Romain Fort1,2,3, Guillaume Monneret,4, Elie Nader2,3, Giovanna Cannas1, Philippe Connes2,3, Fabienne Venet4 and Arnaud Hot1.
1 Department of Internal Medicine, Edouard Herriot University Hospital, Lyon, France.
2 LIBM EA7424 Laboratory, Equipe "Biologie Vasculaire et du Globule Rouge", Claude Bernard University, Lyon, France.
3 Excellence du Globule Rouge (LABEX GR-Ex) Laboratory, PRES Sorbonne, Paris, France.
4 Cellular Immunology Laboratory, Edouard Herriot University Hospital, Lyon, France.
Correspondence to:
Correspondence to: Giovanna Cannas, M.D. Hospices Civils de Lyon,
Department of Internal Medicine, Edouard Herriot University Hospital,
Centre de Référence Constitutif: Syndromes Drépanocytaires Majeurs,
Thalassémies et Autres Pathologies Rares du Globule Rouge et de
l’Erythropoïèse; 5, place d’Arsonval 69437 Lyon cedex 03, France. Tel:
+33 (0)472117412; Fax: +33 (0)472117308. E-mail:
giovanna.cannas@chu-lyon.fr
Published: November 1, 2022
Received: August 28, 2022
Accepted: October 16, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022078 DOI
10.4084/MJHID.2022.078
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Sickle cell anemia (SCA) is a genetic disorder characterized by chronic hemolysis and vascular dysfunction.[1] Patients with SCA are at higher risk of invasive bacterial infections,[2] especially those due to encapsulated germs,[3] leading to specific recommendations for antibiotics prophylaxis and vaccinations.[4,5] The main causes of infections are attributed to splenic dysfunctions,[6] complement activation defects,[7] genetic factors, and micronutrient deficiencies.[8]
Reports from the literature have also suggested a central role in
immune impairment, especially during vaso-occlusive crises (VOC). In
septic shock, a clinical context characterized by an initial systemic
inflammatory response, the down-regulation of human leukocyte
antigen-DR expression on circulating monocytes (mHLA-DR) has been
demonstrated. In this setting, mHLA-DR expression is considered a
pertinent marker to identify patients with an increased risk of
nosocomial infections and, therefore, of deleterious outcome.[9]
The present study evaluates the mHLA-DR expression in SCA patients during and after VOC.
Eighteen
homozygous (HbSS) SCA adults with VOC, seen between October 2017 and
April 2018 at the Edouard Herriot University Hospital in Lyon (France),
were included in this one-center prospective study. A painful episode,
defined as VOC, lasted for more than four hours; the patient felt that
the pain was typical of that of vaso-occlusion, no other etiology of
pain could be identified by the physicians, and the patient required
hospitalization to the Emergency Department to treat the pain with
opioids. SCA patients with VOC were compared to a control group,
including 18 SCA subjects in clinical steady-state seen in the same
institution over the same period. The study was conducted following the
guidelines set by the Declaration of Helsinki. All patients gave
written informed consent prior to inclusion. The study was approved by
the "CPP Sud-Est IV" Ethics Committees (L16-47).
Venous
peripheral blood was drawn to assay mHLADR expression and lymphocyte
subsets counts (T-, B-, and NK-cell) on day 0 (D0) at crisis onset and
then on day 1 (D1) and between day 3 and day 7 (D3-7). In 6 of the 18
patients, mHLA-DR expression was monitored again four months (M4) after
crisis recovery. In the control cohort, peripheral venous blood was
harvested during medical consultation. Blood was sent on ice to the
immunology laboratory within 3 hours, and then the monocyte HLA-DR
expression was assessed by flow cytometry using a standardized
technique. Results are expressed as the number of antibodies bound per
cell (AB/C).
Mann-Whitney tests were used to compare
nonparametric biological values between patients in crisis and patients
in steady-state (control cohort). In contrast, the Wilcoxon test was
used to compare patients in crisis at different time points.
Statistical analyses were performed using SPSS (IBM Statistics) and
GraphPad Prism software. All P values were two-sided and statistical significance was defined as P < 0.05.
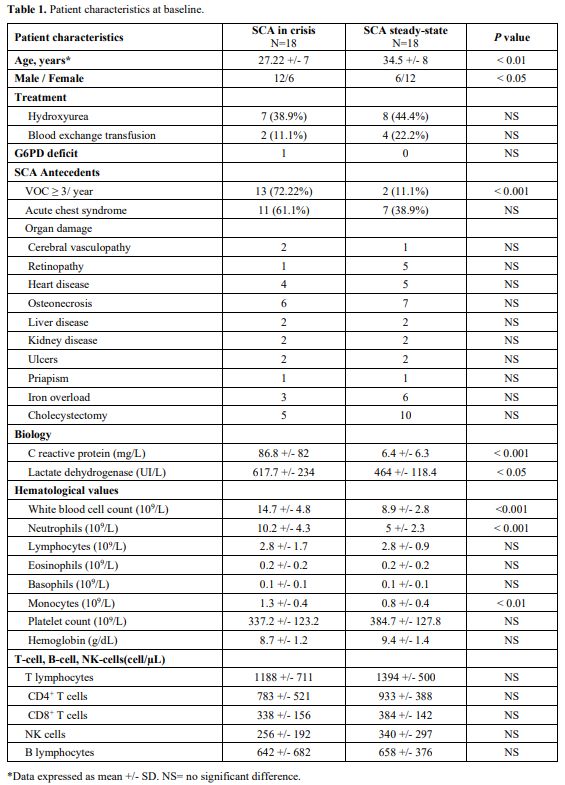
The baseline characteristics of the group in crisis and the control group are detailed in Table 1.
Half of the patients with VOCpresented with fever (>38°C). Patients
in crisis demonstrated significantly higher CRP levels (P < 0.0001, P = 0.0001, and P
= 0.0048, respectively) than patients from the control cohort. During
hospitalization, acute chest syndrome (ACS) occurred in 3 patients
(16.7%), and pneumonia (defined on radiological criteria) was diagnosed
in 2 patients (11.1%). mHLADR expression of the control group was
within the range of normal values. At the onset of VOC (D0), SCA
patients had lower mHLA-DR expression than the control group (P = 0.0001). A lower level of mHLA-DR expression was maintained at D1 (P = 0.052), but the level increased by D3 (P< 0.05). mHLA-DR expressions returned to normal values compared to D0 (P< 0.05) when measured following VOC recovery (Figure 1).
No significant differences were observed among the study and control
groups regarding the absolute counts of total lymphocytes and
lymphocyte subpopulations.
 |
Table
1. Patient characteristics at baseline. |
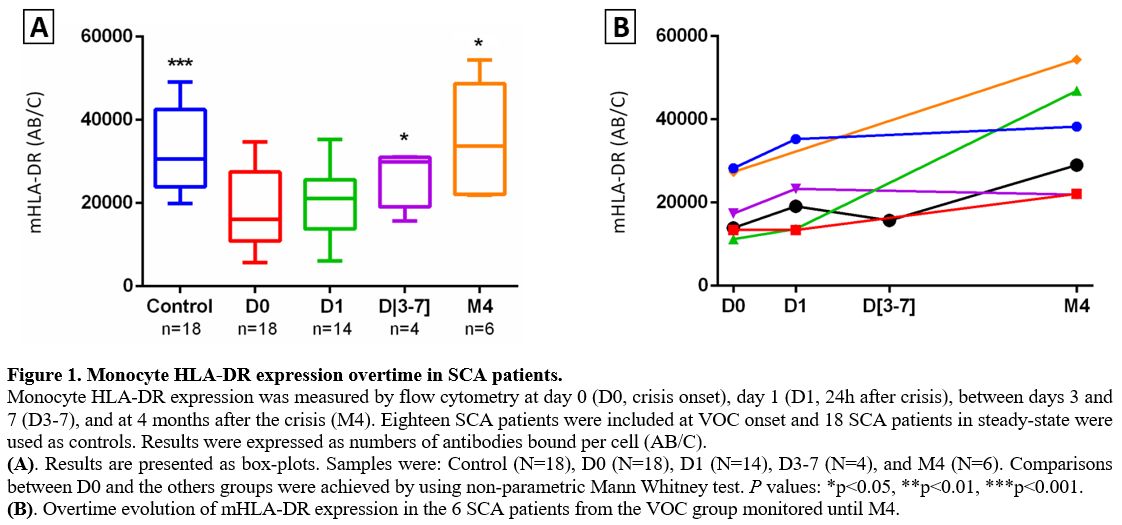 |
Figure 1. Monocyte HLA-DR expression overtime in SCA patients.
Monocyte HLA-DR expression was measured by flow cytometry at day 0 (D0,
crisis onset), day 1 (D1, 24h after crisis), between days 3 and 7
(D3-7), and at 4 months after the crisis (M4). Eighteen SCA patients
were included at VOC onset and 18 SCA patients in steady-state were
used as controls. Results were expressed as numbers of antibodies bound
per cell (AB/C).
(A). Results are presented as box-plots. Samples
were: Control (N=18), D0 (N=18), D1 (N=14), D3-7 (N=4), and M4 (N=6).
Comparisons between D0 and the others groups were achieved by using
non-parametric Mann Whitney test. P values: *p<0.05, **p<0.01,
***p<0.001.
(B). Overtime evolution of mHLA-DR expression in the 6 SCA patients from the VOC group monitored until M4.
|
This
study is the first evaluating mHLA-DR expression in SCA patients with
VOC. The results showed a significant decrease in mHLA-DR levels in
patients in crisis as compared to SCA subjects in clinical
steady-state. The nadir was reached at VOC onset. The mHLA-DR levels
remained low for 24 hours, then increased by D3, to finally normalized
by a few months. Values observed in the VOC group were similar to data
previously described in trauma patients.[10] However, they remained higher than those previously observed in patients with septic shock.[11]
In
SCA patients, VOC is characterized by a systemic inflammatory response,
which is associated with increased levels of biological markers (CRP
and leukocytosis) and cytokines (such as IL-6, IL-8, IL-17, and
TNF-α).[12,13,14] Prior findings showed that circulating monocytes
display an activated phenotype for VOC. The mechanism involves the
activation of the endothelium.[15] In the current study, circulating
monocytes from SCA patients in crisis demonstrated an altered/anergic
phenotype characterized by decreased MHC class II expression. Decreased
HLA-DR expression has previously been proposed as a marker of immune
alterations, and experimental ex-vivo studies have shown a linear
correlation with altered TNF-α production by monocytes.[16]
Similarly, it has been suggested in septic shocks that the decreased
mHLA-DR expression reflects the homeostatic regulation of the immune
response from initial overwhelming inflammation to secondary
immunosuppression.[17]
Despite limitations due
to the small number of patients, our first results suggest that,
following the acute injury associated with VOC, a negative
immunosuppressive feedback response occurs in SCA patients to
compensate for the acute inflammatory response initiated during the
VOC.[13] Furthermore, our findings are supported by
previous data showing that plasma concentrations of immunoregulatory
cytokines, such as IL-10, TGF-β, and PGE2, are also increased in SCA
patients during VOC.[14,18,19] Decreased HLA-DR expression is in accordance with Munford and Pugin's hypothesis[20]
that every systemic inflammatory response is associated with a possible
delayed immunosuppressive mechanism. Similarly, patients with severe
sepsis secondarily develop a 'compensatory anti-inflammatory response
syndrome' in reaction to inflammatory septic stress. Furthermore, the
persistence of low mHLA-DR expression over time in patients with septic
shock is associated with an increased risk of death or secondary
infections.[11,21]
In the
present study, the lowest levels of mHLA-DR expression at D0 were
observed in the two patients who developed pneumonia (5738 AB/C and
9793 AB/C, respectively). In VOC setting, a low mHLA-DR expression
could identify an underlying ongoing infection and contribute to an
early identification of high-risk patients.
Overall, mHLA-DR
expression significantly decreases in SCA patients during VOC
occurrence. Further studies should be conducted to confirm these
preliminary results and to establish relationships between putative
immunosuppression and an increased risk for infection. Upon
confirmation in larger cohorts, mHLA-DR may become an informative
biomarker to monitor patients during the crisis, especially as its cost
and feasibility are not limiting factors in developed countries.
Unfortunately, this biomarker should not be easily affordable in most
tropical African settings where most SCA patients reside.
Author Contributions
RF
designed the study, analyzed and interpreted data, and drafted the
manuscript; GM and FV provided immunologic data; GC provided patients
and control cohort, participated in therapy decision-making and patient
care, reviewed the manuscript, and gave final approval; PC and EN
performed statistical analyses; AH designed the study, analyzed and
interpreted data, participated to therapy decision making and patient
care, reviewed the manuscript, and gave final approval. All authors
approved the final manuscript.
References
- Kato GJ, Piel FB, Reid CD, Gaston MH,
Ohene-Frempong KR, Panepinto JA, Weatherall DJ, Costa FF, Vichinsky EP.
Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. https://doi.org/10.1038/nrdp.2018.10 PMid:29542687
- Booth
C, Inusa B, Obaro SK. Infection in sickle cell disease: A review.
International Journal of Infectious Diseases. 2010;14:e2-e12. https://doi.org/10.1016/j.ijid.2009.03.010 PMid:19497774
- Ramakrishnan
M, Moïsi JC, Klugman KP, Iglesias JM, Grant LR, Mpouti-Etame M, Levine
OS. Increased risk of invasive bacterial infections in African people
with sickle-cell disease: a systematic review and meta-analysis. Lancet
Infect Dis. 2010;10:329-337. https://doi.org/10.1016/S1473-3099(10)70055-4
- Yawn
BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH,
Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE,
Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J.
Management of sickle cell disease: summary of the 2014 evidence-based
report by expert panel members. JAMA. 2014;312:1033- 1048. https://doi.org/10.1001/jama.2014.10517 PMid:25203083
- McCavit
TL, Quinn CT, Techasaensiri C, Rogers ZR. Increase in invasive
Streptococcus pneumoniae infections in children with sickle cell
disease since pneumococcal conjugate vaccine licensure. J. Pediatr.
2011;158:505-507. https://doi.org/10.1016/j.jpeds.2010.11.025 PMid:21193205 PMCid:PMC3062091
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br. J. Haematol.2014;166:165-176. https://doi.org/10.1111/bjh.12950 PMid:24862308
- Gavriilaki
E, Mainou M, Christodoulou I, Koravou EE, Paleta A, Touloumenidou T,
Papalexandri A, Athanasiadou A, Apostolou C, Klonizakis P,
Anagnostopoulos A, Vlachaki E. In vitro evidence of complement
activation in patients with sickle cell disease. Haematologica.
2017;102:e481-e482. https://doi.org/10.3324/haematol.2017.174201 PMid:28912175 PMCid:PMC5709116
- Prasad
AS, Beck FW, Kaplan J, Chandrasekar PH, Ortega J, Fitzgerald JT,
Swerdlow P. Effect of zinc supplementation on incidence of infections
and hospital admissions in sickle cell disease (SCD). Am. J. Hematol.
1999;61:194-202. https://doi.org/10.1002/(SICI)1096-8652(199907)61:33.0.CO;2-C
- Wu
JF, Ma J, Chen J, Ou-Yang B, Chen MY, Li LF, Liu YJ, Lin AH, Guan XD.
Changes of monocyte human leukocyte antigen-DR expression as a reliable
predictor of mortality in severe sepsis. Crit Care. 2011;15:R220. https://doi.org/10.1186/cc10457 PMid:21933399 PMCid:PMC3334765
- Gouel-Chéron
A, Allaouchiche B, Guignant C, Davin F, Flocard B, Monneret G, for the
AzuRea Group. Early interleukin-6 and slope of monocyte human leukocyte
antigen-DR: a powerful association to predict the development of sepsis
after major trauma. PLoS ONE. 2012;7:e33095. https://doi.org/10.1371/journal.pone.0033095 PMid:22431998 PMCid:PMC3303782
- Monneret
G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, Thizy H, Bienvenu J,
Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte
antigen-DR expression predicts mortality in septic shock. Intensive
Care Med.2006;32:1175-1183. https://doi.org/10.1007/s00134-006-0204-8 PMid:16741700
- Okpala
I. The intriguing contribution of white blood cells to sickle cell
disease - a red cell disorder. Blood Rev.2004;18:65-73. https://doi.org/10.1016/S0268-960X(03)00037-7
- Pathare
A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D.
Cytokine profile of sickle cell disease in Oman. Am. J.
Hematol.2004;77:323-328. https://doi.org/10.1002/ajh.20196 PMid:15551290
- Alagbe
AE, Olaniyi JA,Aworanti OW.Adult sickle cell anaemia patients in bone
pain crisis have elevated pro-inflammatory cytokines.Mediterr J Hematol
Infect Dis 2018, 10(1): e2018017 https://doi.org/10.4084/mjhid.2018.017 PMid:29531654 PMCid:PMC5841944
- Belcher
JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes
in sickle cell disease: potential role in the activation of vascular
endothelium and vaso-occlusion. Blood. 2000;96:2451-2459. https://doi.org/10.1182/blood.V96.7.2451 PMid:11001897
- Winkler
MS, Rissiek A, Priefler M, Schwedhelm E, Robbe L, Bauer A, Zahrte C,
Zoellner C, Kluge S, Nierhaus A. Human leucocyte antigen (HLA-DR) gene
expression is reduced in sepsis and correlates with impaired TNFα
response: A diagnostic tool for immunosuppression? PLoS ONE.
2017;12:e0182427. https://doi.org/10.1371/journal.pone.0182427 PMid:28771573 PMCid:PMC5542660
- Monneret G, Venet F. Monocyte HLA-DR in sepsis: shall we stop following the flow? Crit Care. 2014;18:102. https://doi.org/10.1186/cc13179 PMid:24393356 PMCid:PMC4056426
- Graido-Gonzalez
E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma
endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell
disease and acute vaso-occlusive sickle crisis. Blood.
1998;92:2551-2555. https://doi.org/10.1182/blood.V92.7.2551 PMid:9746797
- Keikhaei
B, Mohseni AR, Norouzirad R, Alinejadi M, Ghanbari S, Shiravi F, Solgi
G. Altered levels of pro-inflammatory cytokines in sickle cell disease
patients during vaso-occlusive crises and the steady state condition.
Eur. Cytokine Netw.2013;24:45-52. https://doi.org/10.1684/ecn.2013.0328 PMid:23608554
- Munford
RS, Pugin J. Normal responses to injury prevent systemic inflammation
and can be immunosuppressive. Am. J. Respir. Crit. Care
Med.2001;163:316-321. https://doi.org/10.1164/ajrccm.163.2.2007102 PMid:11179099
- Landelle
C, Lepape A, Voirin N, Tognet E, Venet F, Bohé J, Vanhems P, Monneret
G. Low monocyte human leukocyte antigen-DR is independently associated
with nosocomial infections after septic shock. Intensive CareMed.
2010;36:1859-1866. https://doi.org/10.1007/s00134-010-1962-x PMid:20652682
[TOP]

