Peritoneal
lymphomatosis (PL) is an unusual extranodal presentation of non-Hodgkin
lymphoma (NHL), with relatively few cases reported in the literature.[1] Unlike pleural effusion, which frequently occurs in any NHL, the involvement of the peritoneal cavity is uncommon.[2]
Peritoneal carcinomatosis, tuberculous peritonitis, and metastatic
tumors are the most common causes of peritoneal thickening with
effusion, but PL should be considered in the differential diagnosis.
Peritoneal lymphomatosis is usually related to diffuse large B-cell
lymphoma and refers to the seeding of the parietal peritoneum and
surface of the covered abdominal organs by lymphoma cells. Abdominal
pain and distension are the most frequent clinical symptoms of PL.[1]
We report a case of a 55-years-old male with a prior diagnosis of
peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), who
presented with ascites and suspected peritoneal lymphomatosis. Clinical
signs and imaging were suggestive of a relapse of PTCL-NOS.
In
August 2021, a 55-years-old male was admitted to our hospital because
of abdominal distension, nausea, and vomiting. He had a history of
prostatic cancer treated surgically in 2016 and PTCL-NOS treated in
2017 with six cycles of CHOEP (Cyclophosphamide, Doxorubicin,
Vincristine, Etoposide, and Prednisone) followed by autologous stem
cell transplant (ASCT) obtaining complete disease remission. On
admission, laboratory findings showed anemia (Hb 9.4 g/dL), mild
leucocytosis (WBC 12630/mm3 Neu 11540/mm3),
hypoalbuminemia (2.8 g/dL), high serum creatinine levels (4,9 mg/dL),
hyperuricemia (16,6 mg/dL), and elevated LDH levels (1297 UI/L, normal
value <225 UI/L). On clinical examination, he had ascites but no
palpable superficial lymph nodes. Computed tomography (CT) without
contrast showed peritoneal effusion and thickening at mesenteric and
mesogastric fatty tissue levels. Splenomegaly (16 cm) and the portal
vein of increased caliber (17 mm) were also detected. A paracentesis
was performed, obtaining 4 liters of a cloudy, straw-colored ascitic
fluid (AF) with high protein content (3.9 gr/dL), remarkable LDH values
(4296 UI/L), and glucose (16 mg/dL). We studied the AF with eight-color
multiparametric flow cytometry (MFC) using combinations of monoclonal
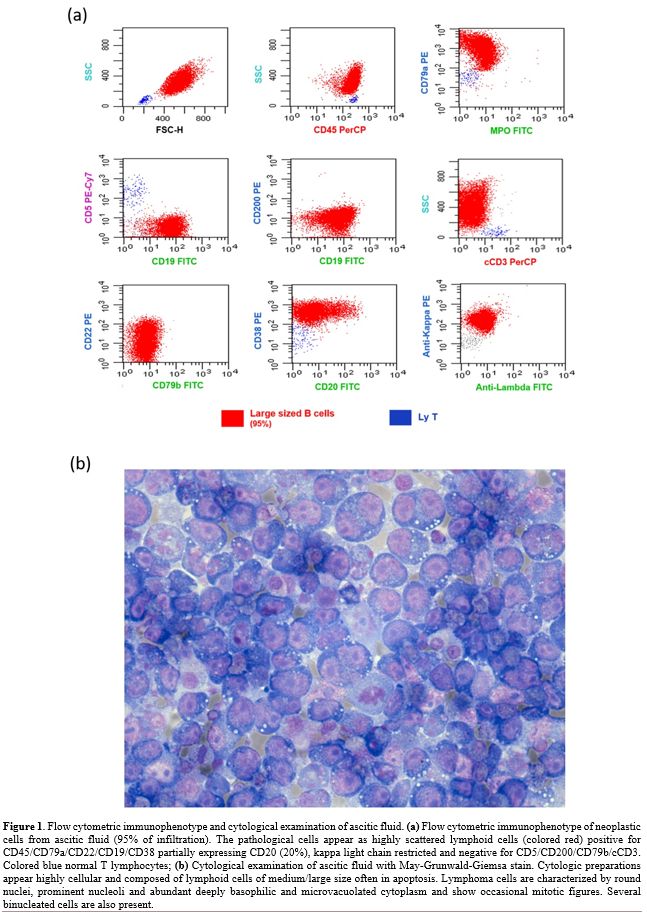
antibodies. MFC analysis of the AF detected 95% of highly scattered
lymphoid B cells, positive for
CD45/CD79a/CD19/CD22/CD38/CD18/CD11a/CD27/CD49d/HLA-DR, CD20 partially
expressed (20%), kappa light chain restricted and negative for
CD5/CD200/FMC7/CD79b/CD10/VS38/CD13/CD33/CD14/CD15/CD11b/CD16/CD56/CD138/MPO/cCD3/NG2/CD123
(Figure 1a). Considering the
previous diagnosis of PTCL-NOS, an accurate assessment of T-cells was
also performed through immunophenotypic analysis of V Beta Repertoire,[3] which did not detect alterations in any of the 24 families studied.
Cytological
examination of ascitic fluid (1000 ml) with May-Grunwald-Giemsa (MGG)
stain revealed numerous lymphoid cells of medium to large size, often
in apoptosis and sometimes in mitosis. Most cells had round nuclei,
prominent nucleoli, and abundant deeply basophilic finely vacuolated
cytoplasm (Figure 1b). The
remaining concentrated sediment was fixed in 10% formalin, embedded in
paraffin, and processed to cell block to perform immunohistochemistry
(IHC). Lymphoma cells were immunoreactive for CD79a, CD20 (about 30%),
PAX5 (weak and diffuse), MUM1, cMyc (>40%) and BCL2 (>50%) and
non-immunoreactive for HHV8, CD3, CD5, CD4, CD8, CD10, CD30, CD138, and
BCL6. We performed a molecular biology investigation to evaluate T-cell
clonality arising out of a previous diagnosis of PTCL-NOS and to study
the B-cell population in the first biopsy at the presentation in 2017.
The lymph node and AF were investigated for the presence of
immunoglobulins (IG) and T-receptor (TR) clonal rearrangements by qualitative
polymerase chain reaction (PCR). All positive targets were sequenced to
identify the type of rearrangement and its clonal feature. The
screening showed different targets: one was immunoglobulin heavy chain
(IGH) gene rearrangements on ascitic fluid, and two resulted in TR
gamma chain positive on lymph node biopsy. No common targets were
identified between the two samples. Based on these findings, the
patient was diagnosed with large B-cell lymphoma of new onset,
presenting as peritoneal lymphomatosis. Gastrointestinal localization
of disease was excluded by esophagogastroduodenoscopy (EGDS) and
recto-colon-sigmoidoscopy (RSCS). Due to the patient poor clinical
conditions, a Gemcitabine-based regimen was chosen to obtain a mild
clinical improvement. After one week, he received a second infusion of
Gemcitabine, but three days later, his clinical conditions worsened
again due to fever and pancytopenia. Considering the normalization of
serum creatinine (1.1 mg/dL) and the recurrence of abdominal
distention, a total body CT scan with contrast confirmed the presence
of an abundant amount of ascitic effusion and mesenteric thickening as
an omental cake pattern, referring to peritoneal localization of the
disease. Small mesenteric lymphadenopathies (15 mm) were observed.
Unfortunately, clinical conditions worsened, and the patient died the
following day.
Peritoneal
lymphomatosis is a rare form of extranodal lymphoproliferative disease,
frequently associated with diffuse large B-cell lymphoma (DLBCL) and
Burkitt's lymphoma.[3] Considering the recent history
of PTCL-NOS in our patient, a relapse of the T-cell disease was
suspected, although T-cell peritoneal lymphomatosis is extremely rare
and usually secondary to intestinal localization.[4]
Thanks to a prompt MFC exam with cytology and IHC afterward, we
obtained an early diagnosis by avoiding a biopsy exam. Although DLBCL
is ideally diagnosed from an excisional biopsy of a suspicious lymph
node or tissue, morphologic and cytometry exams should be considered
when an invasive diagnostic procedure is not possible.
Immunophenotyping by MFC is a rapid and efficient technique adjunct to
conventional diagnostic cytopathology in evaluating serous effusions
for lymphomatous involvement.[5-6] Studies like MFC
and IHC staining help lead to an accurate diagnosis allowing the
identification of the molecular characteristics of the lymphoma and its
immunophenotype, which dictate treatment decisions.[7-10]
This case was interesting as the clinical signs and symptoms could
suggest a relapse of PTCL-NOS diagnosed and treated four years before;
however, FCM, cytology with IHC, and molecular biology investigations
excluded this hypothesis.
In conclusion, MFC could be an extremely
useful and rapid method in the integrated diagnostic process with
cytology and IHC to obtain a proper diagnosis of NHL to quickly start
appropriate therapy, also in the absence of tumor samples for a
complete histopathology examination.