therapy in primary breast
diffuse large B-cell lymphoma in the Rituximab era: a SEER database
analysis. Cancer Med 7 (2018) 1845-1851. https://doi.org/10.1002/cam4.1457 PMid:29624913 PMCid:PMC5943465
- W.
Haque, B. Dabaja, A. Tann, M. Khan, S. Szeja, E.B. Butler, and B.S.
Teh, Changes in treatment patterns and impact of radiotherapy for early
stage diffuse large B cell lymphoma after Rituximab: A population-based
analysis. Radiother Oncol 120 (2016) 150-5. https://doi.org/10.1016/j.radonc.2016.05.027 PMid:27373911
- N.
Zhang, C. Cao, Y. Zhu, P. Liu, L. Liu, K. Lu, J. Luo, and N. Zhou,
Primary breast diffuse large B-cell lymphoma in the era of rituximab.
Onco Targets Ther 9 (2016) 6093-6097. https://doi.org/10.2147/OTT.S108839 PMid:27785056 PMCid:PMC5065257
- S.
Yoshida, N. Nakamura, Y. Sasaki, S. Yoshida, M. Yasuda, H. Sagara, T.
Ohtake, S. Takenoshita, and M. Abe, Primary breast diffuse large B-cell
lymphoma shows a non-germinal center B-cell phenotype. Mod Pathol 18
(2005) 398-405. https://doi.org/10.1038/modpathol.3800266 PMid:15492762
- A.
Aviles, N. Neri, and M.J. Nambo, The role of genotype in 104 cases of
diffuse large B-cell lymphoma primary of breast. Am J Clin Oncol 35
(2012) 126-9. https://doi.org/10.1097/COC.0b013e318209aa12 PMid:21325938
- H.
Nyman, E. Jantunen, E. Juvonen, E. Elonen, J. Bohm, V.M. Kosma, G.
Enblad, M.L. Karjalainen-Lindsberg, and S. Leppa, Impact of germinal
center and non-germinal center phenotypes on overall and failure-free
survival after high-dose chemotherapy and auto-SCT in primary diffuse
large B-cell lymphoma. Bone Marrow Transplant 42 (2008) 93-8. https://doi.org/10.1038/bmt.2008.92 PMid:18391989
- N.
Patil, and M. Girgis, Outcome of germinal center B-cell type compared
to non-germinal center/activated B-cell type diffuse large b-cell
lymphoma as determined by immunohistochemistry using the Hans
algorithm. Journal of Clinical Oncology 38 (2020) e20076-e20076 https://doi.org/10.1200/JCO.2020.38.15_suppl.e20076
- F.
Franco, J. Gonzalez-Rincon, J. Lavernia, J.F. Garcia, P. Martin, C.
Bellas, M.A. Piris, L. Pedrosa, J. Miramon, J. Gomez-Codina, D.
Rodriguez-Abreu, I. Machado, C. Illueca, J. Alfaro, M. Provencio, and
M. Sanchez-Beato, Mutational profile of primary breast diffuse large
B-cell lymphoma. Oncotarget 8 (2017) 102888-102897. https://doi.org/10.18632/oncotarget.21986 PMid:29262531 PMCid:PMC5732697
- X.X.
Cao, J. Li, H. Cai, W. Zhang, M.H. Duan, and D.B. Zhou, Patients with
primary breast and primary female genital tract diffuse large B cell
lymphoma have a high frequency of MYD88 and CD79B mutations. Ann
Hematol 96 (2017) 1867-1871 https://doi.org/10.1007/s00277-017-3094-7 PMid:28803429
- S.
Hu, Y. Song, X. Sun, L. Su, W. Zhang, J. Jia, O. Bai, S. Yang, R.
Liang, X. Li, H. Zhang, Y. Gao, W. Zhang, X. Xiao, H. Bao, N. Wang, H.
Ren, X. Cen, S. Yang, Y. Zhao, Y. Wang, Y. Wang, A. Liu, J. Wang, Y.
Shi, M. Yuan, Y. Li, and X. He, Primary breast diffuse large B-cell
lymphoma in the rituximab era: Therapeutic strategies and patterns of
failure. Cancer Sci 109 (2018) 3943-3952. https://doi.org/10.1111/cas.13828 PMid:30302857 PMCid:PMC6272095
- T.
Wen, J. Wang, Y. Shi, H. Qian, and P. Liu, Inhibitors targeting
Bruton's tyrosine kinase in cancers: drug development advances.
Leukemia 35 (2020) 312-332 https://doi.org/10.1038/s41375-020-01072-6 PMid:33122850 PMCid:PMC7862069
[TOP]




