Alzbeta
Zavrelova1, Pavla Paterova2,
Pavel Zak1, Benjamin Visek1,
Martin Sima3 and Jakub Radocha1.
1
4th Department of Internal Medicine
– Haematology, University Hospital and Charles University Faculty of
Medicine, Hradec Kralove.
2 Department of Clinical Microbiology,
University Hospital and Charles University Faculty of
Medicine, Hradec Kralove.
3 Institute of Pharmacology, First Faculty of
Medicine, Charles University and General University Hospital in Prague,
Prague, Czech Republic
Correspondence to:
Jakub Radocha, MD, Ph.D. University Hospital Hradec Kralove, Sokolska
581, 50005 Hradec Kralove, Czechia. Phone: +420495831111. Mail:
jakub.radocha@fnhk.cz
Published: January 1, 2023
Received: September 8, 2022
Accepted: December 9, 2022
Mediterr J Hematol Infect Dis 2023, 15(1): e2023001 DOI
10.4084/MJHID.2023.001
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: P. aeruginosa
sepsis in immunocompromised patients is a serious complication of
cancer treatment, especially in the case of an Extensively Drug
Resistant (XDR) pathogen.
The aim of the study is to evaluate the efficacy of high-dose
ceftazidime in the treatment of XDR P. aeruginosa
infection and to compare it with the conventionally treated cohort in
hemato-oncological patients.
Methods:
We identified 27 patients with XDR P. aeruginosa
infection during the 2008-2018 period, 16 patients served as a
conventionally treated cohort with an antipseudomonal beta-lactam
antibiotic in standard dose (cohort A), and 11 patients were treated
with high-dose ceftazidime (cohort B). Most of the patients were
neutropenic and under active treatment for their cancer in both cohorts.
Results:
Mortality and related mortality were statistically significantly better
for cohort B than cohort A; it was 18.2% and 9.1% for cohort
B and 68.8% and 68.8% for cohort A, respectively. More patients in
cohort A needed mechanical ventilation and renal replacement therapy,
75% and 50% for cohort A and 27.3% and 9.9% for cohort B, respectively.
It corresponded well with the worst sequential organ failure score
(SOFA) in cohort A compared to cohort B, 16 versus 7, respectively.
Reversible neurotoxicity was seen only in two patients in cohort B.
Conclusion:
Ceftazidime in high doses is a very potent antibiotic (ATB) for
treating XDR P.
aeruginosa infections in neutropenic cancer with
acceptable toxicity.
|
Introduction
Hemato-oncological
patients in active treatment are at high risk of opportunistic
infection, especially during their stay in the intensive care unit.
Their propensity to infections is caused not only by neutropenia but
also by the treatments, which frequently are immunosuppressive and
produce organ damage. In recent years an increase in antimicrobial
resistance, especially in Gram-negative bacilli, has been repeatedly
described.[1-3] These infections
caused by multidrug-resistant
pathogens are burdened by a very high mortality rate.[3-5]
With
multidrug-resistant pathogens, the probability of inadequate antibiotic
coverage is much higher than in susceptible pathogens, which further
increases the mortality rate of these patients.[5-8]
Pseudomonas aeruginosa (P.
aeruginosa) is one of the most life-threatening bacterial
infections in an immunosuppressed host.[3]
It frequently develops as a
breakthrough infection, often MDR or even extensively drug-resistant
(XDR).[9-11] Colonization with MDR P. aeruginosa is
another
well-described factor leading to a higher infection rate.[12-14] It
causes severe and difficult-to-treat infections with reported mortality
rates of 43% and 63% in MDR and XDR pathogens,
respectively.[3,5,6,15-17]
Moreover, P. aeruginosa is prone to
high hospital transmission, and the water supply system often serves as
a reservoir of these MDR pathogens.[18,19]
Ceftazidime is one of the most potent antipseudomonal cephalosporins
with a very acceptable side effect profile compared to other
cephalosporins.[20,21] The most
clinically relevant toxicity is central
nervous system impairment.[22,23]
It manifests itself both
neurologically and psychiatric way and includes encephalopathy,
convulsion, confusion, myoclonus, hallucinations, coma, epilepsy,
tremor, drowsiness, disorientation, and agitation in decreasing order.
Most of these disorders are linked to renal insufficiency.[24] The
pathophysiological explanation is a decreased inhibitory of
gamma-aminobutyric acid and increased excitatory amino acid
release.[25] Ceftazidime has been
described as time-dependent
pharmacokinetics with a pharmacokinetic/pharmacodynamic target for
critical patients with 100% plasma concentration time above minimal
inhibitory concentration (MIC).[26]
It is probably even better when
plasma drug concentration is 4 times higher for 100% time than MIC.[27]
In this study, we wanted to describe the effect of higher-dose
ceftazidime in the treatment of XDR P. aeruginosa,
which occasionally occurs in our intensive care unit and causes
clinically very severe and hard-to-treat infections. Historically, our
patients experienced intolerably high mortality with these infections,
so despite the higher risk of toxicity, we started to use high-dose
ceftazidime treatment for these patients. Here, we want to describe our
experience compared to a historical cohort.
Study
design.
This was a retrospective observational study of our standard clinical
practice in intensive care patients experiencing XDR P. aeruginosa
infection.
Methods
All subsequent
cancer patients who underwent intensive care unit during the years
2008-2018 were retrospectively reviewed, and patients with signs of
sepsis and infection caused by XDR P.
aeruginosa were included in this
study. In addition, the patient charts were reviewed by two independent
clinicians, AZ and JR.
Patients were included if they were confirmed with bloodstream
infection, even if the origin of the infection was unknown. Organ
infection patients were included only if clinical or radiological signs
of infection and concurrent microbial samples from the affected organ
confirmed XDR P. aeruginosa as a causal
pathogen. Patients with sepsis treated with antipseudomonal antibiotics
upon colonization with XDR P. aeruginosa and not
confirmed by causative P. aeruginosa were excluded.
We identified two groups of patients; first, the conventionally treated
control group (cohort A) received a combination of antibiotics
according to the clinical judgment of their clinicians with
antipseudomonal potential (beta-lactam), and everyone received at least
one susceptible antibiotic to XDR P. aeruginosa. All
antibiotics were given in recommended doses. The second group (cohort
B) received off-label ceftazidime 3 g every 6 hours as a prolonged
3-hour infusion with other susceptible antipseudomonal antibiotics
(except for one patient with ceftazidime monotherapy). The choice of a
dosing regimen was entirely within the attending physician's discretion
and was decided after careful evaluation of prior dismal experiences
with this type of infection. This decision was made as all "clinically
safe" effective ATB for treating P.
aeruginosa when resistant.
All clinical samples, including patients' blood cultures, were
processed at the Department of Clinical Microbiology University
Hospital Hradec Kralove. Blood samples were inoculated in aerobic and
anaerobic bottles and, within 2 hours, placed into BACTEC blood culture
system. All pathogens were identified by Maldi TOF (Bruker Daltonics,
Germany). Antimicrobial susceptibility testing was performed by the
disc diffusion method and the broth microdilution method (TRIOS, CZ,
Lachema, CZ). Because patients were evaluated between 2008 and 2018,
susceptibility tests for the ceftolozane/tazobactam and
ceftazidime/avibactam were unavailable then. The results were
interpreted according to The European Committee on Antimicrobial
Susceptibility Testing (EUCAST), and the 2017 tables were chosen as the
most relevant for interpreting the results.[28,29]
P. aeruginosa
was considered XDR if bacterial isolates remained
susceptible to only one or two relevant antimicrobial categories
(aminoglycosides, carbapenems, cephalosporins, fluoroquinolones,
antipseudomonal penicillins with beta-lactamase
inhibitors, polymyxins).[30]
No molecular testing was done to
evaluate possible mechanisms of resistance further. Neutropenia was
defined as an absolute neutrophil count of < 500 cells/mm3.
Mortality was defined as death within this single episode of infection;
related mortality was considered death before the resolution of
symptoms or signs of infection not caused by the progression of the
underlying malignancy. The sequential organ failure score (SOFA) was
used to demonstrate the infection severity.[31,32]
Statistical analysis was performed using GraphPad Prism version 9 for
Windows, GraphPad Software, La Jolla, CA, USA. Fisher's exact test was
used for the comparison of categorical variables, and the T-test or the
Mann-Whitney U
test in the case of an abnormal distribution was used
for continuous variables. All variables were considered statistically
significant at p<0.05.
The study was approved by the Ethics Committee board (Ethics Committee,
Hradec Kralove University Hospital) of our institution.
Results
We were able to
identify 27 patients. 16 patients treated during the years 2008-2015
serve as conventionally treated cohort A. Cohort B consists of 11
patients treated during the years 2015-2018.
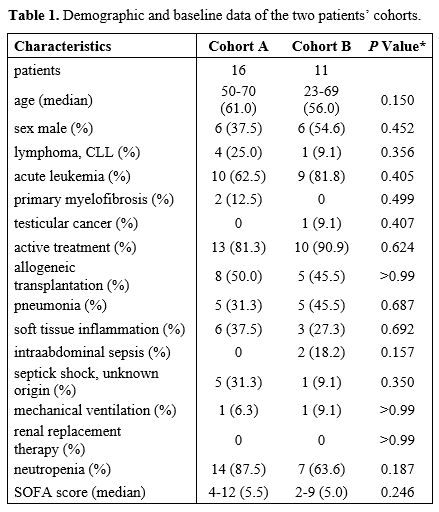
Complete demographic and baseline data are in Table 1.
 |
- Table
1. Demographic and baseline data of the two patients’ cohorts.
|
All of these
patients were treated for a wide range of hematologic
diseases, except one on high-dose chemotherapy for testicular cancer.
Infection complications occurred mainly during active treatment, but
three patients were in the follow-up phase after allogeneic stem cell
transplantation; one patient with progressive chronic lymphocytic
leukemia was just planned to receive immunotherapy. Most patients were
neutropenic during treatment; neutropenia was present in 14 pts (87.5%)
and 7 pts (63.6%) in cohorts A and B, respectively. Their initial SOFA
score was comparable, with a median of 5.5 (4-12) and 5 (2-9) in
cohorts A and B, respectively. However, the SOFA score deteriorated
during treatment due to non-survivor patients, the worst SOFA score was
in the range 5-22 (median 16) and 2-17 (median 7) in cohorts A and B,
respectively, and this reached statistical significance (p 0.008). In
the course of infection, a statistically significant superior number of
cohort A patients required mechanical ventilation and renal replacement
therapy due to the deleterious effect of progressive sepsis. Mechanical
ventilation and renal replacement therapy were administered to 12
patients (75.0%), three patients (27.3%) (p 0,022) and eight patients
(50,0%), one patient (9.1%) (p 0.0417) in cohort A and cohort B,
respectively. The most common source of infection was pneumonia (10
patients) and soft tissue inflammation (9 patients), six patients
developed septic shock, and two patients suffered from intraabdominal
sepsis. Details are described in Table
1 and Table
2.
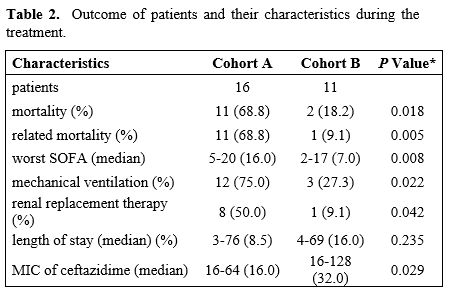 |
- Table
2. Outcome of patients and their characteristics during the treatment.
|
All isolates of
P.
aeruginosa were
susceptible only to one aminoglycoside
and colistin. All antibiotics from the other class of antipseudomonal
antibiotics were resistant. Susceptibility to new antipseudomonal ATB
was not tested, as the test nor new ATBs were yet available. The MICs
of ceftazidime were: median 16 mg/l (8-64 mg/l) and 32 mg/l (16-128
mg/l) in cohort A and B, respectively, which was significantly
different in favor of control Cohort A (p 0.0288). The details of the
susceptibility tests are in Tables
3 and 4.
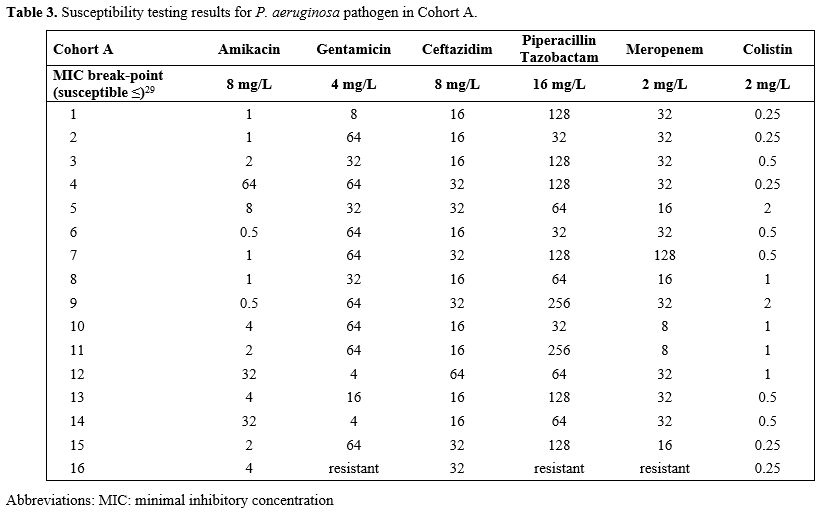 |
Table 3. Susceptibility testing results for P. aeruginosa pathogen
in Cohort A.
|
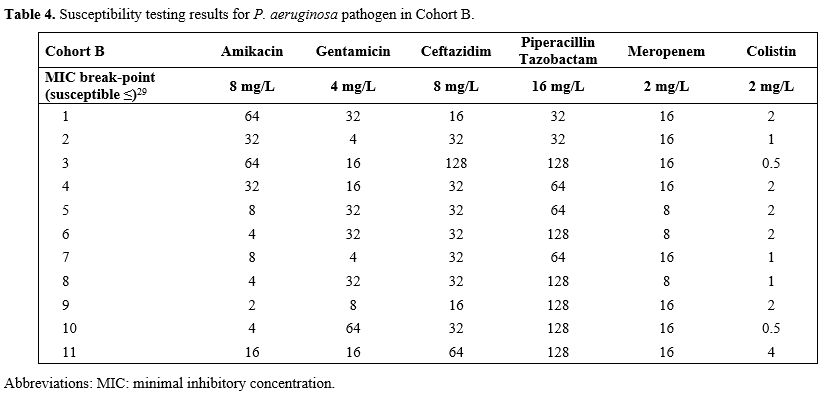 |
Table 4. Susceptibility testing results for P. aeruginosa
pathogen in Cohort B.
|
All patients,
except one, were treated with a combination of
antibiotics (one patient was treated with ceftazidime monotherapy in
cohort B). However, significantly more patients in cohort A were
treated even with the combination of 3 antipseudomonal antibiotics
consisting of beta-lactam, aminoglycoside, and colistin. Data are shown
in Table 5.
 |
- Table
5. Representation of combination of antipseudomonal antibiotics.
|
We experienced
neurological complications after high-dose ceftazidime
treatment in two patients. It was a quantitative disorder of
consciousness that did not lead to serious complications, and lowering
the dose of ceftazidime was sufficient to resolve the symptoms in both
cases. No epilepsy was encountered. Both patients were evaluated for
confusion even before ceftazidime, and septic encephalopathy was
considered.
The mortality rate in both cohorts differed, 68.8% and 18.0% in cohorts
A and B, respectively (p 0.0183). All patients died within 30 days
except one patient from cohort A, who died 76 days after the onset of
soft tissue infection of the neck and pneumonia. The cause of death was
a pulmonary abscess and multiple abscesses in the neck region caused by
P. aeruginosa
complicated by severe colitis. The pulmonary abscess was
verified by
pulmonary biopsy histologically and microbiologically. The patient
underwent pulmonary resection and drainage of neck abscesses
complicated by severe colitis; he died of septic shock with multiorgan
failure. Related mortality was 68.8% and 9.1% in cohorts A and B,
respectively (one patient died of the progression of leukemia) (p
0.047). Data in Table 2.
Discussion
This patient
population with XDR P.
aeruginosa infection in hemato-oncological patients is
unique, and it is difficult to find comparable results in the
literature. The most comparable data on the mortality rate of P.
aeruginosa infections are from studies with MDR P. aeruginosa. The mortality
rate in these studies varies. In the study by Zhao et al., it was 28.9%
with MDR P. aeruginosa infection,
even though 65.8% of the patients with MDR infection had inappropriate
antibiotic coverage.[11] In the
study conducted by Caselli et al., the
mortality rate was slightly higher, 35.8%, and the highest mortality
rate for MDR P. aeruginosa infections was
reported by Trecarichi et al., which was 42.4%. [3,15] In these two
studies, inappropriate treatment was not dated, so we do not know the
percentage of patients adequately treated.[3,11,15]
These results of
mortality rates 28.9%, 35.8 and 42.4% are substantially better than the
results in our cohort A (mortality 68,8%) and, on the other
hand, substantially worse than in our cohort B (mortality
18.2%). If we compare the data only with MDR P. aeruginosa infection and
inadequate antibiotic treatment, mortality was around 63%.[5] This result is already the same as in
our group A. In our group A,
the ATBs coverage should be considered adequate, as susceptible
aminoglycosides and/or colistin were added to all patients. However,
both ATBs should be used just as auxiliary ATBs, so this might be the
reason for the high mortality rate in this group of highly
immunocompromised patients. In one study of cancer patients
with P. aeruginosa infection, the
mortality rate of the XDR infection was 64%, the same rate as in our
group A.[16] In contrast, our
results with the mortality rate
in group B (18,2%) are nearly comparable with the mortality rate of
non-MDR P. aeruginosa infection.
Previously reported mortality was 5.5% in the study by Zhao et al.[11].In the study by Trecarichi et al.,
it was 12.5%, and the same
number was reported by Caselli et al.[3,15]
These results are in line with the recommendation that standard
treatment for P. aeruginosa should be
susceptible to beta-lactams. The results are very good, with expected
mortality as low as 5.5%.[11,33] On the other hand, it supports our
approach, in which we were able to overcome resistance by a higher dose
of ceftazidime in the case of XDR infection and improve the bad
prognosis in cancer patients. It is probably the result of an optimal
blood level and time above the MIC achieved by a higher dose of ATB for
a resistant pathogen.
One can speculate that better survival in cohort B might have been
caused by a beneficial effect of other ATBs added in combination.
Actually, all of these patients were treated with a combination
including aminoglycoside and colistin or both, except one patient in
group B, who was on ceftazidime monotherapy. There were even
significantly more patients covered by a combination of three
anti-pseudomonas antibiotics in group A, probably due to deterioration
of clinical condition as the course of infection was not under control.
However, a nice study with the new antibiotic ceftolozane/tazobactam
supports the idea that higher MIC with the same antibiotics dosing
leads to a worse outcome even in susceptible P. aeruginosa.[34] In this
paper, mortality of the patients was higher with susceptible P. aeruginosa and the same
dose of ATBs if the MIC > 2mg/L than MIC ≤ 2 mg/L; it was
41,2% and 16,2%, respectively.[34]
Hypothetically this might
further support our theory that during the treatment of
life-threatening infections, we should pay more attention to the MIC of
the pathogen. Accordingly, we should adjust the dose of antibiotics to
aim for the ideal situation with 100% time of blood level above MIC,
even in the resistant pathogen; this can lead to better outcomes for
patients.
Ceftazidime is a relatively non-toxic antibiotic that can be used in a
higher dose.[35,36] Despite the
increased risk of toxicity, especially
neurotoxicity, we decided to use a higher dose of ceftazidime in our
patients. This decision followed a careful evaluation of historical
results and experiences, mainly with soft tissue infection and
pneumonia caused by this pathogen. The mortality rate of 68.75% was
considered so huge that we decided to use not standard ceftazidime
dosing, as no new ATBs were available at that time. We chose a uniform
dose because drug monitoring for ceftazidime is not possible 24 hours
per day/7, days per week at our institution and is not routinely
recommended. We experienced toxicity in two patients, which seems to be
an acceptable risk. Furthermore, these two patients suffered only from
a quantitative disorder of consciousness, which did not result in any
medical emergencies, such as the need for tracheal intubation. Lowering
the dose of ceftazidime was sufficient for quick normalization of
consciousness in both patients. In these two patients, a change in
mental status was described even before ceftazidime and was probably
caused by septic encephalopathy. We can speculate that this previous
neurological abnormality might have contributed to ceftazidime
toxicity, as was described previously.[22]
This study has some limitations; the sample size is small, the study is
retrospective, and the data were collected over an extended period. The
biggest limitation is the sample size, where demonstrating superiority
is very difficult. On the other hand, there is not only reduced
mortality but also a worse SOFA score and a need for more mechanical
ventilation in group A, and that seems very convincing to us. It is
also very difficult to collect more patients because our institution's
evaluation to use an off-label dose of ceftazidime was very strict.
Despite these limitations, infection policy regarding bacterial
infections was very similar during this time. No new ATBs were
available, the dose of ATBs was unchanged then, and microbiological
procedures were standardized throughout the period. A single change was
made to off-label high-dose ceftazidime treatment in patients with risk
of XDR P. aeruginosa sepsis. With
the institution of this approach, the mortality rate decreased and
enabled our patients to continue with further cancer treatment. On the
other hand, we have seen some toxicity with our approach, but it was
deemed acceptable concerning the severity of the infection.
An off-label dose of ceftazidime is no longer of use at our institution
after the introduction of new antipseudomonal antibiotics and should
not be considered if other susceptible ATBs can be available. The
standard dose of susceptible ATBs is always prioritized over off label
high dose regimen. Unfortunately, our institution has already
experienced colonisation with resistant P. aeruginosa to all new
antipseudomonal ATBs, including cefiderocol.
Still, the clinical implication of this study seems to be very
important because the patients in cohort B performed well and achieved
their cancer treatment, including planned allogeneic
transplantation.
Acknowledgments
Supported by MH
CZ - DRO (UHHK, 00179906) and supported by the Cooperation Program,
research area O.
References
- Scheich S, Weber S,
Reinheimer C, Wichelhaus TA, Hogardt M, Kempf VAJ, et al. Bloodstream
infections with gram-negative organisms and the impact of multidrug
resistance in patients with hematological malignancies. Ann Hematol
2018;97:2225-34. https://doi.org/10.1007/s00277-018-3423-5
PMid:29974230
- Gudiol C, Bodro M,
Simonetti A, Tubau F, González-Barca E, Cisnal M, et al. Changing
aetiology, clinical features, antimicrobial resistance, and outcomes of
bloodstream infection in neutropenic cancer patients. Clin Microbiol
Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2013;19:474-9. https://doi.org/10.1111/j.1469-0691.2012.03879.x
PMid:22524597
- Trecarichi EM, Pagano L,
Candoni A, Pastore D, Cattaneo C, Fanci R, et al. Current epidemiology
and antimicrobial resistance data for bacterial bloodstream infections
in patients with hematologic malignancies: an Italian multicentre
prospective survey. Clin Microbiol Infect Off Publ Eur Soc Clin
Microbiol Infect Dis 2015;21:337-43. https://doi.org/10.1016/j.cmi.2014.11.022
PMid:25595706
- Trecarichi EM,
Tumbarello M. Antimicrobial-resistant Gram-negative bacteria in febrile
neutropenic patients with cancer: current epidemiology and clinical
impact. Curr Opin Infect Dis 2014;27:200-10. https://doi.org/10.1097/QCO.0000000000000038
PMid:24573013
- Garcia-Vidal C,
Cardozo-Espinola C, Puerta-Alcalde P, Marco F, Tellez A, Agüero D, et
al. Risk factors for mortality in patients with acute leukemia and
bloodstream infections in the era of multiresistance. PloS One
2018;13:e0199531. https://doi.org/10.1371/journal.pone.0199531
PMid:29953464 PMCid:PMC6023133
- Martinez-Nadal G,
Puerta-Alcalde P, Gudiol C, Cardozo C, Albasanz-Puig A, Marco F, et al.
Inappropriate Empirical Antibiotic Treatment in High-risk Neutropenic
Patients With Bacteremia in the Era of Multidrug Resistance. Clin
Infect Dis Off Publ Infect Dis Soc Am 2020;70:1068-74. https://doi.org/10.1093/cid/ciz319
PMid:31321410
- Tumbarello M, Spanu T,
Caira M, Trecarichi EM, Laurenti L, Montuori E, et al. Factors
associated with mortality in bacteremic patients with hematologic
malignancies. Diagn Microbiol Infect Dis 2009;64:320-6. https://doi.org/10.1016/j.diagmicrobio.2009.02.008
PMid:19345033
- Trecarichi EM, Giuliano
G, Cattaneo C, Ballanti S, Criscuolo M, Candoni A, et al. Bloodstream
infections caused by Escherichia coli in onco-haematological patients:
Risk factors and mortality in an Italian prospective survey. PloS One
2019;14:e0224465. https://doi.org/10.1371/journal.pone.0224465
PMid:31661507 PMCid:PMC6818756
- Viasus D, Puerta-Alcalde
P, Cardozo C, Suárez-Lledó M, Rodríguez-Núñez O, Morata L, et al.
Predictors of multidrug-resistant Pseudomonas aeruginosa in neutropenic
patients with bloodstream infection. Clin Microbiol Infect Off Publ Eur
Soc Clin Microbiol Infect Dis 2020;26:345-50. https://doi.org/10.1016/j.cmi.2019.07.002
PMid:31295551
- Gudiol C, Albasanz-Puig
A, Laporte-Amargós J, Pallarès N, Mussetti A, Ruiz-Camps I, et al.
Clinical Predictive Model of Multidrug Resistance in Neutropenic Cancer
Patients with Bloodstream Infection Due to Pseudomonas aeruginosa.
Antimicrob Agents Chemother 2020;64:e02494-19. https://doi.org/10.1128/AAC.02494-19
PMid:32015035 PMCid:PMC7179320
- Zhao Y, Lin Q, Liu L,
Ma R, Chen J, Shen Y, et al. Risk Factors and Outcomes of
Antibiotic-resistant Pseudomonas aeruginosa Bloodstream Infection in
Adult Patients With Acute Leukemia. Clin Infect Dis Off Publ Infect Dis
Soc Am 2020;71:S386-93. https://doi.org/10.1093/cid/ciaa1522
PMid:33367574
- Mizusawa M, Konuma T,
Kato S, Isobe M, Shibata H, Suzuki M, et al. Clinical outcomes of
persistent colonization with multidrug-resistant Gram-negative rods in
adult patients undergoing single cord blood transplantation. Int J
Hematol 2020;111:858-68. https://doi.org/10.1007/s12185-020-02854-5
PMid:32172445
- Girmenia C, Bertaina A,
Piciocchi A, Perruccio K, Algarotti A, Busca A, et al. Incidence, Risk
Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After
Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: An
Italian Prospective Multicenter Survey. Clin Infect Dis Off Publ Infect
Dis Soc Am 2017;65:1884-96. https://doi.org/10.1093/cid/cix690
PMid:29020286
- Cattaneo C, Di Blasi R,
Skert C, Candoni A, Martino B, Di Renzo N, et al. Bloodstream
infections in haematological cancer patients colonized by
multidrug-resistant bacteria. Ann Hematol 2018;97:1717-26. https://doi.org/10.1007/s00277-018-3341-6
PMid:29705860
- Caselli D, Cesaro S,
Ziino O, Zanazzo G, Manicone R, Livadiotti S, et al. Multidrug
resistant Pseudomonas aeruginosa infection in children undergoing
chemotherapy and hematopoietic stem cell transplantation. Haematologica
2010;95:1612-5. https://doi.org/10.3324/haematol.2009.020867
PMid:20305140 PMCid:PMC2930967
- Samonis G, Vardakas KZ,
Kofteridis DP, Dimopoulou D, Andrianaki AM, Chatzinikolaou I, et al.
Characteristics, risk factors and outcomes of adult cancer patients
with extensively drug-resistant Pseudomonas aeruginosa infections.
Infection 2014;42:721-8. https://doi.org/10.1007/s15010-014-0635-z
PMid:24912861
- Kim HS, Park BK, Kim
SK, Han SB, Lee JW, Lee D-G, et al. Clinical characteristics and
outcomes of Pseudomonas aeruginosa bacteremia in febrile neutropenic
children and adolescents with the impact of antibiotic resistance: a
retrospective study. BMC Infect Dis 2017;17:500. https://doi.org/10.1186/s12879-017-2597-0
PMid:28716109 PMCid:PMC5513208
- Slekovec C, Robert J,
van der Mee-Marquet N, Berthelot P, Rogues A-M, Derouin V, et al.
Molecular epidemiology of Pseudomonas aeruginosa isolated from infected
ICU patients: a French multicenter 2012-2013 study. Eur J Clin
Microbiol Infect Dis 2019;38:921-6. https://doi.org/10.1007/s10096-019-03519-w
PMid:30826996
- Breathnach AS, Cubbon
MD, Karunaharan RN, Pope CF, Planche TD. Multidrug-resistant
Pseudomonas aeruginosa outbreaks in two hospitals: association with
contaminated hospital waste-water systems. J Hosp Infect 2012;82:19-24.
https://doi.org/10.1016/j.jhin.2012.06.007
PMid:22841682
- Richards DM, Brogden
RN. Ceftazidime. A review of its antibacterial activity,
pharmacokinetic properties and therapeutic use. Drugs 1985;29:105-61. https://doi.org/10.2165/00003495-198529020-00002
PMid:3884319
- Grill MF, Maganti R.
Cephalosporin-induced neurotoxicity: clinical manifestations, potential
pathogenic mechanisms, and the role of electroencephalographic
monitoring. Ann Pharmacother 2008;42:1843-50. https://doi.org/10.1345/aph.1L307
PMid:19033476
- Deshayes S, Coquerel A,
Verdon R. Neurological Adverse Effects Attributable to β-Lactam
Antibiotics: A Literature Review. Drug Saf 2017;40:1171-98. https://doi.org/10.1007/s40264-017-0578-2
PMid:28755095
- Collins RD, Tverdek FP,
Bruno JJ, Coyle EA. Probable Nonconvulsive Status Epilepticus With the
Use of High-Dose Continuous Infusion Ceftazidime. J Pharm Pract
2016;29:564-8. https://doi.org/10.1177/0897190015608503
PMid:26475124
- Lacroix C, Kheloufi F,
Montastruc F, Bennis Y, Pizzoglio V, Micallef J. Serious central
nervous system side effects of cephalosporins: A national analysis of
serious reports registered in the French Pharmacovigilance Database. J
Neurol Sci 2019;398:196-201. https://doi.org/10.1016/j.jns.2019.01.018
PMid:30683462
- Sugimoto M, Uchida I,
Mashimo T, Yamazaki S, Hatano K, Ikeda F, et al. Evidence for the
involvement of GABAA receptor blockade in convulsions induced by
cephalosporins. Neuropharmacology 2003;45:304-14. https://doi.org/10.1016/S0028-3908(03)00188-6
PMid:12871648
- Gonçalves-Pereira J,
Póvoa P. Antibiotics in critically ill patients: a systematic review of
the pharmacokinetics of β-lactams. Crit Care Lond Engl 2011;15:R206. https://doi.org/10.1186/cc10441
PMid:21914174 PMCid:PMC3334750
- Benko AS, Cappelletty
DM, Kruse JA, Rybak MJ. Continuous infusion versus intermittent
administration of ceftazidime in critically ill patients with suspected
gram-negative infections. Antimicrob Agents Chemother 1996;40:691-5. https://doi.org/10.1128/AAC.40.3.691
PMid:8851594 PMCid:PMC163181
- EUCAST: Previous
versions of documents n.d. https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/
(accessed April 11, 2022).
- v_7.1_Breakpoint_Tables.xls
n.d. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.eucast.org%2Ffileadmin%2Fsrc%2Fmedia%2FPDFs%2FEUCAST_files%2FBreakpoint_tables%2Fv_7.1_Breakpoint_Tables.xls&wdOrigin=BROWSELINK
(accessed June 16, 2022)
- Magiorakos A-P,
Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al.
Multidrug-resistant, extensively drug-resistant and pandrug-resistant
bacteria: an international expert proposal for interim standard
definitions for acquired resistance. Clin Microbiol Infect Off Publ Eur
Soc Clin Microbiol Infect Dis 2012;18:268-81. https://doi.org/10.1111/j.1469-0691.2011.03570.x
PMid:21793988
- Cárdenas-Turanzas M,
Ensor J, Wakefield C, Zhang K, Wallace SK, Price KJ, et al.
Cross-validation of a Sequential Organ Failure Assessment score-based
model to predict mortality in patients with cancer admitted to the
intensive care unit. J Crit Care 2012;27:673-80. https://doi.org/10.1016/j.jcrc.2012.04.018
PMid:22762932
- Vincent JL, Moreno R,
Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA
(Sepsis-related Organ Failure Assessment) score to describe organ
dysfunction/failure. On behalf of the Working Group on Sepsis-Related
Problems of the European Society of Intensive Care Medicine. Intensive
Care Med 1996;22:707-10. https://doi.org/10.1007/BF01709751
PMid:8844239
- Bassetti M, Garau J.
Current and future perspectives in the treatment of multidrug-resistant
Gram-negative infections. J Antimicrob Chemother 2021;76:iv23-37. https://doi.org/10.1093/jac/dkab352
PMid:34849997 PMCid:PMC8632738
- Rodríguez-Núñez O,
Periañez-Parraga L, Oliver A, Munita JM, Boté A, Gasch O, et al. Higher
MICs (>2 mg/L) Predict 30-Day Mortality in Patients With Lower
Respiratory Tract Infections Caused by Multidrug- and Extensively
Drug-Resistant Pseudomonas aeruginosa Treated With
Ceftolozane/Tazobactam. Open Forum Infect Dis 2019;6:ofz416. https://doi.org/10.1093/ofid/ofz416
PMid:31660373 PMCid:PMC6810667
- Moriyama B, Henning SA,
Childs R, Holland SM, Anderson VL, Morris JC, et al. High-dose
continuous infusion beta-lactam antibiotics for the treatment of
resistant Pseudomonas aeruginosa infections in immunocompromised
patients. Ann Pharmacother 2010;44:929-35. https://doi.org/10.1345/aph.1M717
PMid:20371747 PMCid:PMC3148191
- De Boeck K, Breysem L.
Treatment of Pseudomonas aeruginosa lung infection in cystic fibrosis
with high or conventional doses of ceftazidime. J Antimicrob Chemother
1998;41:407-9. https://doi.org/10.1093/jac/41.3.407
PMid:9578170
[TOP]




