Lorenza Torti1, Francesco Sorrentino1, Laura Maffei1, Paolo De Fabritiis1 and Elisabetta Abruzzese1.
1 Hemoglobinopathies Unit, Hematology Department, S. Eugenio Hospital (ASL Roma 2), Rome Italy.
Published: January 1, 2023
Received: July 18, 2022
Accepted: December 20, 2022
Mediterr J Hematol Infect Dis 2023, 15(1): e2023007 DOI
10.4084/MJHID.2023.007
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Coronavirus
disease 2019 (COVID-19), the highly contagious viral illness caused by
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a
catastrophic effect on the world’s demographics, emerging as the most
consequential global health crisis.[1]
RNA
viruses constantly evolve through the emergence of new variants,
acquiring a selective advantage with greater transmissibility and a
different severity, circumventing immunity previously acquired either
by natural infection or vaccination.
The clinical course of
SARS-Cov2 might be asymptomatic or vary from a typical presentation
like fever, cough, or respiratory symptoms to atypical presentations
such as gastrointestinal symptoms or peculiar symptoms like loss of
smell (anosmia), taste (ageusia), or a change in taste (dysgeusia).
Data indicate that COVID-19 has a wide range of presentations, and its
severity varies from asymptomatic disease to life-threatening
complications.
At the beginning of the pandemic, a study on SARSCov2 infected monkeys seemed to rule out the reinfection risk.[2] Nevertheless, cases in humans have demonstrated this possibility.[3-4]
The
prevalence of SARSCoV-2 infection among β-thalassemia patients seems to
be lower than in the general population; however, associated
comorbidities confer the risk of more severe disease with a poorer
prognosis. Regular transfusion therapy leads to a deficit in immune
response and, thus, to higher susceptibility to infectious events.[5]
Patients
with B-thalassemia show a 5-fold increase in age-standardized lethality
due to SARSCov2, representing a high-risk population compared with age
and sex-matched healthy subjects.[6]
A long-lived
protective immunity after primary infection/immunization seems
unlikely, and the immune response generated to earlier variants may not
cover newer ones.
We conducted a retrospective cohort
epidemiological investigation at our Center of all documented cases of
SARSCov2 reinfection among hemoglobinopathic patients already studied,[7] in chronic transfusional support from February 2020 to the present.
A
total of 162 hemoglobinopathic patients were followed; among them, 127
suffered from B-thalassemia (114 with major and 13 with intermediate
thalassemia) and 35 with sickle cell disease. Forty-five thalassemic
and five sickle cell disease patients were infected. The first
infection was documented by a positive reverse-transcriptase polymerase
chain reaction (RT-PCR) test; and/or a baseline positive serology of
SARSCov2 IgG/IgM antibodies (Ab).
Four thalassemic patients had symptomatic
reinfection, as demonstrated by a time elapse of >90 days between
the first and second positive COVID RT-PCR test, with >1
intermediate negative swab between the two positive tests, according to
Center Disease Control guidelines.
Case-Descriptions and Results
Patient 1.
A 54-year-old female patient, with intermediate B-Thalassemia double
heterozygosity Hb Lepore and an IVS II and alpha 3.7 genotype profile,
was found to be positive for SARSCov2 in November 2020. During this
first episode, she developed arthralgias and asthenia with intermittent
cough. Her symptoms did not require hospitalization or activation of
home transfusional therapy. Afterward, she was vaccinated with two
Pfizer-BioNTech COVID-19 doses in May and November 2021. Antibody
titers measured three months after the first vaccination appeared to be
protective (IgG spike S 1415 AU/ml, BAU 201/ml) in accordance with FDA
guidelines regarding convalescent plasma use demonstrating protective
values of more than 160 BAU/ml.[8,9] In January 2022,
she developed a mild sore throat and headache. A nasal swab RT-PCR
confirmed the infection, and her paucisymptomatic clinical course was
managed at home as mild COVID-19.
Patient 2.
The second case was an unvaccinated 48-year-old female with
B-thalassemia (CD39/IVS1-110) in chronic transfusional support. She
suffered from a severe first COVID-19 episode in August 2021, with
life-threatening bilateral pneumonia and acute respiratory distress
syndrome requiring high-flow oxygen therapy and in-patient hospital
care. Genomic analysis of the SARSCov2 variant of this first infection
revealed the presence of the Omicron (B.1.1.529) variant. Transfusion
therapy was required during hospitalization, together with intravenous
antibiotic therapy to treat gram-negative sepsis due to a
central-venous-catheter-infection. Post-infection antibody titers were
not available. Seven months later, she developed diffuse arthralgias
and flu-like symptoms, with confirmation of SARSCov2 infection by a
nasal swab RT-PCR. Molecular characterization this time was
unavailable, and she was managed at home without transfusion. She was
never vaccinated because of personal choice and is currently tested
with nasal swabs before Day Hospital access.
Patient 3.
A 45-year-old female suffering from intermediate B-thalassemia
(Cod39-homozygosis/Alfa 3.7 type -1-heterozygosis) was found to have
COVID-19 twice, confirmed by RT-PCR.
On March 8, 2020, at the
beginning of the pandemic, she developed a fever, cough, asthenia, and
multiple arthralgias. At this time, it was not easy to be tested, if
not in selected hospitals; thus, the patient was not investigated
regarding the COVID-19 test. Three months later, however, first contact
with the virus was determined once with a serological examination,
showing previous SARSCov2 infection with positive IgG antibodies. She
was vaccinated with 2 BNT162b2-Pfizer doses in March 2021. Antibody
values three months after the first vaccination appeared protective
(IgG spike S of 13460 AU/ml, BAU 1911/ ml).
The second COVID-19
episode occurred in February 2022, with a four-day paucisymptomatic
flu-like course. Due to worsening anemia, she received a transfusion of
filtered red blood cells in the COVID-19 area of the Emergency
Department.
Patient 4.
A 59-year-old female with Major B-thalassemia (Codon 39 Β homozygosity)
suffered from a first infection in January 2020, presenting with
asthenia and headache. A positive serological test for SARSCov2 IgM and
IgG antibodies identified COVID-19.
She was vaccinated with 2
BNT162b2-Pfizer doses in February 2020, 15 days after the first mild
infection. She also received a BNT162b2-Pfizer third dose in November
2021. Antibody values three months after the last vaccination were
present at the high title (IgG spike S of 7688 AU/ml, BAU 1091/ml). The
second episode occurred in June 2022, with a paucisymptomatic flu-like
course, confirmed by an RT-PCR positive swab.
The patient was transfused in a COVID-19 Emergency Department.
Detailed features of all patients can be found in Tables 1-2-3.
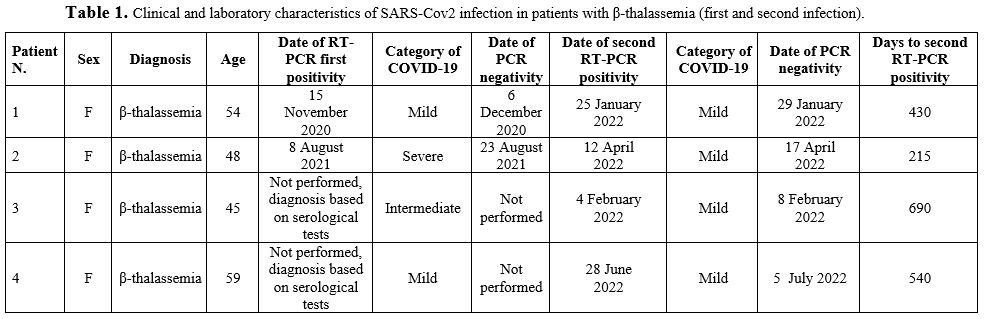 |
Table
1. Clinical and laboratory characteristics of SARS-Cov2 infection in patients with β-thalassemia (first and second infection). |
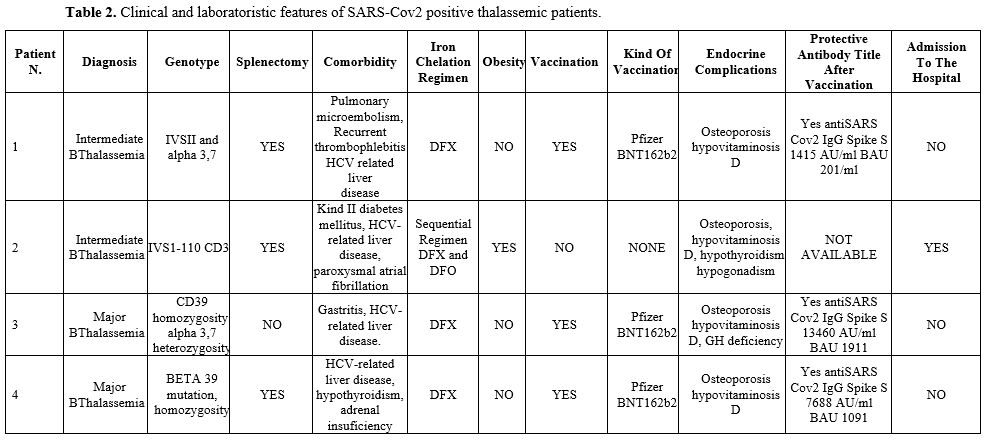 |
Table 2. Clinical and laboratoristic features of SARS-Cov2 positive thalassemic patients.
|
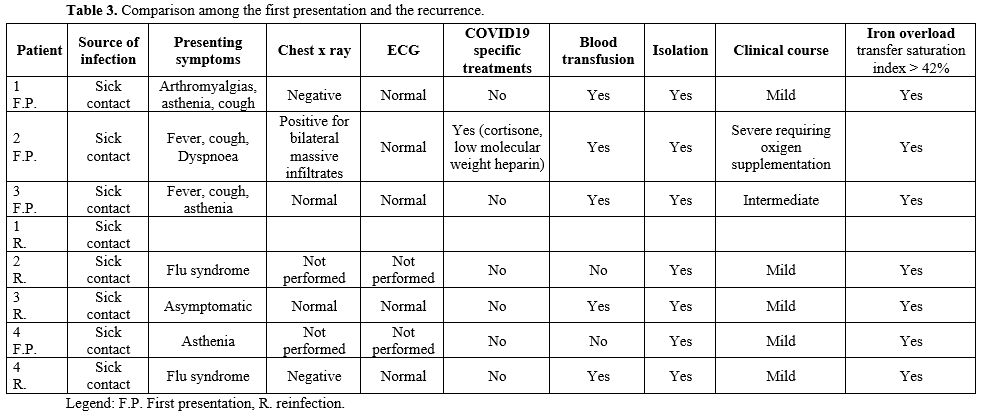 |
Table 3. Comparison among the first presentation and the recurrence.
|
Discussion
We
performed a retrospective cohort study in 127 hemoglobinopathic
patients to estimate the incidence of SARSCov2 reinfection over two
years and their clinical outcomes.
The rate of reinfection in
COVID-19 patients who have recovered and had a long-lasting negative
RT-PCR test is an emerging topic.
After the first documented case
in August 2020 in Hong Kong, many studies have reported SARSCov2
reinfection after a primary episode.
Up to now, except for a
single case report, this is the first cohort study screening
thalassemic patients for SARSCov2 reinfection and confirms that
patients with thalassemia who have recovered from COVID-19 can be
reinfected.[10] All reinfections were less severe
than primary and with a shorter duration of the second episode. 3/4
were diagnosed incidentally through random and routine testing.
Unfortunately, we were only able to chacarterize the viral genotype in
one case. The reinfection incidence is relatively rare, accounting for
0,088% (45/127 infected patients and four reinfections) and rapid virus
clearance.
These findings are consistent with other studies in the
general population (incidence variable from 0.061% to 0.66%), showing
reinfection prevalence among female, unvaccinated patients and omicron
variant.[11-12]
According to
Thalassemia-International-Federation-guidelines (TIF), patients with
hemoglobinopathies are frail and prone to COVID-19 reinfection due to
multiple factors such as periodic blood transfusion, splenectomy, and
iron chelation therapy.[13]
The outcome at
reinfection may reflect the balance between friability and immune
response. However, the exact role played by the various adaptive immune
responses in previously infected/immunized patients during reinfection
is unclear, and very little is known about their mechanisms.
Assessment
of SARSCov2 memory B and T cell-mediated responses in patients exposed
to the virus could help to define the risk of future SARS-Cov2
infection; however, unlike other hematological diseases, as of our
knowledge, only the humoral immune response has been investigated in
thalassemia.
Carsetti et al. have shown antibodies titers
significantly lower than controls in patients with
transfusion-dependent thalassemia 12 weeks after the second dose,
reaching comparable results only after a third additional dose.[14]
As a matter of fact, evidence of premature aging of the immune system
has been demonstrated in patients with thalassemia, maybe due to
multiple transfusions and circulating interleukin with a detrimental
effect on immune response, resulting in an immunosenescent profile.
However,
splenectomized patients seem to have higher antibodies against the
viral Spike protein than non-splenectomized thalassemic patients,
probably related to a compensatory mechanism of antibody production by
peripheral lymphatic tissue and bone marrow. Indeed, as our patients,
thalassemic are good responders to the Pfizer BNT 162b2 vaccine in
terms of clinical outcome and humoral response.[15]
Certain immunocompromised people, such as those with primary immunodeficiency or recipients of immunosuppressive therapy,[16]
often with an inadequate antibody response, experience months of
positivity at different viral loads, alternating symptomatic to a
clinical-recovery period. In contrast, efficient response with normal
virus clearance in primary infection and rapid negativization at
reinfection was proven in our patients.
Thus, immune memory
protects from clinical symptoms and reduces viral shedding as vaccines
do, but it does not seem to protect against reinfection.[17]
Pathophysiological
mechanisms underlying the development of a second infectious episode
remain not fully understood, involving both true reinfection or virus
reactivation from sanctuaries due to a decreased cellular immune
function.
The sensitivity and specificity of diagnostic methods
should also be considered to identify true reinfections or long-lasting
virus persistence.
All our patients presented with >90 days
between first and second positivity, suggesting a real reinfection.
Further, at least two RT-PCR swabs should be available to confirm the
absence or presence of SARSCov2.
False-negative results have been
reported, mainly due to sampling procedures. Consequently, when
asymptomatic patients are tested, it is not always easy to discriminate
between the recurrence of COVID-19 infection, intermittent shedding of
RNA fragments, or new onset infections. However, this is unlikely in
our cases due to the long-time interval between the two infections.
With
the challenges associated with developing an effective COVID-19
immunization and the probability of reinfection by SARS-CoV-2, the risk
of severe disease in susceptible hosts may persist.
For this
reason, several Monoclonal Antibodies (mAbs) have been developed
against SARCov2 and have proven their ability in therapeutic and
prophylactic fields. Most of them have indications for use in
thalassemia and sickle cell disease. They represent an alternative
prevention route for COVID-19, offering short-term protection to those
who are not yet vaccinated or lack a proper response to vaccination.[18]
The
mildness of reinfection in our 4 cases may suggest that severe disease
manifestations are rare once some immunity against the virus has been
elicited.[19-20]
Conclusions
The
true prevalence of COVID-19 reinfection may be difficult to estimate as
people with paucisymptomatic or asymptomatic reinfections are less
likely to be identified. Variants of concern and the title decline can
lead to a higher burden of reinfection in the future, because genetic
flexibility may lead to escape humoral immune responses. Ongoing
surveillance will be critical, and newer vaccines covering variants
could help. Much remains to be learned regarding coronavirus immunity,
including the maintenance of immunity against this virus and the
etiology of the COVID-19 relapse. Vaccination against SARSCov2 remains
crucial to reduce mortality and morbidity of frail patients.
Despite
the limitations of a small study sample, the present study is one of
the few works providing information on SARSCov2 reinfection among frail
hemoglobinopathic patients during the two epidemic waves.
References
- Cascella M, Rajnik M, Aleem A, Dulebohn SC,
Di Napoli R. Features, Evaluation, and Treatment of Coronavirus
(COVID-19). 2022 May 4. In: StatPearls [Internet]. Treasure Island
(FL): StatPearls Publishing; 2022 Jan-. PMID: 32150360.
- Bao, L, Deng W, Gao H. Reinfection could not occur in SARSCov2 infected rhesus macaques. Biorxiv 2020. https://www.biorxiv.org/content/10.1101/2020.03.13.990226v1
- Bongiovanni
M, Vignati M, Giuliani G. The dilemma of COVID19 recurrence after
clinical recovery. J Infect. 2020 Dec; 81(6): 979–997. https://doi.org/10.1016/j.jinf.2020.08.019
- Marie
Gousseff, Pauline Penot, Laure Gallay, Dominique Batisse, Nicolas
Benech, Kevin Bouiller, Rocco Collarino, Anne Conrad, Dorsaf Slama,
Cédric Joseph, Adrien Lemaignen, François-Xavier Lescure, Bruno Levy,
Matthieu Mahevas, Bruno Pozzetto, Nicolas Vignier, Benjamin Wyplosz,
Dominique Salmon, Francois Goehringer, Elisabeth Botelho-Nevers.
Clinical recurrences of COVID-19 symptoms after recovery: Viral
relapse, reinfection or inflammatory rebound? Journal of Infection 2020
81(5), 816-846 https://doi.org/10.1016/j.jinf.2020.06.073
- De
Sanctis V., Canatan D., Corrons J.L.V., Karimi M., Daar S., Kattamis C.
et Al. Preliminary data on COVID-19 in patients with
hemoglobinopathies: a multicentre icet-a study. Mediterr J Hematol
Infect Dis 2020, 12(1): e2020046 https://doi.org/10.4084/mjhid.2020.046 PMid:32670524 PMCid:PMC7340245
- Longo
F, Gianesin B, Voi V, Motta I, Pinto VM, Piolatto A, Spasiano A, Ruffo
GB, Gamberini MR, Barella S, Mariani R, Fidone C, Rosso R, Casale M,
Roberti D, Dal Zotto C, Vitucci A, Bonetti F, Pitrolo L, Quaresima M,
Ribersani M, Quota A, Arcioni F, Campisi S, Massa A, De Michele E, Lisi
R, Miano M, Bagnato S, Gentile M, Carrai V, Putti MC, Serra M, Gaglioti
C, Migone De Amicis M, Graziadei G, De Giovanni A, Ricchi P, Balocco M,
Quintino S, Borsellino Z, Fortini M, Denotti AR, Tartaglione I,
Beccaria A, Marziali M, Maggio A, Perrotta S, Piperno A, Filosa A,
Cappellini MD, De Franceschi L, Piga A, Forni GL.Italian patients with
hemoglobinopathies exhibit a 5-fold increase in age-standardized
lethality due to SARSCov2 infection. Am J Hematol. 2022 Feb
1;97(2):E75-E78. doi: 10.1002/ajh.26429. Epub 2021 December 10. https://doi.org/10.1002/ajh.26429 PMid:34861054 PMCid:PMC9011434
- Torti
L, Maffei L, Sorrentino F. , De Fabritiis P, Miceli R. , Abruzzese E .
Impact of SARS Cov2 in Hemoglobinopathies with immune disfunction and
Epidemiology. A protective mechanism from Β chain defects? . Mediter J
Hematol Infect Diseases 2020 jul, 1;12: e2020052 https://doi.org/10.4084/mjhid.2020.052 PMid:32670530 PMCid:PMC7340215
- Barone
P, DeSimone RA. Convalescent plasma to treat coronavirus disease 2019
(COVID-19): considerations for clinical trial design. Transfusion. 2020
Jun;60(6):1123-1127. doi: 10.1111/trf.15843. Epub 2020 May 12 https://doi.org/10.1111/trf.15843 PMid:32374891 PMCid:PMC7267607
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigational-covid-19-convalescent-plasma
- Okar
L, Ahmad R, Yassin M. First report of COVID19 reinfection in a patient
with β thalassemia major. Clin Case Rep.2021, 9, 861-865. https://doi.org/10.1002/ccr3.3682 PMid:33598260 PMCid:PMC7869313
- Flacco
ME, Soldato G, Acuti Martellucci C, Di Martino G, Carota R, Caponetti
A, Manzoli L. Risk of SARS-CoV-2 Reinfection 18 Months After Primary
Infection: Population-Level Observational Study. Front Public Health.
2022 May 2;10:884121. doi: 10.3389/fpubh.2022.884121. https://doi.org/10.3389/fpubh.2022.884121 PMid:35586006 PMCid:PMC9108359
- Flacco
ME, Acuti Martellucci C, Baccolini V, De Vito C, Renzi E, Villari P,
Manzoli L. Risk of reinfection and disease after SARS-CoV-2 primary
infection: Meta-analysis. Eur J Clin Invest. 2022 Oct;52(10):e13845.
doi: 10.1111/eci.13845. Epub 2022 August 8. https://doi.org/10.1111/eci.13845 PMid:35904405 PMCid:PMC9353414
- Farnakis
D, Giakoumis A, Cannon L, Angastiniotis M, Eleftheriou A. COVID19 and
thalassemia: a position statement of the Thalassemia International
Federation. Eur J Haematol. 2020; 1-9.
https://doi.org/10.1111/ejh.13410 PMid:32198891
- Carsetti
R, Agrati C, Pinto VM, Gianesin B, Gamberini R, Fortini M, Barella S,
Denotti R, Perrotta S, Casale M, Maggio A, Pitrolo L, Tartaglia E,
Mortari EP, Colavita F, Puro V, Francalancia M, Marini V, Caminati M,
Mazzi F, De Franceschi L, Forni GL, Locatelli F. Premature aging of the
immune system affects the response to SARS-CoV-2 mRNA vaccine in
β-thalassemia: role of an additional dose. Blood. 2022 Oct
13;140(15):1735-1738. doi: 10.1182/blood.2022017594. https://doi.org/10.1182/blood.2022017594 PMid:36004936 PMCid:PMC9420073
- Anastasi
E, Marziali M, Preziosi A, Berardelli E, LoSardo A, Ribersani M,
Pugliese P, Farina A, Mancini P, Angeloni A. Humoral immune response to
Comirnaty SARS-Cov2 mRNA vaccine in Thalassemia Major patients.
Microbes and infection 2022. MICINF 2022. https://doi.org/10.1016/j.micinf.2022.104976 PMid:35381359 PMCid:PMC8977376
- Choi
B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo
HH, Boucau J, Bowman K, Adhikari UD, Winkler ML, Mueller AA, Hsu TY,
Desjardins M, Baden LR, Chan BT, Walker BD, Lichterfeld M, Brigl M,
Kwon DS, Kanjilal S, Richardson ET, Jonsson AH, Alter G, Barczak AK,
Hanage WP, Yu XG, Gaiha GD, Seaman MS, Cernadas M, Li JZ. Persistence
and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med.
2020 December 3;383(23):2291-2293. doi: 10.1056/NEJMc2031364. Epub 2020
November 11. https://doi.org/10.1056/NEJMc2031364 PMid:33176080 PMCid:PMC7673303
- Harvey
RA, Rassen JA, Kabelac CA, Turenne W, Leonard S, Klesh R, Meyer WA 3rd,
Kaufman HW, Anderson S, Cohen O, Petkov VI, Cronin KA, Van Dyke AL,
Lowy DR, Sharpless NE, Penberthy LT. Association of SARS-CoV-2
Seropositive Antibody Test With Risk of Future Infection. JAMA Intern
Med. 2021 May 1;181(5):672-679. doi: 10.1001/jamainternmed.2021.0366. https://doi.org/10.1001/jamainternmed.2021.0366 PMid:33625463 PMCid:PMC7905701
- Ju, B, Zhang, Q, Ge J, Wang R, Sun J. Human neutralizing antibodies elicited by SARS-Cov2 infection. Nature, 2020, 484: 115-9. https://doi.org/10.1038/s41586-020-2380-z PMid:32454513
- Hansen
CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of
protection against reinfection with SARS-CoV-2 among 4 million
PCR-tested individuals in Denmark in 2020: a population-level
observational study. Lancet. 2021 Mar 27;397(10280):1204-1212. doi:
10.1016/S0140-6736(21)00575-4. Epub 2021 March 17. https://doi.org/10.1016/S0140-6736(21)00575-4 PMid:33743221 PMCID: PMC7969130.
- Abu-Raddad
LJ, Chemaitelly H, Coyle P, Malek JA, Ahmed AA, Mohamoud YA,
Younuskunju S, Ayoub HH, Al Kanaani Z, Al Kuwari E, Butt AA,
Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul Rahim HF,
Nasrallah GK, Yassine HM, Al Kuwari MG, Al Romaihi HE, Al-Thani MH, Al
Khal A, Bertollini R. SARS-CoV-2 antibody-positivity protects against
reinfection for at least seven months with 95% efficacy.
EClinicalMedicine. 2021 May;35:100861. doi:
10.1016/j.eclinm.2021.100861. Epub 2021 April 28. https://doi.org/10.1016/j.eclinm.2021.100861 PMid:33937733 PMCid:PMC8079668


