Sneha Tandon1, Sheila Weitzman2, Brooklyn Joyce2, Bryan Mcguire4, Derek Stephens4, James Whitlock2, Cynthia Hawkins3, Bo Yee Ngan3 and Oussama Abla2.
1 Division of Paediatric Hematology/Oncology, The Royal London Hospital, Barts Health NHS Trust, London, United Kingdom.
2 Division of Hematology/Oncology, The Hospital for Sick Children, University of Toronto, Canada.
3
Division of Pathology, Department of Paediatric Laboratory Medicine,
The Hospital for Sick Children, University of Toronto, Canada.
4 Department of Biostatistics, The Hospital for Sick Children, University of Toronto, Canada.
Correspondence to:
Sneha Tandon, MD, Division of Paediatric Hematology/Oncology,Department
of Paediatric, The Royal London Hospital, Barts Health NHS Trust,
London, United Kingdom. E-mail:
sneha.tandon@nhs.net
Published: May 1, 2023
Received: September 27, 2022
Accepted: April 24, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023035 DOI
10.4084/MJHID.2023.035
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background And Objectives:
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasm
with a wide spectrum of clinical presentations. Programmed Cell Death-1
(PD-1) receptor and its ligand (PD-L1) are overexpressed in LCH, but
their clinical significance is unknown. We performed a clinical
correlation study of PD-1/PD-L1 and VE1(BRAFp.V600E) expression in 131
children with LCH.
Methods: A total of 111 samples were tested for PD-1/PD-L1 and 109 for VE1(BRAFp.V600E) mutant protein by immunohistochemistry.
Results:
PD-1, PD-L1 and VE1(BRAFp.V600E) positivity was observed in 40.5%,
31.53% and 55%, respectively. PD-1/ PD-L1 expression showed no
significant effect on the rate of disease reactivations, early response
to therapy or late sequelae. The 5-year EFS was not statistically
different between patients with PD-1 positive compared to those with
PD-1 negative tumours (47.7% vs.58.8%, p=0.17). Similar 5-year EFS
rates were also seen in those who were PD-L1 positive compared to PD-L1
negative cases (50.5% vs.55.5%, p=0.61). VE1(BRAFp.V600E) positivity
was associated with a significantly higher frequency of risk-organ
involvement (p=0.0053), but no significant effect on early response to
therapy or rates of reactivations or late sequelae.
Conclusions:
Our study showed no significant correlation between VE1(BRAFp.V600E)
expression, PD-1 and PD-L1 and clinical outcome in pediatric LCH.
|
Introduction
Langerhans
cell histiocytosis (LCH) is a rare disorder characterized by the
accumulation of CD1a+/CD207+ dendritic cells with an inflammatory
infiltrate in many organs including bone, skin, lungs, liver, spleen,
bone marrow, pituitary gland and the central nervous system (CNS).[1-4]
LCH has a widely variable clinical presentation ranging from single
indolent lesions to severe multisystem (MS) disease. Over the past two
decades, the survival of children with MS-LCH has improved to nearly
90%.[5] Nevertheless, there remains significant
long-term morbidity in both high and low-risk patients, with late
sequelae like diabetes insipidus (DI), anterior pituitary dysfunction
and neurodegenerative disease (CNS-ND) being increasingly challenging
to treat.[6]
A major breakthrough in the understanding of LCH pathogenesis came with the discovery[7] and validation[8] of recurrent BRAF-V600E mutations in over 50% of LCH lesions.[7,8] Subsequently, additional MAPK pathway gene mutations in MAP2K1, ARAF, NRAS, KRAS and in-frame deletions, fusions and duplications of BRAF have been reported in LCH.[9,10]
Thus, LCH is now considered a myeloid neoplasm with a strong
inflammatory component with a median of 8% cells as Langerhans cells in
lesions and the remainder being inflammatory infiltrate.[11,12] These discoveries have provided scope for targeted therapy of LCH and other histiocytic disorders with BRAF and MEK inhibitors.[13,14]
The
programmed cell death-1 (PD-1) receptor and programmed cell
death-ligand (PDL-1) immune checkpoint pathway has been implicated in
the pathogenesis of different malignancies. Cancer-intrinsic
inflammation is involved in cancer progression via recruitment and
activation of inflammatory cells. The PD-1/PDL-1 pathway normally
inhibits T-cell function thereby resulting in reduced activation and
cytokine production by T cells. Tumor-associated T-cells and NK-cells
secrete cytokines like IFN-ℽ which leads to increased PDL-1 expression
on tumor cells.[15] Increased infiltration of
regulatory T cells (Tregs) as well as PD-L1 expression on CD207+
Langerhans cells have been previously reported in patients with LCH.[12,16,17] In addition, PD-1 blockade and targeted MAPK inhibition were found to be synergistic in a recent LCH mouse model.[18]
In
the current study, we explored the expression and clinical correlations
of PD-1/PD-L1 and VE1(BRAF p.V600E) mutant protein in archived
pathology samples of 131 children with LCH.
Methods
We
conducted an exploratory, single-centre retrospective study with chart
and pathology review of all cases of LCH treated at the Hospital for
Sick Children, Toronto from the year 2000 until 2018. The study was
approved by our institutional ethics board. During the study period,
164 children were treated for LCH at our center; most of these were
diagnosed locally while few were referred from other centers.
Thirty-three patients were excluded from the study due to unavailable
pathology samples, and the remaining 131 patients with available
samples were enrolled. Biopsy sites in these cases were bone 69(52.7%),
skin 35(26.7%), lymph node 6(12.2%), bone marrow 5(3.8%) and others
16(12.2%). Among the 131 cases, 22 and 20 were unable to be tested for
VE1(BRAFp.V600E) and PD-1/PD-L1, respectively, due to either missing
pathology samples (slides returned to outside referral centers),
insufficient samples or tissue exhausted or degraded from
decalcification. Therefore, 111 and 109 samples were successfully
tested for PD-1/PD-L1 and VE1(BRAFp.V600E) mutant protein, respectively
(Figure 1).
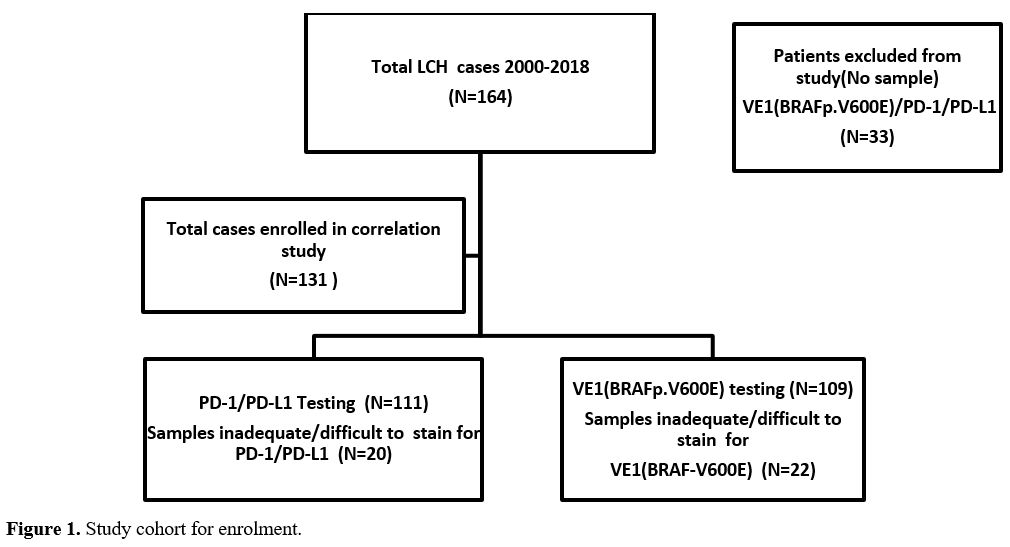 |
- Figure
1. Study cohort for enrolment.
|
PD-1,
PD-L1 and VE1(BRAFp.V600E) mutant proteins were tested using
immunohistochemistry (IHC) and reported by 2 staff pathologists at our
institution (BY-N, CH). VE1 antibody was used to detect BRAFp.V600E
expression. The anti -V600 BRAF antibody immunostaining procedure
was validated using tissues that is known to contain the BRAF mutation.
The percentages of PD-1+ infiltrating lymphocytes and PD-L1+-Langerin+
tumour cells and staining intensity were evaluated for each sample.
Staining intensity was scored considering 0 as negative or trace, 1 as
weak, 2 as moderate and 3 as high. Similarly, to previous studies,[13,20] all cases with staining intensity ≥2+ in ≥5% of tumour cells were considered as positive.[13,20] To identify the significance of PD-1+
infiltrating lymphocytes, the presence of any stained PD-1 cells in a
selected field were counted by the same pathologists. VE1(BRAFp.V600E)
mutant protein was considered either positive or negative by IHC
irrespective of staining intensity. Data recorded included patient
demographics (age, sex), disease classification using the criteria
defined by the Histiocyte society,[21] and treatment
protocols. Disease was classified as single system (SS) or multi-system
(MS) LCH. Risk organ (RO) involvement was used to classify MS LCH as
high risk (RO+) or low risk disease (RO-). Response to treatment at
weeks 6 and 12, disease reactivation and long-term sequelae were also
captured. Slow early response (SER) at week 6 of therapy was defined as
active disease intermediate or worse as per standard response criteria.
PD-1/PD-L1 and VE1(BRAFp.V600E) Immunostaining. Immunostains were performed on deparaffinised tissue slides (Figure 2 a-d).
For PD-1 and PD-L1 immunostaining, an automated staining system from
Dako Omnis (Agilent, Santa Clara, USA) was used. Slides were prepared
and stained following procedure protocols and reagents from the
supplier. Antibody staining reaction was detected using the Envision
Flex detection kit from Dako. Antibody for PD-1 (Clone NAT105, applied
at 1/75 dilution) was purchased from Cell Marque, Netherlands,
(marketed by Cedarlane, Ontario, Canada). For PD-L1, antibody (Clone
26.6, applied at 1/500 dilution) was purchased from AbCam, Ontario,
Canada. Immunostaining for VE1(BRAFp.V600E) was performed using
the Ventana Benchmark XT automated staining system (Roche Diagnostics,
USA). Staining conditions were used as recommended by the supplier and
staining reaction was detected by using the Optiview Amplifier,
supplied by Roche diagnostics. Antibody was purchased from Spring
Bioscience, clone VE1 (via distributor AbCam, Ontario, Canada). 1/800
dilution of this antibody was used. Immunostain results were evaluated
by light microscopy and images were captured with digital camera
(Infinity 3) supplied with a calibration software supplied by Lumenera,
Ontario Canada.
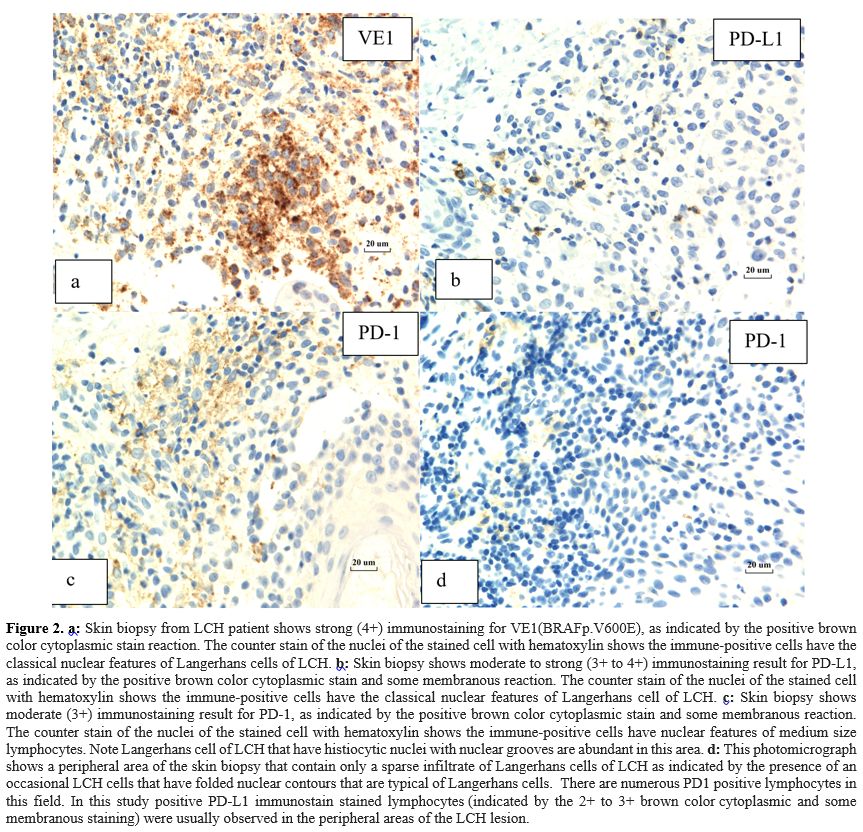 |
- Figure 2. a:
Skin biopsy from LCH patient shows strong (4+) immunostaining for
VE1(BRAFp.V600E), as indicated by the positive brown color cytoplasmic
stain reaction. The counter stain of the nuclei of the stained cell
with hematoxylin shows the immune-positive cells have the classical
nuclear features of Langerhans cells of LCH. b:
Skin biopsy shows moderate to strong (3+ to 4+) immunostaining result
for PD-L1, as indicated by the positive brown color cytoplasmic stain
and some membranous reaction. The counter stain of the nuclei of the
stained cell with hematoxylin shows the immune-positive cells have the
classical nuclear features of Langerhans cell of LCH. c:
Skin biopsy shows moderate (3+) immunostaining result for PD-1, as
indicated by the positive brown color cytoplasmic stain and some
membranous reaction. The counter stain of the nuclei of the stained
cell with hematoxylin shows the immune-positive cells have nuclear
features of medium size lymphocytes. Note Langerhans cell of LCH that
have histiocytic nuclei with nuclear grooves are abundant in this area.
d: This photomicrograph shows a
peripheral area of the skin biopsy that contain only a sparse
infiltrate of Langerhans cells of LCH as indicated by the presence of
an occasional LCH cells that have folded nuclear contours that are
typical of Langerhans cells. There are numerous PD1 positive
lymphocytes in this field. In this study positive PD-L1 immunostain
stained lymphocytes (indicated by the 2+ to 3+ brown color cytoplasmic
and some membranous staining) were usually observed in the peripheral
areas of the LCH lesion.
|
Statistical Analyses.
Overall Survival (OS) was defined as survival from diagnosis time until
last follow-up time, and Event Free Survival (EFS), being the primary
outcome measure, was defined as absence of reactivations, late sequelae
or death. Differences in the OS and EFS between the two groups with or
without PD-1 or PD-L1 expression or VE1(BRAFp.V600E) expression were
tested using Cox proportional hazards regression and the hazard ratios,
5-year survival rate, and p-values presented. For the categorical
outcomes, RO+, disease reactivation, and slow early response (SER)
differences were assessed using Fisher's exact test and the odds ratios
and p-values presented. Results with a p-value <0.05 were considered
significant.
Results
Patient Characteristics.
Among the 131 enrolled patients, the median age at diagnosis was 4
years (range,1.62-8) with a male: female ratio of 1.5:1. SS-LCH was
diagnosed in 73% (n=95) patients and 27% (n=36) had MS disease. The
majority of MS patients (64%) were RO+ (corresponding to 17% of the
total cohort), of whom liver involvement was seen in 9, spleen in 10
and bone marrow in 4 patients. Bone was the commonest site (76.3%)
followed by skin (32%). Slow early response at week 6 of therapy was
seen in 26% and disease reactivation in 21.4% of patients; median time
to reactivation was 1.68 years (range, 0.88-2.28 years). Detailed
patient characteristics are shown in Table 1.
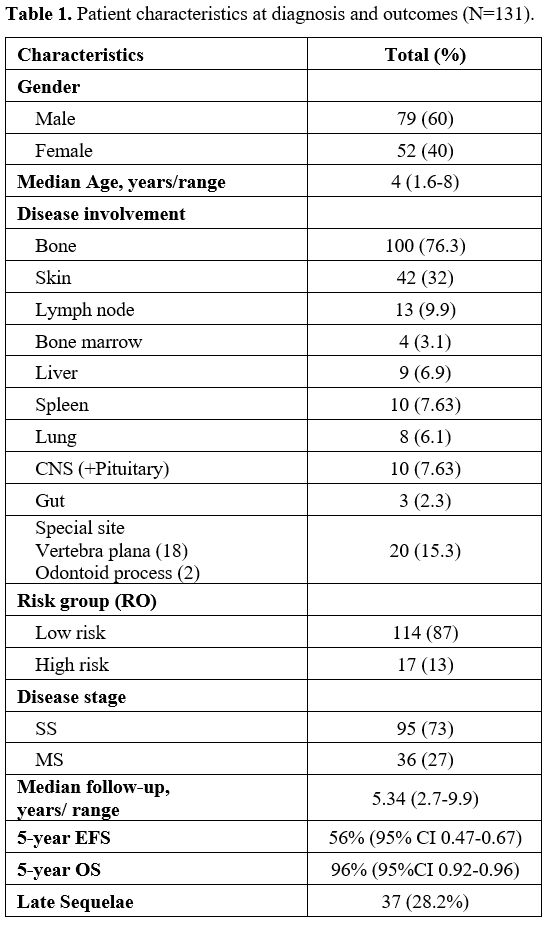 |
- Table
1. Patient characteristics at diagnosis and outcomes (N=131).
|
Treatment strategies.
Over the 18-year study period, a number of different therapies were
used upfront and for disease reactivation or refractory disease. The
commonest were LCH II/III protocols as upfront therapy, and
cladribine-cytarabine, vincristine-cytarabine, or clofarabine for
reactivations and refractory disease. BRAF inhibitor (Dabrafenib)
and/or MEK inhibitor (Trametinib) were used as salvage therapy in
5(3.8%) and 2(1.5%) patients, respectively.
Survival outcomes.
The median follow-up duration of our cohort was 5.34 years (range,
2.76-9.93years). The 5-year EFS and OS were 56% (95% CI 0.47-0.67) and
96% (95%CI 0.92-0.96) respectively. The 5-year EFS was not
statistically different between PD-1 positive compared to PD-1 negative
tumours (47.7% vs.58.8%, p=0.33). Similar 5-year EFS rates were also
seen in those who were PD-L1 positive compared to PD-L1 negative cases
(50.5% vs.55.5%, p=0.61) (Figure 3 a, b).
Further, there was no statistically significant difference in the
5-year OS and EFS of the VE1(BRAFp.V600E) negative compared to the
VE1(BRAFp.V600E) positive patients with a hazard ratio of 2.3 (95% CI
0.24, 22.1, p=0.47), and 1.67 (95% 0.907, 3.057, p=0.10) respectively (Figure 3c).
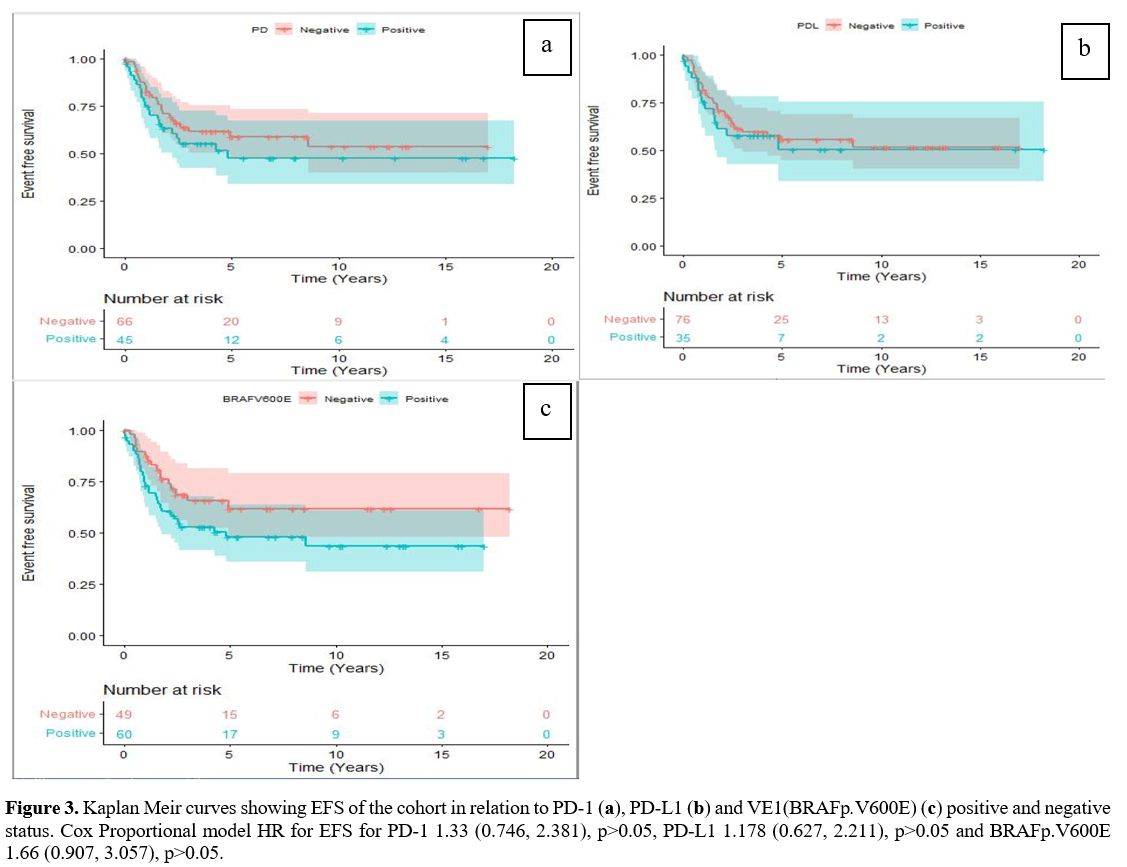 |
- Figure 3. Kaplan Meir curves showing EFS of the cohort in relation to PD-1 (a), PD-L1 (b) and VE1(BRAFp.V600E) (c)
positive and negative status. Cox Proportional model HR for EFS for
PD-1 1.33 (0.746, 2.381), p>0.05, PD-L1 1.178 (0.627, 2.211),
p>0.05 and BRAFp.V600E 1.66 (0.907, 3.057), p>0.05.
|
PD-1/PD-L1 status and clinical correlations.
Of the 111 samples successfully tested for PD-1/PD-L1; PD-1 positivity
was seen in 45 (40.5%) and PD-L1 in 35 (31.53%) patients. There was no
significant difference in the expression of PD-1/PDL-1 between bony and
non-bony LCH cases. RO+ was not different in those who were PD-1
positive compared to those who were negative (15.6% vs. 13.6%,
p>0.05). Similarly, RO+ did not correlate with PD-L1 positivity
(17.1% vs 13.2%, p>0.05). Neither PD-1 nor PD-L1 positivity were
correlated with disease response at week 6 of therapy (Table 2). Disease reactivation was seen in 31.1% and 28.6% of those who were PD-1+ and PD-L1+, respectively, and was not significantly different compared to PD-1/PD-L1 negative patients.
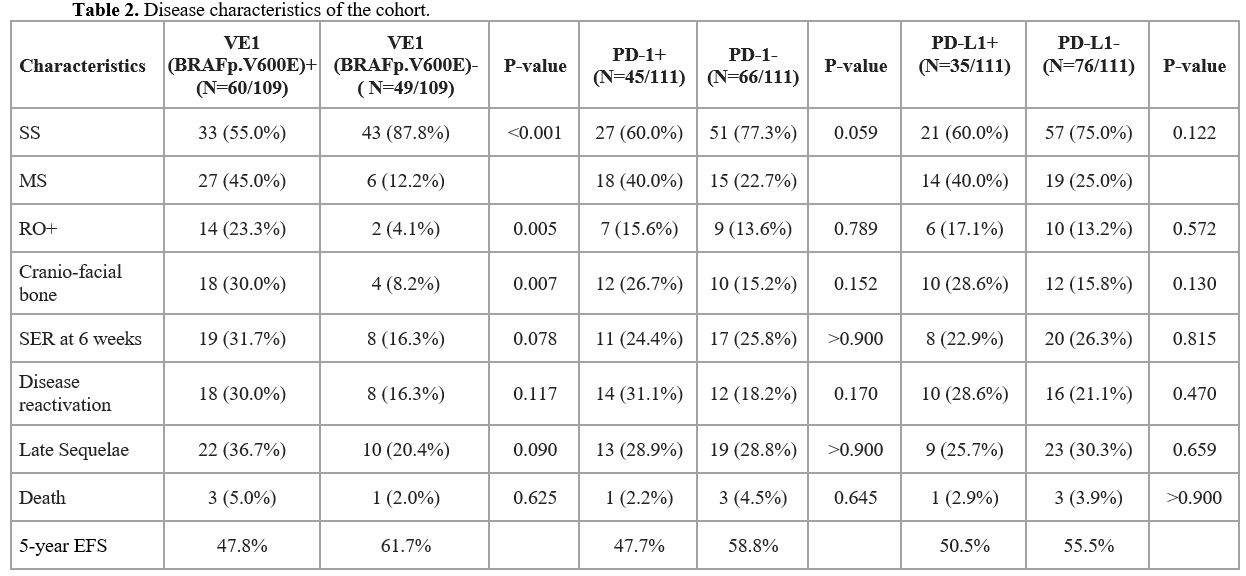 |
- Table 2. Disease characteristics of the cohort.
|
VE1(BRAFp.V600E) status and clinical correlations.
Of the total 109 patients tested for VE1(BRAFp.V600E), 60 (55%) were
positive and 49 (45%) were negative. Among the VE1(BRAFp.V600E) +
cases, 45% had MS LCH, and RO+ was significantly higher compared to the
VE1(BRAFp.V600E) negative cases (23.3% vs.4.1%, p=0.0053; OR=7.207, 95%
CI: 1.517-69.18) (Table 2).
Cranio-facial bone involvement was seen in a large proportion of those
who were VE1(BRAFp.V600E) positive 18/60 (30%). There was no
statistically significant difference in the rates of SER at week 6
(31.7% vs 16.3%; p= 0.073; OR=2.357, 95% CI: 0.865-6.964) or disease
reactivation (30% vs 16.3%; p>0.05; OR=2.181, 95% CI: 0.795-6.476)
in the VE1(BRAFp.V600E) + vs. VE1(BRAFp.V600E) negative patients.
Late Sequelae and correlation with PD-1/PDL-1 and VE1(BRAFp.V600E) status.
Late sequelae related to LCH were observed in 37(28.2%) patients, and
were more frequent in those with MS-LCH and in the VE1(BRAFp.V600E)
positive cases (36.7% vs.20.4%, p=0.0551). CNS-ND was seen in 7(19%)
patients; median duration from LCH diagnosis to the onset of CNS -ND
was 3 years (range, 0.1-14 years); 6 of the 7 (86%) had MS disease.
CNS-ND was noted to be higher in those with VE1(BRAFp.V600E) (24% vs.
5.8%) (p=0.195) and PD-1 (18.8% vs. 12.5%) (p=1.0) positivity compared
those who were negative. Endocrine complications noted were DI
11(29.7%), short stature 5(13.5%) and delayed puberty 1(2.7%). Of those
developing DI, 10(91%) had MS disease. VE1(BRAFp.V600E) + (24%, p=0.70)
and PD-1+(25%, p=0.54) expression were associated with increased risk
of DI. The median time for the onset of DI from LCH diagnosis was
1.6years (range, 0-4 years). Sclerosing cholangitis was noted in
6(16.2%) cases; 5 had MS disease, 5 were VE1(BRAFp.V600E) +, 3 were
PD-L1+ and 4 subsequently underwent a liver transplant. Hearing loss
was seen in 4(10.8%). Musculoskeletal complications were seen in a
significant proportion of our patients 28(75.6%); scoliosis 1(2.7%),
kyphosis 1(2.7%), facial asymmetry 4(10.8%), and pathological fracture
4(10.8%). These tended to be more prevalent in patients who were
VE1(BRAFp.V600E) +. Overall, there was no statistically significant
correlation between the incidence of late sequelae and VE1(BRAFp.V600E)
(p=0.0551) or PDL-1(p=0.708) positivity.
Discussion
The
involvement of the PD-1/PDL-1 pathway in the pathogenesis of LCH is not
well defined. We assessed the expression and prognostic impact of
PD-1/PDL-1 molecules and VE1(BRAFp.V600E) protein, using IHC, in
childhood LCH.
A recent study showed a PD-1 positivity in 5% to 20% and PDL-1 positivity in 5% of pulmonary LCH.[19] A
study by Gatalica et al. demonstrated a PD-L1 positive rate of 88% in
LCH samples, and showed that both PD-L1 and VE1(BRAFp.V600E) proteins
co-localized to the same multinucleated Langerhans cell.[16]
Another study detected PD-L1 expression in 20% of LCH cases (3/15), in
18% of Rosai-Dorfman cases (2/11) and in 50% of histiocytic sarcoma
cases (7/14).[20] Another report suggested that
PD-1/PDL-1 pathway may have some role in the microenvironment and
pathogenesis of bone LCH; however, the study had a small sample size of
6 patients and PD-1/PDL-1 positivity was quite low (16.6%).[21] In the current study, we observed 40.5% (45/111) positive rate for PD-1, and 31.5% (35/111) positive rate for PDL-1.
A recent report, including 97 children and adults with LCH, showed that BRAF-V600E
mutation correlated with higher levels of PD-L1 expression, and that
both proteins were independent prognostic factors of poor outcomes.[17]
In addition, accumulating evidence shows that PD-L1 expression is
frequently upregulated in tumours by activation of key oncogenic
pathways such as the class A phosphoinositide 3-kinases (PI3KCA)– AKT and RAS– RAF– MAPK pathways.[20]
This has therapeutic implications for LCH, especially in the
MS/relapsed-refractory settings where conventional chemotherapy could
lead to significant toxicity. An LCH mouse model showed a decrease in
the size of LCH lesions with the use of anti-PD-1 monoclonal antibodies
via reduction in the lymphoid component; further, combination therapy
with a MEK inhibitor proved synergistic in reducing the size of the lesion as well as restored T-cell effector function.[18] Other studies have shown that BRAF-V600E
expression results in immune suppression in melanoma and papillary
thyroid carcinoma via expression of PD-L1 and forkhead box protein 3
(FOXP3), which translates into disruption of endogenous host immune
surveillance and tumour immune escape.[16,22,23]
In
the present study, there was no significant association between risk
category, early disease response or late sequelae with PD-1/PD-L1
expression, and EFS in those who were PD-1 positive vs. PD-1 negative
cases (47.7% vs.58.8%, p>0.05). The reactivation rates in PD-1
positive (29%) and PD-L1 positive cases (25%) were not different from
the overall cohort and were similar to those reported by Gadner et al.
on the LCH-III trial.[5]
VE1(BRAFp.V600E) mutant
protein expression was associated with a 30% reactivation rate, a 31.7%
resistance to frontline therapy (Table 2),
lower EFS in VE1(BRAFp.V600E)+ cases, 36.7% rate of late
sequelae, and was more frequent in high-risk RO+ patients
(p=0.0053). Our results are similar to the BRAFV600E mutated French
cohort.[24]
CNS -ND occurred in a higher
proportion of children VE1(BRAFp.V600E) positive vs. VE1(BRAFp. V600E)
negative ones (24% vs. 5.8%), which is comparable to the published
literature.[24-26] This could be related to migration of BRAF-V600E positive myeloid cells to particular regions of the brain via perivascular accumulation and parenchymal infiltration.[27]
CNS-ND LCH has inferior outcomes and can have devastating sequelae in
the long-term, affecting the clinical outcomes as well as quality of
life of both patients and their families.[25-26] Previously, these were not reversible with chemotherapy,[28] but a recent report[29] suggested that BRAF
inhibitor therapy may improve CNS-ND symptomatology. This has
implications for prognosis and could warrant more aggressive follow-up
of patients who are BRAF-V600E
positive, as well as the potential to have a lower threshold for using
targeted agents in such patients. Successful targeted therapy against BRAF-V600E mutation has been shown in patients with relapsed/refractory LCH across various case reports or series,[30,31] including cases of CNS-ND.[14]
To
the best of our knowledge, the present study is the largest one to
analyse the clinical significance of PD-1/PD-L1 expression in a
pediatric LCH cohort, mostly treated with LCH-III like protocols.
Further, the long-term follow-up of 18 years allowed the capture of
early and late reactivations as well as late sequelae. However, our
study has few limitations. Firstly, although we have analysed a large
cohort, this study is retrospective and there is a potential for
selection bias. Secondly, 30% of the archived bone samples could not be
tested, due to difficulties in IHC staining of bone biopsies for
PD-1/PD-L1. Thirdly, only 17% of our patients were RO+, which could
contribute to the lack of correlation between PD-1/PD-L1 expression
with EFS and OS. Lastly, BRAF-V600E
status was examined by IHC as opposed to genotyping, and IHC may not be
as sensitive as genotyping in the detection of BRAFp.V600E; however,
previous studies have shown a strong correlation between IHC and PCR
testing of BRAF-V600E.[32]
Conclusions
Our
study did not find a significant correlation between VE1(BRAFp.V600E)
mutation, PD-1, PD-L1 expression and clinical outcomes in pediatric
LCH. Thus, it remains to be determined whether checkpoint inhibitors
with or without MAPK inhibition might be effective in high-risk
patients with LCH, such as refractory or relapsed RO+ cases. The
expression and prognostic impact of PD-1/PD-L1 should be explored in
all types of pediatric LCH, including MS disease, in large prospective
clinical trials.
Funding
This
project was funded by Division of Paediatric Haematology-Oncology New
Project Funding grant by The Hospital for Sick Children (SickKids),
Toronto, Canada.
Author Contributions
All
authors contributed to the study design, data collection and
interpretation, writing and reviewing the final manuscript. Oussama
Abla, Sneha Tandon, Bo Yee Ngan conceptualized the study and design,
initial data collection, interpretation of data and critically revising
the manuscript. Bo- Yee Ngan and Cynthia Hawkins provided,
BRAFp.V600E(VE1), PDL-1 and PD-1 antibodies, reported the
immunohistochemistry on samples, and critically reviewed the
manuscript. Brooklyn Joyce did initial data collection and
interpretation. Derek Stephens, Bryan McGuire helped with study design,
data analysis and interpretation and critical review of the manuscript.
James Whitlock and Sheila Weitzman made substantial contributions to
study design and critical review of the manuscript for important
intellectual content.
References
- Allen CE, Merad M, McClain KL. Langerhans Cell Histiocytosis. N Engl J Med.2018;379(9):856-868. https://doi.org/10.1056/NEJMra1607548 PMid:30157397 PMCid:PMC6334777
- Abla O, Rollins B, Ladisch S. Langerhans cell histiocytosis: progress and controversies. Bri J Haematol.2019;187:559-562. https://doi.org/10.1111/bjh.16099 PMid:31309539
- Fernandez
LF, Menor SF, Alonso CS, Arenas JJ. Langerhans cell histiocytosis of
the temporal bone in paediatric patients. Am J Roentgenol.2000;
174:217-21. https://doi.org/10.2214/ajr.174.1.1740217 PMid:10628482
- Gadner
H, Grois N, Potschger U, et al. Improved outcome in multisystem
Langerhans cell histiocytosis is associated with therapy
intensification. Blood.2008;111:2556-62. https://doi.org/10.1182/blood-2007-08-106211 PMid:18089850
- Gadner
H, Grois N, Potschger U, Minkov M,Arico M, Braier J,Broadbent V,
Donadieu J, Henter JI, McCarter R,Ladisch S, Histiocyte society.
Therapy prolongation improves outcome in multisystem Langerhans cell
histiocytosis. Blood.2013;121:5006-5014. https://doi.org/10.1182/blood-2012-09-455774 PMid:23589673
- Haupt
R, Nanduri V, Calevo MG, Bernstrand C, Braier JL, Broadbent V, Rey G,
McClain KL., Schaub J, Egeler RM. Permanent consequences in Langerhans
cell histiocytosis patients. A pilot study from the Histiocytic
Society-Late Effects Study Group. Pediatr Blood Cancer.2004;42:438-444.
https://doi.org/10.1002/pbc.20021 PMid:15049016
- Badalian-Very
G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo
FC, Ligon AH, Stevenson KE, Kehoe SM, Garraway LA, Hahn WC, Meyerson M,
Fleming MD, Rollins BJ. Recurrent BRAF mutations in Langerhans cell
histiocytosis. Blood. 2010 Sep 16;116(11):1919-23. https://doi.org/10.1182/blood-2010-04-279083. PMID: 20519626; PMCID: PMC3173987.
- Berres
ML, Lim KP, Peters T, Price J, Takizawa H, Salmon H, Idoyaga J, Ruzo A,
Lupo PJ, Hicks MJ, Shih A, Simko SJ, Abhyankar H, Chakraborty R,
Leboeuf M, Beltrão M, Lira SA, Heym KM, Bigley V, Collin M, Manz MG,
McClain K, Merad M, Allen CE. BRAF-V600E expression in precursor versus
differentiated dendritic cells defines clinically distinct LCH risk
groups. J Exp Med. 2014 Apr 7;211(4):669-83. https://doi.org/10.1084/jem.20130977 PMID: 24638167; PMCID: PMC3978272.
- Diamond
EL, Durham BH, Haroche J, Yao Z, Ma J, Parikh SA, Wang Z, Choi J, Kim
E, Cohen-Aubart F, Lee SC, Gao Y, Micol JB, Campbell P, Walsh MP,
Sylvester B, Dolgalev I, Aminova O, Heguy A, Zappile P, Nakitandwe J,
Ganzel C, Dalton JD, Ellison DW, Estrada-Veras J, Lacouture M, Gahl WA,
Stephens PJ, Miller VA, Ross JS, Ali SM, Briggs SR, Fasan O, Block J,
Héritier S, Donadieu J, Solit DB, Hyman DM, Baselga J, Janku F, Taylor
BS, Park CY, Amoura Z, Dogan A, Emile JF, Rosen N, Gruber TA,
Abdel-Wahab O. Diverse and Targetable Kinase Alterations Drive
Histiocytic Neoplasms. Cancer Discov. 2016 Feb;6(2):154-65. https://doi.org/10.1158/2159-8290.CD-15-0913 PMID: 26566875; PMCID: PMC4744547.
- Chakraborty
R, Hampton OA, Shen X, Simko SJ, Shih A, Abhyankar H, Lim KP, Covington
KR, Trevino L, Dewal N, Muzny DM, Doddapaneni H, Hu J, Wang L, Lupo PJ,
Hicks MJ, Bonilla DL, Dwyer KC, Berres ML, Poulikakos PI, Merad M,
McClain KL, Wheeler DA, Allen CE, Parsons DW. Mutually exclusive
recurrent somatic mutations in MAP2K1 and BRAF support a central role
for ERK activation in LCH pathogenesis. Blood. 2014 Nov
6;124(19):3007-15. https://doi.org/10.1182/blood-2014-05-577825 PMID: 25202140; PMCID: PMC4224195.
- Abla
O, Weitzman S. Treatment of Langerhans cell histiocytosis: role
of BRAF/MAPK inhibition. Hematology Am Hematol Educ Program.
2015:565-570. https://doi.org/10.1182/asheducation-2015.1.565
- Allen
CE, Li L, Peters TL, Leung HC, Yu A, Man TK, Gurusiddappa S, Phillips
MT, Hicks MJ, Gaikwad A, Merad M, McClain KL. Cell-specific gene
expression in Langerhans cell histiocytosis lesions reveals a distinct
profile compared with epidermal Langerhans cells. J Immunol. 2010 Apr
15;184(8):4557-67. https://doi.org/10.4049/jimmunol.0902336 PMID: 20220088; PMCID: PMC3142675.
- Haroche
J, Cohen-Aubart F, Emile JF, Maksud P, Drier A, Tolédano D, Barete S,
Charlotte F, Cluzel P, Donadieu J, Benameur N, Grenier PA, Besnard S,
Ory JP, Lifermann F, Idbaih A, Granel B, Graffin B, Hervier B, Arnaud
L, Amoura Z. Reproducible and sustained efficacy of targeted therapy
with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester
disease. J Clin Oncol. 2015 Feb 10;33(5):411-8. https://doi.org/10.1200/JCO.2014.57.1950 PMID: 25422482.
- Eckstein
OS, Visser J, Rodriguez-Galindo C, Allen CE; NACHO-LIBRE Study Group.
Clinical responses and persistent BRAF V600E+ blood cells in children
with LCH treated with MAPK pathway inhibition. Blood. 2019 Apr
11;133(15):1691-1694. https://doi.org/10.1182/blood-2018-10-878363. PMID: 30718231; PMCID: PMC6460419.
- Hashimoto
K, Nishimura S, Ito T, Akagi M. Characterization of PD-1/PDL-1 immune
checkpoint expression in soft tissue sarcomas. European Journal of
Histochemistry 2021; 65:3203. https://doi.org/10.4081/ejh.2021.3203
- Gatalica
Z, Bilalovic N, Palazzo JP, Bender RP, Swensen J, Millis SZ, Vranic S,
Von Hoff D, Arceci RJ. Disseminated histiocytoses biomarkers beyond
BRAFV600E: frequent expression of PD-L1. Oncotarget. 2015 Aug
14;6(23):19819-25. https://doi.org/10.18632/oncotarget.4378 PMID: 26110571; PMCID: PMC4637323.
- Zeng
K, Wang Z, Ohshima K, Liu Y, Zhang W, Wang L, Fan L, Li M, Li X, Wang
Y, Yu Z, Yan Q, Guo S, Wei J, Guo Y. BRAF V600E mutation correlates
with suppressive tumor immune microenvironment and reduced disease-free
survival in Langerhans cell histiocytosis. Oncoimmunology. 2016 Jun
14;5(7): e1185582. https://doi.org/10.1080/2162402X.2016.1185582 PMID: 27622040; PMCID: PMC5006923.
- Sengal
A, Velazquez J, Hahne M, Burke TM, Abhyankar H, Reyes R, Olea W, Scull
B, Eckstein OS, Bigenwald C, Bollard CM, Yu W, Merad M, McClain KL,
Allen CE, Chakraborty R. Overcoming T-cell exhaustion in LCH: PD-1
blockade and targeted MAPK inhibition are synergistic in a mouse model
of LCH. Blood. 2021 Apr 1;137(13):1777-1791. https://doi.org/10.1182/blood.2020005867 PMID: 33075814; PMCID: PMC8020265.
- Wang
J, Xie L, Miao Y, et al. Adult pulmonary Langerhans cell histiocytosis
might consist of two distinct groups: isolated form and extrapulmonary
recidivism type. Ann Transl Med. 2021; 9:357.
- Xu
J, Sun HH, Fletcher CD, Hornick JL, Morgan EA, Freeman GJ, Hodi FS,
Pinkus GS, Rodig SJ. Expression of Programmed Cell Death 1 Ligands
(PD-L1 and PD-L2) in Histiocytic and Dendritic Cell Disorders. Am J
Surg Pathol. 2016 Apr;40(4):443-53. https://doi.org/10.1097/PAS.0000000000000590 PMID: 26752545.
- Hashimoto
K, Nishimura S, Sakata N, et al. Characterization of PD-1/PDL-1 immune
checkpoint expression in the pathogenesis of musculoskeletal Langerhans
cell histiocytosis: A retroespective study. Medicine 2021;
100:43(e27650).
- Leslie
C, Bowyer SE, White A, Grieu-Iacopetta F, Trevenen M, Iacopetta B,
Amanuel B, Millward M. FOXP3+ T regulatory lymphocytes in primary
melanoma are associated with BRAF mutation but not with response to
BRAF inhibitor. Pathology. 2015 Oct;47(6):557-63. https://doi.org/10.1097/PAT.0000000000000314 PMID: 26308130.
- Angell
TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E
in papillary thyroid carcinoma is associated with increased programmed
death ligand 1 expression and suppressive immune cell infiltration.
Thyroid. 2014 Sep;24(9):1385-93. https://doi.org/10.1089/thy.2014.0134 PMID: 24955518; PMCID: PMC4148060.
- Héritier
S, Emile JF, Barkaoui MA, Thomas C, Fraitag S, Boudjemaa S, Renaud F,
Moreau A, Peuchmaur M, Chassagne-Clément C, Dijoud F, Rigau V, Moshous
D, Lambilliotte A, Mazingue F, Kebaili K, Miron J, Jeziorski E, Plat G,
Aladjidi N, Ferster A, Pacquement H, Galambrun C, Brugières L, Leverger
G, Mansuy L, Paillard C, Deville A, Armari-Alla C, Lutun A,
Gillibert-Yvert M, Stephan JL, Cohen-Aubart F, Haroche J, Pellier I,
Millot F, Lescoeur B, Gandemer V, Bodemer C, Lacave R, Hélias-Rodzewicz
Z, Taly V, Geissmann F, Donadieu J. BRAF Mutation Correlates With
High-Risk Langerhans Cell Histiocytosis and Increased Resistance to
First-Line Therapy. J Clin Oncol. 2016 Sep 1;34(25):3023-30. https://doi.org/10.1200/JCO.2015.65.9508 PMID: 27382093; PMCID: PMC5321082.
- Imashuku
S, Okazaki N', Nakayama M, Fujita N, Fukuyama T, Koike K, Minato T,
Kobayashi R, Morimoto A; Japan LCH Study Group. Treatment of
neurodegenerative CNS disease in Langerhans cell histiocytosis with a
combination of intravenous immunoglobulin and chemotherapy. Pediatr
Blood Cancer. 2008 Feb;50(2):308-11. https://doi.org/10.1002/pbc.21259 PMID: 17458874.
- Idbaih
A, Donadieu J, Barthez MA, Geissmann F, Bertrand Y, Hermine O,
Brugières L, Genereau T, Thomas C, Hoang-Xuan K. Retinoic acid therapy
in "degenerative-like" neuro-langerhans cell histiocytosis: a
prospective pilot study. Pediatr Blood Cancer. 2004 Jul;43(1):55-8. https://doi.org/10.1002/pbc.20040 PMID: 15170890.
- McClain
KL, Picarsic J, Chakraborty R, Zinn D, Lin H, Abhyankar H, Scull B,
Shih A, Lim KPH, Eckstein O, Lubega J, Peters TL, Olea W, Burke T,
Ahmed N, Hicks MJ, Tran B, Jones J, Dauser R, Jeng M, Baiocchi R,
Schiff D, Goldman S, Heym KM, Wilson H, Carcamo B, Kumar A,
Rodriguez-Galindo C, Whipple NS, Campbell P, Murdoch G, Kofler J,
Heales S, Malone M, Woltjer R, Quinn JF, Orchard P, Kruer MC, Jaffe R,
Manz MG, Lira SA, Parsons DW, Merad M, Man TK, Allen CE. CNS Langerhans
cell histiocytosis: Common hematopoietic origin for LCH-associated
neurodegeneration and mass lesions. Cancer. 2018 Jun
15;124(12):2607-2620. https://doi.org/10.1002/cncr.31348 PMID: 29624648; PMCID: PMC6289302.
- McClain
KL, Allen CE, Rauch R, et al. Cytosine arabinoside can ameliorate the
symptoms of CNS -LCH in some patients: Proceedings of the 24th annual
meeting of the histiocyte society, October 1-3, Berlin, Germany (2008).
- Donadieu
J, Larabi IA, Tardieu M, Visser J, Hutter C, Sieni E, Kabbara
N,Barkaoui M, Miron J, Chalard F, Milne P, Haroche J, Cohen F,
Hélias-Rodzewicz Z, Simon N, Jehanne M, Kolenova A, Pagnier A, Aladjidi
N, Schneider P, Plat G, Lutun A, Sonntagbauer A, Lehrnbecher T, Ferster
A, Efremova V, Ahlmann M, Blanc L, Nicholson J, Lambilliote A, Boudiaf
H, Lissat A, Svojgr K, Bernard F, Elitzur S, Golan M, Evseev D, Maschan
M, Idbaih A, Slater O, Minkov M, Taly V, Collin M, Alvarez JC, Emile
JF, Héritier S. Vemurafenib for Refractory Multisystem Langerhans Cell
Histiocytosis in Children: An International Observational Study. J Clin
Oncol. 2019 Nov 1;37(31):2857-2865. https://doi.org/10.1200/JCO.19.00456 PMID: 31513482; PMCID: PMC6823889.
- Héritier
S, Jehanne M, Leverger G, Emile JF, Alvarez JC, Haroche J, Donadieu J.
Vemurafenib Use in an Infant for High-Risk Langerhans Cell
Histiocytosis. JAMA Oncol. 2015 Sep;1(6):836-8. https://doi.org/10.1001/jamaoncol.2015.0736 PMID: 26180941.
- Charles
J, Beani JC, Fiandrino G, Busser B. Major response to vemurafenib in
patient with severe cutaneous Langerhans cell histiocytosis harboring
BRAF V600E mutation. J Am Acad Dermatol. 2014 Sep;71(3):e97-9. https://doi.org/10.1016/j.jaad.2014.03.038 PMID: 25128147.
- Long
GV, Wilmott JS, Capper D, Preusser M, Zhang YE, Thompson JF, Kefford
RF, von Deimling A, Scolyer RA. Immunohistochemistry is highly
sensitive and specific for the detection of V600E BRAF mutation in
melanoma. Am J Surg Pathol. 2013 Jan;37(1):61-5. https://doi.org/10.1097/PAS.0b013e31826485c0 PMID: 23026937.
[TOP]




