Aziz Sidi Aristide Tapsoba1, Florencia Wendkuuni Djigma1,2, Bagora Bayala1,2, Pegdwendé Abel Sorgho1,2, Lassina Traore1, Théodora Mahoukèdè Zohoncon3, Shoukrat Ohuwa Toyin Bello1, Prosper Bado1, Bapio Valérie Elvira Jean Télesphore Bazie4, Fiffou Yougbare1, Marius Ayaovi Setor1, Esther Mah Alima Traore4, Dorcas Obiri-Yeboah5, Albert Théophane Yonli1,2 and Jacques Simpore1,2.
1 Université
Joseph KI-ZERBO, Laboratoire de Biologie Moléculaire et de Génétique
(LABIOGENE), P.O. Box 7021, Ouagadougou 03, Burkina Faso.
2 Centre de Recherche Biomoléculaire Pietro Annigoni (CERBA), P.O. Box 364, Ouagadougou 01, Burkina Faso.
3 Université Saint Thomas d’Aquin, Saaba 06 BP 10212 Ouagadougou 06.
4 Centre National de la Recherche Scientifique et Technologique (CNRST), 03 BP. 7047, Ouagadougou, Burkina Faso.
5 Department of Microbiology and Immunology, School of Medical Sciences, University of Cape Coast, PMB, Cape Coast, Ghana.
Correspondence to: Dr.
Florencia Wendkuuni Djigma. Université Joseph KI-ZERBO, Laboratoire de
Biologie Moléculaire et de Génétique (LABIOGENE), P.O. Box 7021,
Ouagadougou 03, Burkina Faso, Burkina Faso. Tel; 00226 70 58 56 33;
E-mail:
florencia.djigma@gmail.com
Published: November 1, 2022
Received: July 4, 2022
Accepted: October 13, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022075 DOI
10.4084/MJHID.2022.075
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives:
Dengue fever (DF), an emerging and re-emerging viral disease, is a
major public health problem. The aim of this study was to investigate
the influence of KIRs genes polymorphism and KIRs
genotypes in susceptibility to dengue virus infection and disease
severity in a population from Burkina Faso through a case-control study.
Methods:
KIRs genes determination was performed using PCR-SSP in 50 patients
infected by dengue virus (DENV) and 54 Healthy controls (HC) subjects
who had never been infected.
Results: Data analysis showed significant association between frequencies of three KIR genes and dengue virus infection (DF): KIR2DL2 (OR: 7.32; IC: 2.87-18.65; P < 0.001); KIR2DL5A (OR: 15.00, IC: 5.68-39.59; P < 0.001) and KIR2DL5B (OR: 11.43; IC: 4.42-29; P < 0.001). While, KIR3DL3 (OR: 0.13, IC: 0.052-0.32; P < 0.001) and KIR2DS5 (OR: 0.12; IC: 0.04-0.30; P < 0.001) were associated with protection against DF. KIR2DL4 (OR: 9.75; IC95%: 1.33-70.97; p: 0.03) and KIRD3DL1
(OR: 12.00; IC95%: 1.60-90.13; p: 0.02) were associated with an
increased risk in the development of secondary dengue infection (SDI).
Conclusion: The results suggest a contribution of KIR2DL2, KIR2DL5A, and KIR2DL5B genes in the susceptibility of DF development. In contrast, KIR3DL3 and KIR2DS5 were associated with protection against DF development by enhancing both innate and acquired immune responses.
|
Introduction
Dengue
fever is widespread in the tropics and subtropical regions; it is the
first public health problem caused by arboviruses. According to the
World Health Organization,1 around 40-50% or 3.9 billion people in 128
countries are exposed to the dengue virus (DENV); each year, there are
390 million cases of dengue fever with 96 million presenting symptoms
and more than 3,000 deaths in the world.[1] Recently,
outbreaks of the Dengue Fever (DF) epidemic were reported in many
European countries and Africa, including Burkina Faso where 1061
probable cases and 15 deaths were reported in 2016.[2] In August 2019, Burkina Faso once again experienced cases of DF observed in hospitals of Ouagadougou and its surroundings.[3]
In their study, Ouattara et al. (2017) reported, in Burkina Faso, that
the prevalence of dengue virus infection was 23.5% in 2016 and 13.3% in
2017.[4] Dengue virus (DENV) is a member of the
flavivirus family comprising at least four distinct serotypes.
Transmitted by the mosquito Aedes aegypti,
DENV is endemic in the tropics/subtropics and causes an acute febrile
illness known as dengue fever (DF). However, a small percentage of
individuals experience a more severe syndrome known as dengue
hemorrhagic fever (DHF). The key features of DHF are plasma leakage and
a bleeding tendency, which develop as the fever subsides with clearance
of viremia.[5,6] There are four serotypes of dengue viruses (DENV-1, DENV-2, DENV-3 and DENV-4) which share 65–70% sequence homology.[1,7]
The
onset of the severe form of Dengue is due to increased endothelial
dysfunction and vascular leakage. It could be explained by an increase
in viremia but also by the phenomenon of antigenic sin linked to the
genetics of the host.[8] As the vaccine or effective
antiviral therapy is not yet available to everyone to prophylactically
or therapeutically treat DENV infection, Dengue's incidence is
increasing globally, worldwide, especially in the endemic area.[9]
Many
studies have shown the influence of the KIR genes on the host's
susceptibility and resistance to infectious diseases, such as AIDS,
Hepatitis B, C, and leprosy.[10-12]
Studies
conducted in many countries revealed the importance of KIR and HLA
ligands in innate immune responses to Dengue viral infections and, in
particular, their effect on clinical outcomes and disease severity.[13-16]
The human KIR gene locus is located on chromosome19q13.4 and extends approximately 150KB, encoding more than 15 KIR genes.[17]
The KIR genes are grouped into two major haplotypes, namely haplotype A
consisting of the KIR3DL3, 2DL3, 2DL1, 2DP1, 3DP1, 2DL4, 3DL1, 2DS4,
3DL2 genes, and haplotype B, the composition of which is variable
including several genes and alleles which are not part of haplotype A.
Each haplotype (A or B) consists of four framework genes (KIR3DL3,
3DP1, 2DL4, and 3DL2) which, with very rare exceptions, are present in
each individual.[18,19] All human populations have
haplotypes of groups A and B with varying frequencies. Individuals with
only the genes of the group A KIR haplotypes (KIR3DL3, 2DL3, 2DL1,
2DP1, 3DP1, 2DL4, 3DL1, 2DS4, 3DL2) were considered to be homozygous
for haplotype A and received the AA genotype of KIR. Individuals
without one of the four genes associated with haplotype A (KIR2DL1,
2DL3, 3DL1, and 2DS4), which have a known function and vary from one
individual to another, are considered to be homozygous for haplotypes
of group B and have received the KIR BB genotype. All other individuals
considered heterozygous for haplotypes A and B were assigned the KIR
genotype AB.[19,20] Either AB or BB genotypes were referred to as KIR genotype Bx, which contains more activating KIR genes.[21]
The human leukocyte antigen (HLA) class I molecules on target cells are
ligands for some KIRs. The presence or absence of KIR genes and their
HLA class I ligands are associated with susceptibility to or protection
against infectious diseases.[22] In Burkina Faso, there are yet no studies in the literature showing the influence of KIRs
genes on the development of dengue fever. Therefore, the aim of this
study was to investigate the impact of KIRs genes polymorphisms on
susceptibility and resistance to dengue virus infections and disease
severity from a population of Burkina Faso.
Material and Methods
Type and Population of the Study.
This is a case-control study that was conducted from June to December
2018. A total of 104 individuals were included in this study, which
consisted of 50 patients of Dengue virus and 54 Healthy Controls
recruited at the laboratories of Saint Camille Hospital in Ouagadougou
(HOSCO), National Center for Blood Transfusion (CNTS) and Pietro
Annigoni Biomolecular Research Center (CERBA/LABIOGENE) respectively.
All subjects were seronegative for Human Immunodeficiency Virus (HIV),
hepatitis B (HBV), and C (HCV) infections and had not also other
pathology history reported. Patients of all ages, including children
and blood donors, came from all professions and social categories. All
patients seen for consultations during the sample collection period and
presenting at least two signs suggestive of dengue fever were included
after giving their free consent. In addition, voluntary blood donors
received during the collection period were also included with no known
history of Dengue. The subjects with no contact with DENV from the same
geographical area were included as Healthy Controls after giving their
free consent. Healthy controls were screened for exposure to DENV
(AgNS1, IgM, and IgG).
Ethical Consideration.
The present study received the approval of the Ministry of Health of
Burkina-Faso through its Ethics Committee for Health Research (CERS)
(Deliberation N°2017-01-004), and the institutional ethics committee of
CERBA/LABIOGENE approved this study. According to the Helsinki
declarations, written informed consent was obtained from the study
participants for adult persons and tutors for children.
Dengue Virus Diagnostic.
Serological markers for DENV were detected using Dengue Duo Comb Test
Kits (Abon Biopharm Guangzhou, Co., Ltd. China). The AgNS1, IgM
and IgG were detected directly from blood samples obtained by taking
venous blood from the bend of the elbow. The results were read between
15 and 20 minutes.
Definition of Primary and Secondary Dengue Infection.
There are four distinct serotypes of the dengue virus which infect
humans. An individual infected with one of them is immunized for life
against this serotype but only acquires transient and partial immunity
against the other serotypes. Consequently, this disease has no
cross-protective immunity,[35] so a single person can
have up to four episodes of dengue fever in their lifetime. Primary
dengue fever is thus distinguished from secondary Dengue through the
analysis or diagnostics of the kinetics of anti-IgM and anti-IgG
antibodies and of viremia.[36]
Primary infection
of DENV is defined as the cases where we have the immuno-serological
AgNS1 (+) / IgM (+/-) / IgG (-), and secondary infection of DENV is the
cases of reinfection by another serotype, therefore on the
immune-serological level we translate it by AgNS1 (+)/ IgM (+/-) IgG
(+).
Genomic DNA Extraction and Determination of KIR Genes by SSP-PCR.
Genomic DNA was extracted from the serum or plasma using the commercial
kit called "DNA-Sorb-B" from Sacace Biotechnologies®, Italy, according
to the manufacturer's protocol. DNA purity and concentration were
determined using a Biodrop (Isogen Life Science, NV/S.A, Temse,
Belgium). Approximately 100 ng/μl of DNA was used to amplify the subset
of 12 targeted KIR genes using the SSP-PCR method as previously
described.[22] The PCR reactions were performed in 60
μL of the reaction mixture containing 100 ng/μL of DNA (variable
volume), 7.5 μL of 10 × PCR buffer, 2.25 μL MgCl2; 0.6 μL of dNTPs and
0.375 μL of PlatinumTM DNA Taq
polymerase in nuclease-free water. The PCR reactions were performed as
follows: after initial denaturation for 3 minutes at 94°C, the
amplifications were carried out respectively for 5 cycles, 21 cycles
and 4 cycles of denaturation at 94°C, annealing at primer-specific
temperature for 15 seconds (65°C and 60°C) or 1 minute (55°C for 4
cycles), and extension at 30 seconds at 72°C or 2 minutes for 4 cycles
step with a final extension at 72°C for 7 minutes. The PCR products
were separated on 3% agarose gel and visualized under UV light at 312
nm using the Gene flash apparatus (Gene Flash syngenge Bio-Imaging,
USA). PCR products were validated against a positive internal control
corresponding to the DRB1 gene fragment.
Prediction of KIR Haplogroups from Genotypes.
The KIR gene content of a given individual is conventionally called
"KIR genotype", which is variable among individuals. The KIR gene
content was used to infer group A and B KIR Haplotypes and to assign
each person to one of three genotypes: AA, BB, and AB. Individuals
having only genes of the group A KIR haplotypes
(KIR3DL3-2DL3-2DL1-2DP1-3DP1-2DL4-3DL1-2DS4-3DL2) were considered to be
homozygous for the A haplotype and assigned the KIR genotype AA.
Individuals lacking any of the four A haplotype-associated genes
(KIR2DL1, 2DL3, 3DL1, and 2DS4) that have a known function and vary
among individuals in their existence were regarded to be homozygous for
group B haplotypes and assigned the KIR genotype BB. All other
individuals were considered heterozygous for A and B haplotypes and
assigned the KIR genotype AB. The individuals with AB genotypes had all
nine genes on the A haplotype and one or more B haplotype-specific
genes (2DL2, 2DL5, 2DS1, 2DS2, 2DS3, 2DS5, and 3DS1).[19,20]
Therefore, the AB genotypes were considered heterozygous, carrying both
haplogroup genes. However, due to the difficulty in differentiation
between AB and BB genotypes, the current system annotated them as Bx
genotypes according to Allele Frequency Net Database (AFND).
Statistical Analysis. The data was analyzed using the standard Statistical Package for Social Sciences (SPSS) version 20.0. The χ2
test was used to compare variant frequencies between groups. The risk
was estimated with an Odds Ratio (OR) and 95% of confidence interval
(95% CI). P-values < 0.05 were considered statistically significant.
Association between KIRs genes and dengue virus infection was
established by comparing frequencies between cases and controls using
the χ2 test.
Results
The
study population consisted of 104 subjects, with 50 patients of DENV
presenting clinical signs of dengue fever, which were confirmed by
diagnostic, and 54 Healthy Controls who had never been infected by the
DENV. The percentage of men was 40.38% (42/104) and 59.62% (62/104)
for women. Among 50 dengue virus patients, women represented the most
(52%). The sex ratio of the study population was 0.68 (42/62). In the
study population, the youngest was 4 years old, and the majority had an
age between 20 to 39 years. The average age of the patients was 26.58 ±
12.01 years. The highest frequency of dengue fever (64.00%) was
noted in patients aged between 20 to 39 years (Table 1).
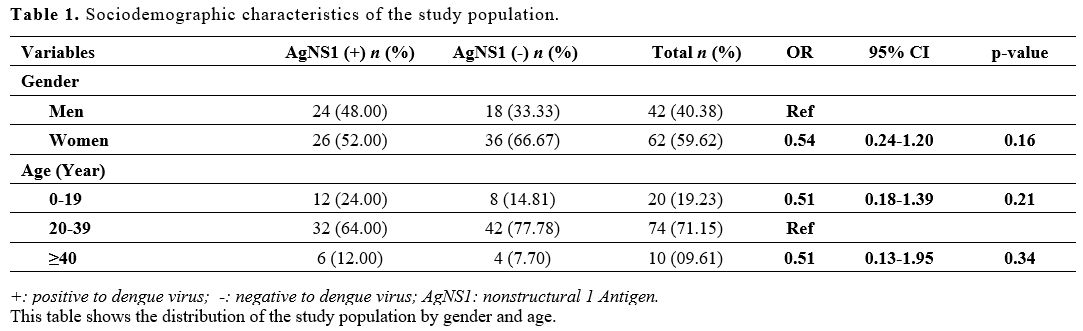 |
Table 1. Sociodemographic characteristics of the study population. |
The
serological diagnostic of Dengue virus revealed 10.00% (5/50) of
primary infection with dengue virus and 90% (45/50) of secondary
infection to DENV in the study population. The proportion of dengue
fever was 48.08% (50/104), with a rate of at least one contact with
DENV (Table 2).
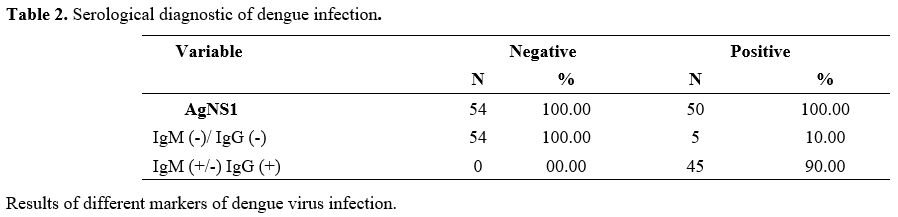 |
Table 2. Serological diagnostic of dengue infection. |
A
total of 16 KIR genes were genotyped by using the SSP-PCR method. The
results showed the different frequencies of KIR genes between dengue
patients (DF) and Healthy Controls. The frequencies of KIR2DL2 (OR: 7.32; IC: 2.87-18.65; P < 0.001); KIR2DL5A (OR: 15.00, IC: 5.68-39.59; P < 0.001); KIR2DL5B (OR: 11.43; IC: 4.42-29; P < 0.001); KIR2DS2 (OR: 2.40; IC: 1.06-5.41; P = 0.04) were more frequent in dengue patients (DF) while the frequencies of KIR3DL3 (OR: 0.13, IC: 0.052-0.32; P < 0.001) and KIR2DS5 (OR: 0.12; IC: 0.04-0.30; P < 0.001) were more frequent in Healthy controls subjects (Table 3).
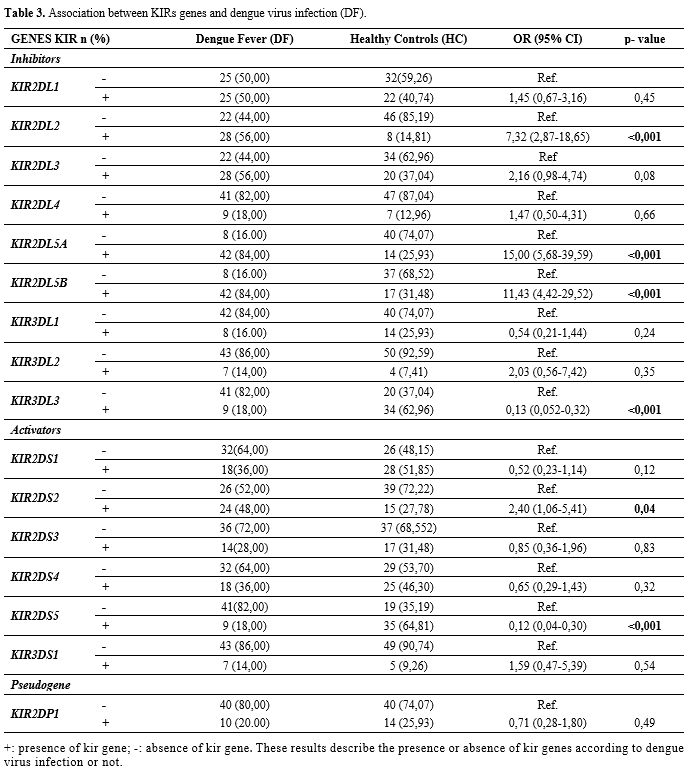 |
Table 3. Association between KIRs genes and dengue virus infection (DF). |
When the DENV primary infection group was compared to DENV secondary infection group, we found that KIR2DL4 (OR: 9.75; IC95%: 1.33-70.97; p: 0.03), KIRD3DL1
(OR: 12.00; IC95%: 1.60-90.13; p: 0.02) were associated with an
increased risk in the development of dengue secondary infection. In
contrast KIRD2DLB (OR: 0.08; IC95%: 0.08-0.62; P: 0.02) was associated in the protection of secondary dengue development (Table 4).
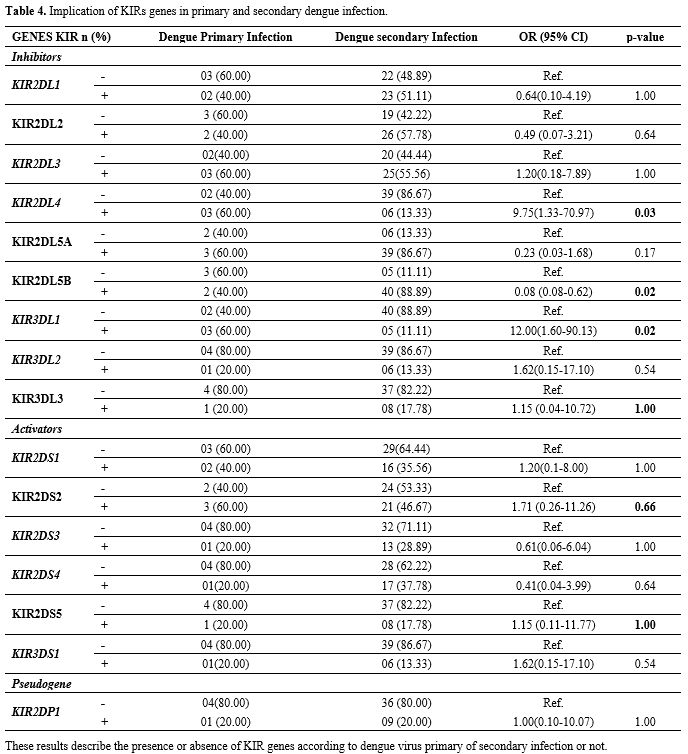 |
Table 4. Implication of KIRs genes in primary and secondary dengue infection. |
The
content of the KIR genes from our study population was used to infer
the different KIR haplotypes and assign a genotype to each person.
Three genotypes, notably the AA, AB, and BB genotypes, were identified
from the study population. The AB and BB genotypes were both referred
to as KIR genotype Bx which contains more activating KIRs genes. In the
general population study, we found 5.77% of AA genotypes and 92.23% of
Bx genotypes. In DENV patients, we recorded an AA genotype frequency of
8.00% and a Bx genotype frequency of 92.00%. The AA and Bx genotypes
frequencies were 3.70% and 96.30%, respectively, in Healthy Controls
subjects. No association was established between the frequencies of AA
and Bx genotypes in DENV patients and Healthy controls. However, the Bx
genotype was the predominant genotype in the total population study (Table 5).
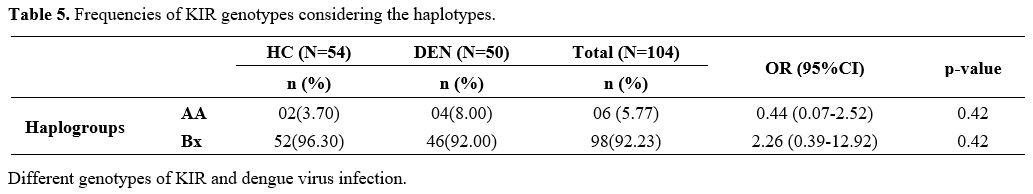 |
Table 5. Frequencies of KIR genotypes considering the haplotypes. |
Discussion
This
study identified KIRs genes and haplotypes in dengue patients and
healthy control subjects for the first time in a population of Burkina
Faso. Given the damage caused by this arbovirus in the country's health
system with its share of deaths as well as psychosis in the previous
years, we did not hesitate to carry out this investigation despite the
modest size of our sample compared to the general population of the
Burkina Faso an endemic country. This pilot study will help to
understand how human genetic factors are involved in cases of viral
dengue infection.
Many previous studies have shown that KIRs
receptors, a group of Natural Killer receptors, play an important role
in controlling the severity of viral diseases and infections in humans.[12,23-26] Previous studies have already established a relationship between KIRs genes and certain infectious diseases and cancers, such as hepatitis B,[12,23,26] hepatitis C with hepatocellular carcinoma,[27-29] and AIDS with Lymphomas.[11,24] The 20-39 year age group of patients had the highest frequency of dengue fever (64.00%) (Table 1), and 90% (45/50) of patients had a secondary dengue infection in our population of the study (Table 2).
This proportion of young people who contracted the dengue virus and the
rate of secondary dengue infection in the study population justifies
that dengue infection represents a major health problem in tropical
areas, according to WHO.[1]
KIR receptors
influence susceptibility or protection from certain diseases through a
balance between the signals of activation or inhibition that regulate
the function of NK cells. These cells interact with target cells that
express HLA class I molecules on their surface, which are ligands for
KIR.[14]
The study showed that KIR2DL2, KIR2DL5A, KIR2DL5B and KIR2DS2 were susceptibility genes associated with DF development, while KIR3DL3 and KIR2DS5
were associated with protection from DF development. These
susceptibility genes were present in greater number in the group of
dengue patients; it would seem that these genes are potential factors
of susceptibility to infection by the dengue virus; many additional
studies are needed to confirm this observation. Among these genes, we
observed that KIR2DL2, KIR2DL5 and KIR2DL5B were inhibitory, and KIR2DS2
was an activator. Inhibitory and activators KIRs genes act in
complementary, non-infected, healthy cells expressing HLA class I
proteins are preserved through inhibitory "self-recognition" mechanisms
that prevent their lysis. In contrast, infected cells and cancer cells,
lacking the HLA class I molecules on their surfaces, are recognized and
destroyed by lysis activating receptors;[30] this
could be justified by assuming that the infected cells do not lack
their HLA ligands, thus allowing the inhibitory KIR receptors to
protect the infected cells. A study conducted in India found that KIR3DL1/KIR3DS1 locus might be associated with the risk of developing DF;[13] in our investigation, this gene KIR3DL1
was associated with the development of secondary dengue infection.
Another study conducted in Southern Brazil found that inhibitory KIR2DL5 and activator KIR2DS5 were associated with the development of DF;[14] in our study, we found KIR2DL5 associated with the development of DF and KIR2DS5 associated with the protection of DF development. The KIR2DL1
and its related ligand HLA-C2 were significantly associated with
susceptibility to infection with CHIKV arbovirus transmitted by the
same mosquitoes in Gabon;[15] we do not find KIR2DL1 associated with DF development in the study. In our study population, KIR2DL4 and KIR3DL1 were associated with the development of secondary dengue infection, and KIRD2L5B was associated with the protection of secondary dengue infection. Furthermore, in their study,[26] Zhi-Ming et al. suggested that the KIR2DS3
gene favors infection by inducing a persistent inflammatory reaction
and chronic hepatitis in the case of the hepatitis B virus. However,
the KIR2DS1 and KIR2DL5 genes may contribute to protection against this virus.[26]
The
extensive polymorphism of the KIR genes may suggest the possibility of
pleiotropic effects in different diseases, i.e., a KIR gene that
confers protection against one disease may predispose the organism to
another.[31] Activating KIR receptors, which
stimulate the secretion of cytokines and the lysis of target cells by
NK cells, might be beneficial in response to infectious diseases and
tumors. However, these diseases have a variety of etiologies, so immune
activation is not necessarily beneficial in all phases of the disease
process. KIR genotypes that stimulate strong activation may increase
the risk of developing tumors associated with localized inflammation,
as in the case of cervical cancer. They have also been connected to the
pathogenesis of autoimmune diseases.[32] It emerges from this work that the inhibitory KIR genes are more numerous among those associated with the development of dengue fever, with the KIR2DL5
genes showing very high frequencies in dengue cases compared to
controls. Indeed, the expression of these genes would cause an
inhibition on the NKs, hence their inaction and the progression of the
disease. The paradox is that in this group, there is an activator gene
whose frequency is statistically significant; it is KIR2DS2;
this could be explained by the fact that a ligand defect or a
difficulty of recognition could make null the action of this gene and
consequently have an effect contrary to what is expected: that of
activating the NKs against the pathogen. In the group of genes
associated with protection against the development of dengue fever
there is an activator KIR gene which is KIR2DS5; the receptors
resulting from the latter activate the NK cells, which in turn act in
the form of a cytolytic action against DENV. In the Dengue Fever group,
the Bx (AB+BB) genotype frequency was 92%, and the AA genotype was 8%.
There was not any association between Healthy Controls and dengue
patients. In Brazil, Beltrame et al., in their study, showed a possible
protective factor against dengue fever in individuals with the AA
genotype.[14] In a study on Ebola infection, Wauquier
et al. showed that the AA profile was more frequent in survivors and a
control group compared to fatal cases.[33] Based on
these findings, it could be that an inhibitory KIR repertoire,
represented in this case by the AA genotype, is conferring a protective
effect on the individuals that possess it against such infections.
According to Lu et al. (2008), genotypes and haplotypes containing more
activating genes may play an important role in the infection or
clearance of certain viruses.[34]
The main
limitation of this study is that we only characterized the KIR genes,
but not the KIR/HLA combination, and we did not notify Dengue
Hemorrhagic Fever cases (DHF).
This study showed the implication
of the KIRs genes in the immune pathogenicity of dengue fever in
Burkina Faso. For the first time in Burkina Faso, it has been
demonstrated that the susceptibility to dengue fever is related to the
individual's KIR genotype; there is also a significant association
between certain KIR genes and dengue fever. KIR inhibitors genes such
as KIR2DL2, KIR2DL5A, and KIR2DL5B and the activating KIR2DS2 were associated with a risk of development and progression of the disease, while KIR2DS5 and KIR3DL3
would confer protection against this disease. However, KIR/HLA/cytokine
studies combined with further genotyping of DENV are needed to
investigate the molecular mechanisms by which KIR genes contribute to
infection or clearance or even progression to severe forms of the
disease dengue fever.
Acknowledgments
We
want to thank The World Academy of Sciences and the Swedish
International Development Cooperation Agency (Sida) for funding this
research through grant №17-403 RG/BIO/AF/AC_I – FR3240297757. We are
also grateful to the Saint Camille Hospital staff, Biomolecular
research center Pietro Annigoni and the national blood sanguine
transfusion center for their collaboration.
References
- WHO. Dengue and severe dengue. https://wwwwhoint/news-room/fact-sheets/detail/dengue-and-severe-dengue. 2022.
- Ministère de la Santé. Situation Report : Flambée de cas de dengue au Burkina Faso. 2016. (no No. 018, p. 2).
- S.
Ouédraogo SD, S. A. Barro, P. A. Somé, E. Bonnet, et V.
Ridde. Recurrence of dengue epidemics in Burkina Faso: Community
preference
for an intervention to prevent the disease. Rev Epidemiol Sante
Publique. 2019;vol. 67, no 6, p. 375‑382, doi:
10.1016/j.respe.2019.08.002.
https://doi.org/10.1016/j.respe.2019.08.002 PMid:31645291
- Abdoul
Karim OUATTARA CN, Birama DIARRA, Theodora ZOHONCON, Albert YONLI,
Dorcas OBIRI-YEBOAH, Marius BELEMGNEGRE, Paul OUEDRAOGO, Virginio
PIETRA and Jacques SIMPORE. Serological diagnosis in suspected dengue
cases at saint camille hospital of ouagadougou: high prevalence of
infection among Ouagadougou.
https://wwwijramrcom/issue/serological-diagnosis-suspected-dengue-cases-saint-camille-hospital-ouagadougou-high.
2018 ( no December 2017, 2018.).
- Vaughn
DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et
al. Dengue in the early febrile phase: viremia and antibody responses.
The Journal of Infectious Diseases. 1997;176(2):322-30.
https://doi.org/10.1086/514048 PMid:9237696
- Vaughn
J, Wolford JK, Prochazka M, Permana PA. Genomic structure and
expression of human KCNJ9 (Kir3.3/GIRK3). Biochemical and Biophysical Research Communications. 2000;274(2):302-9.
https://doi.org/10.1006/bbrc.2000.3136 PMid:10913335
- Anoop
M, Mathew AJ, Jayakumar B, Issac A, Nair S, Abraham R, et al. Complete
genome sequencing and evolutionary analysis of dengue virus serotype 1
isolates from an outbreak in Kerala, South India. Virus Genes.
2012;45(1):1-13. https://doi.org/10.1007/s11262-012-0756-3 PMid:22729802
- Deparis
X, Maréchal V, Matheus S. [Pathophysiological mechanisms of dengue
fever: critical review of current concepts]. Medecine Tropicale: Revue
du Corps de Sante Colonial. 2009;69(4):351-7.
- AW
T-R. A Putative Fifth Serotype of Dengue - Potential Implications for
Diagnosis, Therapy and Vaccine Design. Int J Clin Med Microbiol 1: 101
- Franceschi
DS, Mazini PS, Rudnick CC, Sell AM, Tsuneto LT, de Melo FC, et al.
Association between killer-cell immunoglobulin-like receptor genotypes
and leprosy in Brazil. Tissue Antigens. 2008;72(5):478-82.
https://doi.org/10.1111/j.1399-0039.2008.01127.x PMid:18778326
- Pelak
K, Need AC, Fellay J, Shianna KV, Feng S, Urban TJ, et al. Copy number
variation of KIR genes influences HIV-1 control. PLoS Biology.
2011;9(11):e1001208. https://doi.org/10.1371/journal.pbio.1001208
PMid:22140359 PMCid:PMC3226550
- Sorgho PA,
Martinson JJ, Djigma FW, Yonli AT, Nagalo BM, Compaore TR, et al.
Insights into the Interplay between KIR Gene Frequencies and Chronic
HBV Infection in Burkina Faso. Mediterranean Journal of Hematology and Infectious Diseases. 2018;10(1):e2018060.
https://doi.org/10.4084/mjhid.2018.060 PMid:30416692 PMCid:PMC6223576
- Alagarasu
K, Bachal RV, Shah PS, Cecilia D. Profile of killer cell
immunoglobulin-like receptor and its human leucocyte antigen ligands in
dengue-infected patients from Western India. International Journal of Immunogenetics. 2015;42(6):432-8. https://doi.org/10.1111/iji.12231
PMid:26385514
- Beltrame LM, Sell AM,
Moliterno RA, Clementino SL, Cardozo DM, Dalalio MM, et al. Influence
of KIR genes and their HLA ligands in susceptibility to Dengue in a
population from southern Brazil. Tissue Antigens. 2013;82(6):397-404.
https://doi.org/10.1111/tan.12256 PMid:24498996
- Petitdemange
C, Wauquier N, Jacquet JM, Theodorou I, Leroy E, Vieillard V.
Association of HLA class-I and inhibitory KIR genotypes in Gabonese
patients infected by Chikungunya or Dengue type-2 viruses. PloS One.
2014;9(9):e108798. https://doi.org/10.1371/journal.pone.0108798
PMid:25264760 PMCid:PMC4181859
- Townsley
E, O'Connor G, Cosgrove C, Woda M, Co M, Thomas SJ, et al. Interaction
of a dengue virus NS1-derived peptide with the inhibitory receptor
KIR3DL1 on natural cells. Clinical and Experimental Immunology.
2016;183(3):419-30. https://doi.org/10.1111/cei.12722 PMid:26439909
PMCid:PMC4750593
- Salim PH, Jobim M,
Bredemeier M, Chies JA, Schlottfeldt J, Brenol JC, et al. Killer cell
immunoglobulin-like receptor (KIR) genes in systemic sclerosis killer.
Clinical and Experimental Immunology. 2010;160(3):325-30.
https://doi.org/10.1111/j.1365-2249.2010.04095.x PMid:20082621
PMCid:PMC2883102
- Robinson J, Halliwell
JA, McWilliam H, Lopez R, Marsh SG. IPD--the Immuno Polymorphism
Database. Nucleic Acids Research. 2013;41(Database issue):D1234-40.
https://doi.org/10.1093/nar/gks1140 PMid:23180793 PMCid:PMC3531162
- Uhrberg
M, Parham P, Wernet P. Definition of gene content for nine common group
B haplotypes of the Caucasoid population: KIR haplotypes contain
between seven and eleven KIR genes. Immunogenetics. 2002;54(4):221-9.
https://doi.org/10.1007/s00251-002-0463-7 PMid:12136333
- Ashouri
E, Farjadian S, Reed EF, Ghaderi A, Rajalingam R. KIR gene content
diversity in four Iranian populations. Immunogenetics.
2009;61(7):483-92. https://doi.org/10.1007/s00251-009-0378-7
PMid:19521696 PMCid:PMC2706385
- McQueen
KL, Dorighi KM, Guethlein LA, Wong R, Sanjanwala B, Parham P.
Donor-recipient combinations of group A and B KIR haplotypes and HLA
class I ligand affect the outcome of HLA-matched, sibling donor
hematopoietic cell transplantation. Human Immunology.
2007;68(5):309-23. https://doi.org/10.1016/j.humimm.2007.01.019
PMid:17462498 PMCid:PMC1937576
- Kulkarni
S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human
disease. Seminars in Immunology. 2008;20(6):343-52.
https://doi.org/10.1016/j.smim.2008.06.003 PMid:18635379
PMCid:PMC3501819
- Kibar
F, Goruroglu
Ozturk O, Ulu A, Erken E, Inal S, Dinkci S, et al. Role of KIR genes
and genotypes in susceptibility to or protection against hepatitis B
virus infection in a Turkish cohort. Medical science monitor:
International Medical Journal of Experimental and Clinical Research.
2014;20:28-34. https://doi.org/10.12659/MSM.889893 PMid:24407110
PMCid:PMC3894916
- Sorgho PA, Djigma FW,
Martinson JJ, Yonli AT, Nagalo BM, Compaore TR, et al. Role of Killer
cell immunoglobulin-like receptors (KIR) genes in stages of HIV-1
infection among patients from Burkina Faso. Biomolecular Concepts.
2019;10(1):226-36. https://doi.org/10.1515/bmc-2019-0024 PMid:31863692
- Yindom
LM, Mendy M, Bodimeade C, Chambion C, Aka P, Whittle HC, et al. KIR
content genotypes associate with carriage of hepatitis B surface
antigen, e antigen and HBV viral load in Gambians. PloS One.
2017;12(11):e0188307. https://doi.org/10.1371/journal.pone.0188307
PMid:29149205 PMCid:PMC5693433
- Zhi-ming
L, Yu-lian J, Zhao-lei F, Chun-xiao W, Zhen-fang D, Bing-chang Z, et
al. Polymorphisms of killer cell immunoglobulin-like receptor gene:
possible association with susceptibility to or clearance of hepatitis B
virus infection in Chinese Han population. Croatian Medical Journal.
2007;48(6):800-6. https://doi.org/10.3325/cmj.2007.6.800 PMid:18074414
PMCid:PMC2213808
- De Re V, Caggiari L, De
Zorzi M, Repetto O, Zignego AL, Izzo F, et al. Genetic diversity of the
KIR/HLA system and susceptibility to hepatitis C virus-related
diseases. PloS One. 2015;10(2):e0117420.
https://doi.org/10.1371/journal.pone.0117420 PMid:25700262
PMCid:PMC4336327
- Joshita S, Ota M,
Kobayashi H, Wakabayashi SI, Yamashita Y, Sugiura A, et al. Association
analysis of KIR/HLA genotype with liver cirrhosis, hepatocellular
carcinoma, and NUC freedom in chronic hepatitis B patients. Scientific Reports. 2021;11(1):21424. https://doi.org/10.1038/s41598-021-01014-x
PMid:34728722 PMCid:PMC8563771
- Umemura T,
Joshita S, Saito H, Wakabayashi SI, Kobayashi H, Yamashita Y, et al.
Investigation of the Effect of KIR-HLA Pairs on Hepatocellular
Carcinoma in Hepatitis C Virus Cirrhotic Patients. Cancers.
2021;13(13). https://doi.org/10.3390/cancers13133267 PMid:34209910
PMCid:PMC8267716
- Selvakumar A, Steffens
U, Dupont B. Polymorphism and domain variability of human killer cell
inhibitory receptors. Immunological reviews. 1997;155:183-96.
https://doi.org/10.1111/j.1600-065X.1997.tb00951.x PMid:9059894
- Carrington
M NP. The KIR gene cluster. US Natl Library Med 2003 Available at:
http://wwwncbinlmnihgov/books/bookresfcgi/mono_003/ch1d1pdf. 2003.
- Bashirova
AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like
receptor gene cluster: tuning the genome for defense. Annual review of
genomics and Human Genetics. 2006;7:277-300.
https://doi.org/10.1146/annurev.genom.7.080505.115726 PMid:16824023
- Wauquier
N, Padilla C, Becquart P, Leroy E, Vieillard V. Association of KIR2DS1
and KIR2DS3 with fatal outcome in Ebola virus infection.
Immunogenetics. 2010;62(11-12):767-71.
https://doi.org/10.1007/s00251-010-0480-x PMid:20878400 PMCid:PMC2978320
- Lu
Z, Zhang B, Chen S, Gai Z, Feng Z, Liu X, et al. Association of KIR
genotypes and haplotypes with susceptibility to chronic hepatitis B
virus infection in Chinese Han population. Cellular & Molecular Immunology. 2008;5(6):457-63. https://doi.org/10.1038/cmi.2008.57
PMid:19118512 PMCid:PMC4072426
- Poltep K,
Phadungsombat J, Nakayama EE, Kosoltanapiwat N, Hanboonkunupakarn B,
Wiriyarat W, et al. Genetic Diversity of Dengue Virus in Clinical
Specimens from Bangkok, Thailand, during 2018-2020: Co-Circulation of
All Four Serotypes with Multiple Genotypes and/or Clades. Tropical Medicine and Infectious Disease. 2021;6(3).
https://doi.org/10.3390/tropicalmed6030162 PMid:34564546
PMCid:PMC8482112
- Matheus S, Deparis X,
Labeau B, Lelarge J, Morvan J, Dussart P. Discrimination between
primary and secondary dengue virus infection by an immunoglobulin G
avidity test using a single acute-phase serum sample. Journal of Clinical Microbiology. 2005;43(6):2793-7.
https://doi.org/10.1128/JCM.43.6.2793-2797.2005 PMid:15956399
PMCid:PMC1151893
[TOP]




