Mariam Markouli1#, Sevastianos Chatzidavid1#, Dimitra Vlachopoulou1, Nefeli Giannakopoulou1, Amalia Anastasopoulou1, Nora-Athina Viniou1 and Panagiotis Diamantopoulos1.
1 First Department of Internal Medicine, Laikon General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
# These authors equally contributed to the article.
Correspondence to:
Panagiotis Diamantopoulos, MD, PhD. First Department of Internal
Medicine, Hematology Unit, Laikon General Hospital, National and
Kapodistrian University of Athens. Athens 11527, Greece. Tel: +30 213
206 1643, Mobile: +30 697 677 6260, Fax: +30 213 206 1795. E-mail:
pandiamantopoulos@gmail.com
Published: September 1, 2022
Received: July 12, 2022
Accepted: August 21, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022071 DOI
10.4084/MJHID.2022.071
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the
editor
After reading the publication entitled “Infections in Myelodysplastic Syndrome in Relation to Stage and Therapy” (Mediterr J Hematol Infect Dis. 2018; 10(1): e2018039)
that was published in your journal, we would like to congratulate the
authors for this interesting review article and make some contributions
specifically concerning Bacillus cereus infections in patients with hematological malignancies.
Bacillus cereus
is a spore-building, Gram-positive rod that may cause three distinct
syndromes: food intoxication, localized infection, or bacteremia with
potential hematogenous complications (e.g., liver and cerebral
abscesses).[2,3] Furthermore, patients with hematological diseases are at greater risk for invasive B. cereus infections.[2]
Herein, we present an interesting case of fulminant B. cereus
septicemia in a patient with myelodysplastic syndrome
(MDS).
Α 74-year-old woman was diagnosed with
MDS upon assessment of severe pancytopenia. Bone marrow (BM)
examination revealed a blast percentage of 12% compatible with MDS with
excess blasts 2 (MDS-EB-2) per the 2016 World Health Organization (WHO)
classification,[4] whereas the BM cytogenetic analysis was normal (46, XX). She was started on treatment with 5-azacytidine at a dose of 75 mg/m2/day subcutaneously (IV) for 7 days in 28-day cycles. On day 20 of Cycle 2, while the patient was neutropenic (0.5x109/L),
she developed a fever of 39°C accompanied by chills, fatigue, and
fainting. Her physical examination and initial chest X-ray did not
reveal any specific findings. Computed tomography (CT) scan of the
brain, conducted to investigate fainting, did not suggest central
nervous system (CNS) involvement. Within one hour from the febrile
episode, IV piperacillin/tazobactam at a dose of 4,5 g q6h was started
along with filgrastim at a dose of 300 mcg daily. The patient reported
diarrhea within the next 20 hours, and stool cultures were obtained. By
that time, gram-positive, rod-shaped bacteria were isolated from both
blood cultures, and vancomycin was added to the regimen. B. cereus
was identified in the blood but was not isolated from the stool. Her
central venous catheter was considered to be the source of her
infection. The patient remained febrile for an additional 3 days
after B. cereus isolation. A transthoracic echocardiogram did not reveal findings compatible with B. cereus
endocarditis. Two days later, the fever subsided, and clinical
improvement was noted within four days, as diarrhea and fatigue
ameliorated.
In our case, a 74-year-old patient with MDS was diagnosed with isolated B. cereus bacteremia while on cycle 2 of chemotherapy. Studies have shown that immunocompromised patients with isolated B. cereus
bacteremia usually follow a more benign course compared to
organ-involved cases, having a more severe clinical presentation and
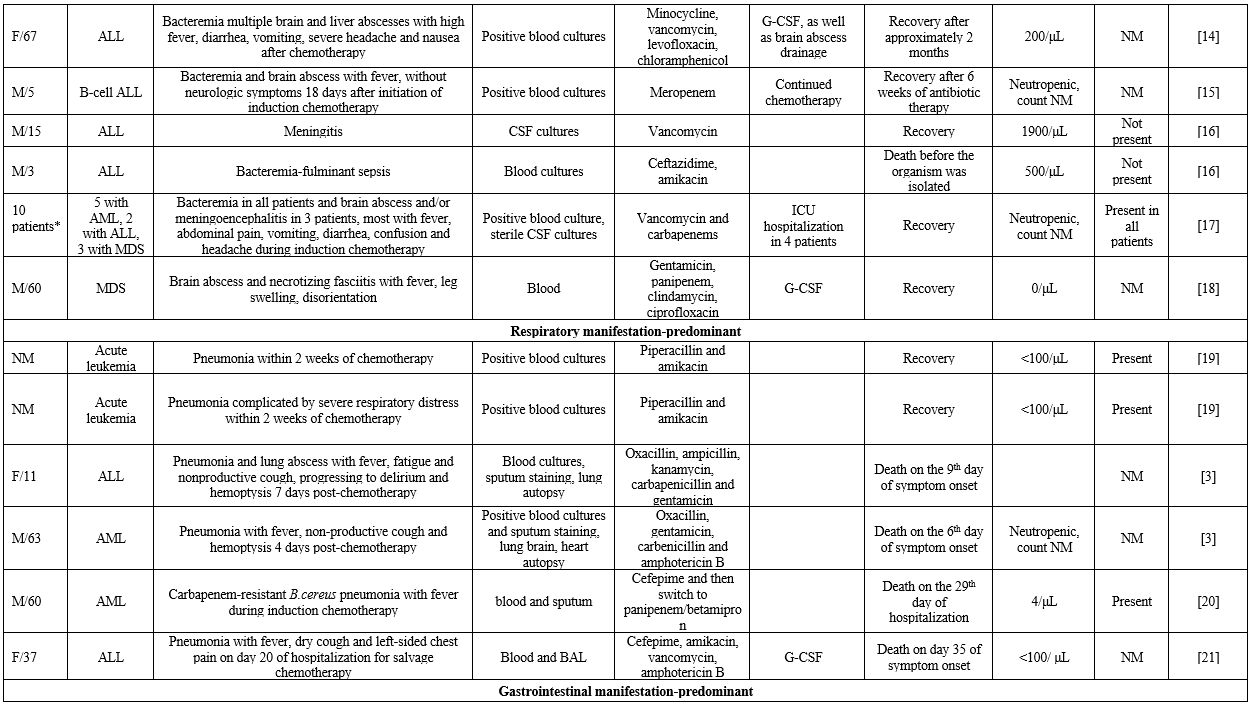
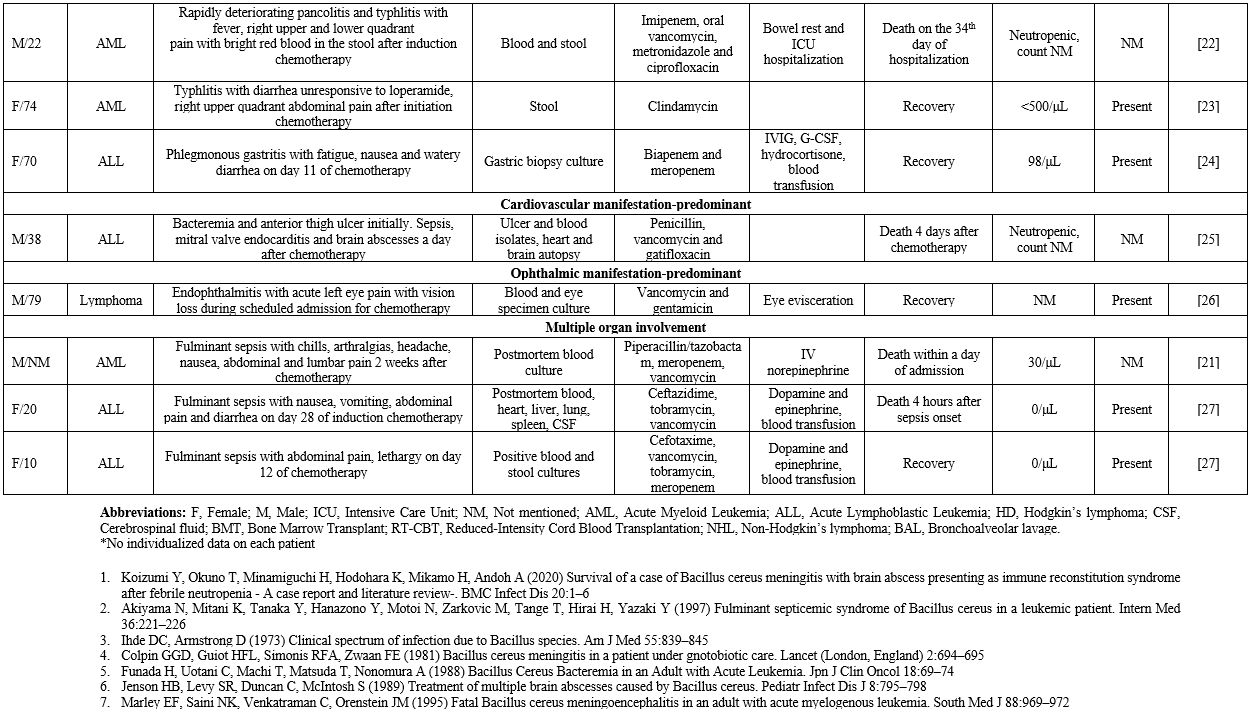
life-threatening course.[3] In this context, we gathered reported cases of B. cereus bacteremia with contemporary manifestations from various organ systems in patients with hematological malignancies (Supplementary file 1).
Concerning
general patient characteristics, 30 out of 73 patients were female
(41%) and 31 were male (42.4%), while sex was not mentioned in 12
cases. Most patients were middle-aged, with the median age of 36 and
the interquartile range being 45 years. Concerning risk factors for
infection, all patients were neutropenic, and 45 had a diagnosis of
acute leukemia (61.6%). Among patients with acute leukemia, 66.6% had
acute myeloid leukemia (AML). The percentage of patients with
intravascular catheters, an important risk factor since B. cereus can adhere to foreign bodies by producing biofilms,[3]
was 76.7%. Notably, 56.1% of these patients had concurrent
gastrointestinal symptoms, such as abdominal pain and diarrhea.
However, CNS involvement was the most common manifestation (80.8% of
patients). Other common symptoms included fever in 46.5% and headache
in 23.1% of patients. Although the issue of ICU hospitalization was not
mentioned in all cases, it was reported in 10.1% of patients. The death
occurred in 34.2% of patients, and 92% of these deaths occurred within
30 days of symptom onset. Of note, 76.7% of patients received
vancomycin, and 26.7% of those died, whereas 61.1% of patients who did
not receive vancomycin died.
This outcome is in line with the well-studied susceptibility pattern of B. cereus, which is characterized by susceptibility to vancomycin but is resistant to penicillins and cephalosporins.[5]
The presence of a CVC in most patients, which can be a source of
infection similarly to our case, highlights the importance of early
central catheter removal within 72 hours from the onset of B. cereus bacteremia, as previously recommended.[3]
In addition, catheter infection may be associated with a worse outcome
with frequent neurologic complications. Regardless of the presence of a
central catheter, B. cereus
infections should be included in the differential diagnosis of
neutropenic patients with hematologic malignancies who have recently
received chemotherapy and present with neurological symptoms.
Inappropriate
antibiotic treatment is predictive of higher mortality rates in
patients with bacteremia compared to appropriate therapy.[6]
It is, therefore, crucial to select the right antimicrobial agents for
empirical treatment according to the antimicrobial susceptibility of
the pathogen. In the presence of CNS disease, abscess drainage in large
and accessible abscesses should also be encouraged.[7] Most B. cereus
isolates produce beta-lactamases and are resistant to penicillins and
cephalosporins. Therefore, vancomycin should be included in empirical
treatment regimens.[8] Alternative agents having in vitro activity against Bacillus spp include aminoglycosides, carbapenems, and fluoroquinolones.[9]
However, reports of carbapenem resistance have recently been reported,
and carbapenems are no longer considered appropriate as an empiric
treatment.[10]
In conclusion, B. cereus
should always be taken into consideration as a potential threat for
patients with hematological malignancies, and a low threshold for
prompt diagnosis and treatment should be placed.
References
- Leone G, Pagano L. Infections in Myelodysplastic
Syndrome in Relation to Stage and Therapy. Mediterr J Hematol Infect
Dis. 2018 Jul 1;10(1):e2018039 https://doi.org/10.4084/mjhid.2018.039 PMid:30002795 PMCid:PMC6039080
- Inoue
D, Nagai Y, Mori M, et al (2010) Fulminant sepsis caused by Bacillus
cereus in patients with hematologic malignancies: analysis of its
prognosis and risk factors. Leuk Lymphoma 51:860-869 https://doi.org/10.3109/10428191003713976 PMid:20367571
- Tusgul
S, Prod'hom G, Senn L, Meuli R, Bochud PY, Giulieri SG (2016) Bacillus
cereus bacteraemia: comparison between haematologic and nonhaematologic
patients. New microbes new Infect 15:65-71 https://doi.org/10.1016/j.nmni.2016.11.011 PMid:28050250 PMCid:PMC5192042
- IARC Publications Website - WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017. Accessed 1 Jun 2022
- Bottone EJ (2010) Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382-398 https://doi.org/10.1128/CMR.00073-09 PMid:20375358 PMCid:PMC2863360
- Dellinger
RP, Levy M, Rhodes A, et al (2013) Surviving Sepsis Campaign:
international guidelines for management of severe sepsis and septic
shock, 2012. Intensive Care Med 39:165-228 https://doi.org/10.1007/s00134-012-2769-8 PMid:23361625 PMCid:PMC7095153
- Sakai
C, Iuchi T, Ishii A, Kumagai K, Takagi T (2001) Bacillus cereus brain
abscesses occurring in a severely neutropenic patient: successful
treatment with antimicrobial agents, granulocyte colony-stimulating
factor and surgical drainage. Intern Med 40:654-657 https://doi.org/10.2169/internalmedicine.40.654 PMid:11506311
- Savić
D, Miljković-Selimović B, Lepšanović Z, Tambur Z, Konstantinović S,
Stanković N, Ristanović E (2016) Antimicrobial susceptibility and
β-lactamase production in Bacillus cereus isolates from stool of
patients, food and environment samples. Vojnosanit Pregl 73:904-909 https://doi.org/10.2298/VSP150415134S PMid:29327895
- Tuazon CU, Yu VL, Weber R, Raoult D (2002) Bacillus species. Antimicrobial therapy and vaccines. Antimicrob Ther vaccines I 73
- Uchino
Y, Iriyama N, Matsumoto K, et al (2012) A case series of Bacillus
cereus septicemia in patients with hematological disease. Intern Med
51:2733-2738 https://doi.org/10.2169/internalmedicine.51.7258 PMid:23037464
Supplementary files
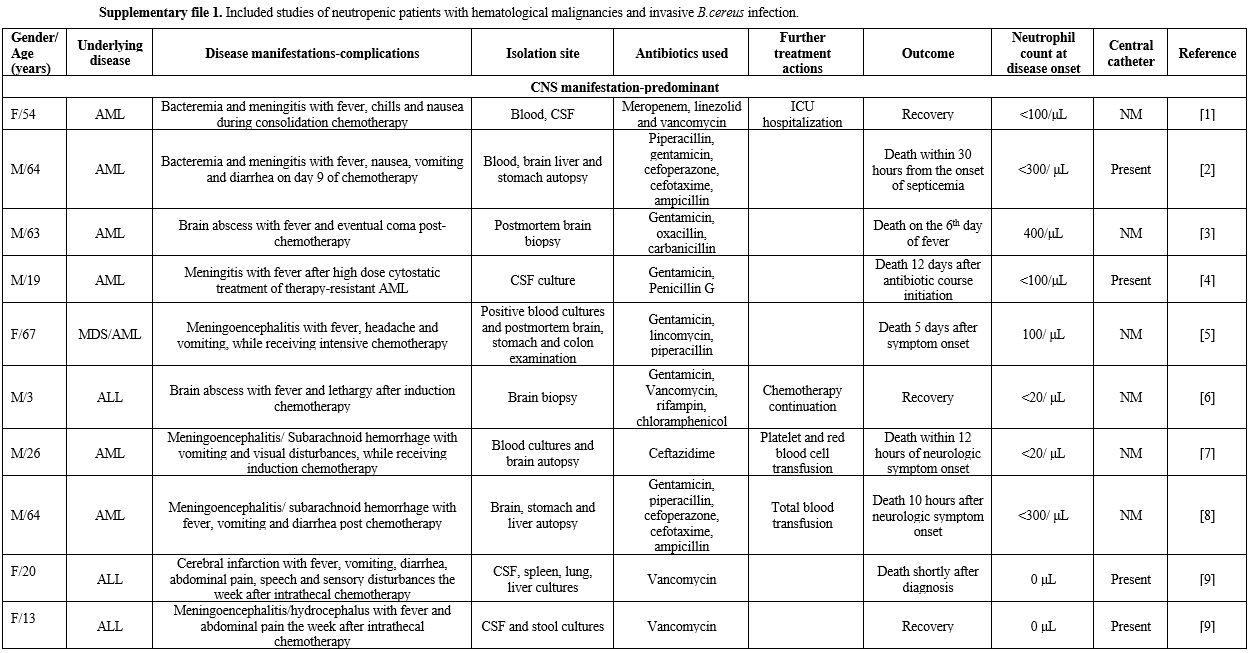 |
Supplementary file 1. Included studies of neutropenic patients with hematological malignancies and invasive B.cereus infection. |
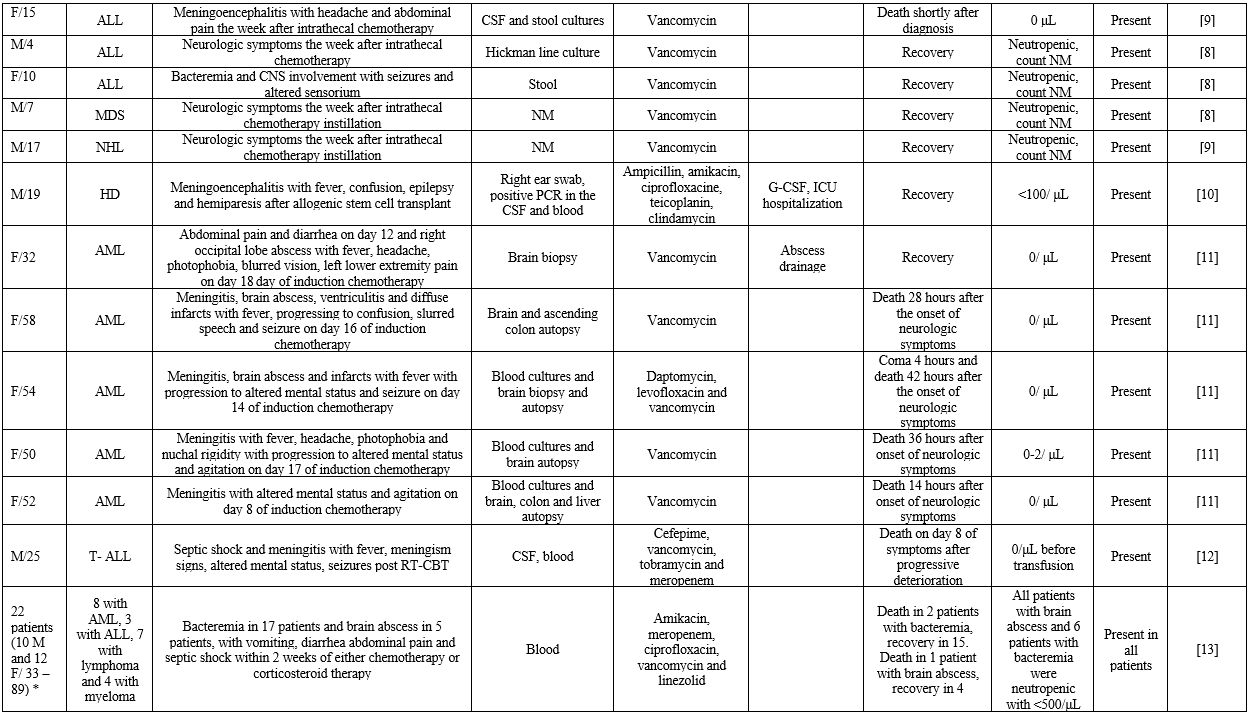 |
. |
[TOP]