Luca Guarnera1, Gentiana Elena Trotta1, Valentina Boldrini1, Lucia Cardillo1, Ilaria Cerroni1, Valeria Mezzanotte1, Gianmario Pasqualone1, Arianna Savi1, Beatrice Borsellino1, Elisa Buzzatti1, Raffaele Palmieri1, Giovangiacinto Paterno1, Luca Maurillo1, Francesco Buccisano1, Adriano Venditti1 and Maria Ilaria Del Principe1.
1 Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy.
Correspondence to:
Professor Adriano Venditti. Department of Biomedicine and Prevention,
University of Rome Tor Vergata, Rome, Italy. E-mail:
adriano.venditti@uniroma2.it
Published: January 1, 2023
Received: November 10, 2022
Accepted: December 31, 2022
Mediterr J Hematol Infect Dis 2023, 15(1): e2023013 DOI
10.4084/MJHID.2023.013
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Colonization by multidrug-resistant organisms (MDRO) is a frequent
complication in hematologic departments, which puts patients at risk of
life-threatening bacterial sepsis. Fever of unknown origin (FUO) is a
condition related to the delivery of chemotherapy in hematologic
malignancies, in which the use of antibiotics is debated. The
incidence, risk factors, and influence on the outcome of these
conditions in patients with acute myeloid leukemia (AML) are not
clearly defined.
Methods:
We retrospectively analyzed 132 consecutive admissions of
non-promyelocytic AML patients at the Hematology Unit of the University
Tor Vergata in Rome between June 2019 and February 2022. MDRO
swab-based screening was performed in all patients on the day of
admission and once weekly after that. FUO was defined as fever with no
evidence of infection.
Results:
Of 132 consecutive hospitalizations (69 AML patients), MDRO
colonization was observed in 35 cases (26%) and resulted independently
related to a previous MDRO colonization (p=0.001) and length of
hospitalization (p=0.03). The colonization persistence rate in
subsequent admissions was 64%. MDRO-related bloodstream infection was
observed in 8 patients (23%) and correlated with grade III/IV mucositis
(p=0.008) and length of hospitalization (p=0.02). FUO occurred in 68
cases (51%) and correlated with an absolute neutrophilic count
<500μ/L at admission (0.04).
Conclusion:
In our experience, MDRO colonization is a frequent and
difficult-to-eradicate condition that can arise at all stages of
treatment. Prompt discharge of patients as soon as clinical conditions
allow could limit the spread of MDRO. In addition, the appropriate use
of antibiotics, especially in the case of FUO, and the contraction of
hospitalization length, when feasible, are measures to tackle the
further spread of MDRO.
|
Introduction
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy of the myeloid lineage.
The choice of treatment requires a careful analysis of the biological characteristics of the disease[1] and a proper assessment of patients’ fitness;[2]
patients deemed eligible for aggressive treatment are to receive
anthracycline-based induction chemotherapy followed by cytarabine
and/or hematopoietic stem cell transplantation (HSCT) as consolidation.
Patients not eligible for this approach undergo less intensive
therapies, such as hypomethylating agents (HMA) (± venetoclax) or other
forms of low-intensity chemotherapy (i.e., low-dose cytarabine).
Patients ineligible for active therapy are referred to palliative care.[1]
Immunosuppression
caused by these treatments and prolonged hospitalizations expose AML
patients to life-threatening infections, which can be sustained by
multidrug-resistant organisms (MDROs), accounting for one of the major
causes of mortality.[3]
Given the complex profile
of antibiotic resistance and the rapid worldwide diffusion of MDROs,
epidemiological surveillance of the microbiological colonization of
patients has become a critical step. Actually, early detection of
colonization prevents MDROs from spreading, through patients’ isolation
and delivery of targeted therapy, in case of fever.[4]
In
the treatment of febrile neutropenia, the European Conference on
Infections in Leukemia suggests a wise use of antibiotics to avoid
further selection of resistance:[5-7] non-colonized
patients should be treated with empirical therapy, not including
carbapenems, while colonized patients should be treated with a
"de-escalation" approach, choosing the antibiotics based on the MDRO
antibiogram. Any modification of the therapeutic strategy at 72-96
hours should rely on the patient's clinical evaluation and the results
of microbiologic culture tests.[7]
Fever, in the
absence of non-infectious causes and clinical focus of infection and
negativity of blood cultures or pathological microbiological findings
related to a possible focus of infection, is defined as of unknown
origin (FUO).[8] The onset of FUO is frequently
described in hematologic malignancies; the underlying mechanisms are
poorly understood, and the use of antibiotics is a matter of debate.[7,8]
This
retrospective study aims to analyze the incidence of Colonization by
MDRO and FUO in a consecutive series of AML patients and assess these
factors' effects on the outcome.
Material and Methods
Patients.
We retrospectively analyzed 132 consecutive admissions for a total of
69 adult patients (≥18 years old) with non-promyelocytic AML seen at
the Hematology Unit of the University Tor Vergata in Rome between June
2019 and March 2022. AML diagnosis and treatment schedules were defined
according to the European LeukemiaNet guidelines.[1]
Baseline
data were recorded for each patient at admission and included age,
gender, ECOG, white blood cell count (WBCc), absolute neutrophil count
(ANC), hemoglobin (Hb), lymphocytes count (Lyc), and lactate
dehydrogenase (LDH). In addition, Patients on AML treatment regimens
received antibiotic exposure in the previous six months before
admissions to hematology departments. MDRO colonization at previous
admissions, incidence and severity of neutropenia, grade III/IV
mucositis according to WHO grading scale,[9] MDRO colonization, FUO occurrence, and outcome at 30 and 60 days from colonization were also recorded.
MDROs
were defined as vancomycin-resistant enterococcus (VRE),
methicillin-resistant Staphylococcus aureus (MRSA),
Carbapenem-resistant Enterobacteriaceae (CRE), and Extended-spectrum
beta-lactamases (ESBLs).
Nasal, oropharyngeal, anal, perianal, and
urethral or vaginal MDRO screening culture swabs were performed in all
patients on the same day of admission, and anal and perianal swabs once
weekly thereafter. Colonized patients were isolated to contain the
spread of the pathogen.
Bloodstream infection (BSI) was defined
as the detection of a bacterium in one blood culture; two positive
cultures were required for diagnosing coagulase-negative staphylococci
or Corynebacterium spp. In addition, BSI was defined as related to MDRO
(MDROrel BSI) in case of identification in blood culture of the same
pathogen detected in screening culture swabs.
FUO was defined as
fever (≥ 38.3°C once or ≥ 38.0°C lasting for at least 1 h or being
measured twice within 12 h) in the absence of identified causes and
negativity of blood cultures from both peripheral vein and central
venous catheter (if present).
During neutropenia, no
fluoroquinolone prophylaxis (FP) was used. In the case of febrile
neutropenia, antibiotic therapy was started: in colonized patients, the
choice of the antibiotic was driven by the sensitivity profile of MDRO,
whereas non-colonized patients were treated empirically with a
first-line β-lactam antibiotic piperacillin/tazobactam.
The study
was approved by the Institutional Review Board and all patients
provided informed consent to the processing of their sensitive data.
Statistical Analysis.
Univariate and multivariate analyses were used to establish the
connections between the variables. Chi-square or Fisher exact test was
used for dichotomous variables; the independent test or Mann-Whitney
test were used for continuous variables as appropriate. A p-value less
than 0.05 was considered significant. All analyses were performed using
the IBM SPSS Statistics 27 software.
Results
Characteristics of the study population are shown in table 1.
One hundred thirty-two admissions were analyzed (for a total of 69
adult patients); intensive chemotherapy was administered in 74,
non-intensive treatment in 29, and supportive therapy in 29. Table 2
summarizes the therapeutic regimens. MDRO colonization was detected in
35 admissions (26%) and correlated with previous exposure to Vancomycin
(p=0.002) and Carbapenem (p=0.03), previous MDRO colonization
(p<0.001), mucositis (p=0.04) and days of hospitalization (p=0.001).
A near-significance correlation with FUO (p=0.1), ECOG (p=0.08), ≥2
previous intensive chemotherapies (p=0.05), and the absence of previous
treatment with HMA (p=0.05) was also observed. In multivariate
analysis, previous MDRO colonization (p=0.001) and days of
hospitalization (p=0.03) remained independent factors significantly
associated with MDRO colonization. Among these patients, the
colonization persistence rate in subsequent admissions was 64%. CRE was
the most frequently identified MDRO (in 29 cases, 22%); VRE was
detected in 8 cases (6%), MRSA in 4 (3%), and ESBL in 2 (1.5%) (Figure 1).
Two patients developed anal abscesses; CRE colonized both, presented
mucositis, and had a long hospitalization (59 and 46 days).
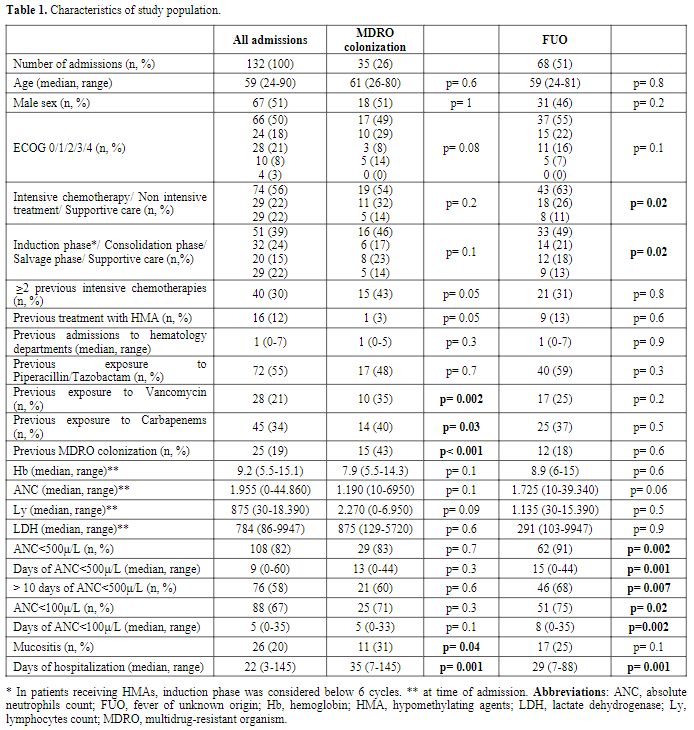 |
Table 1. Characteristics of study population. |
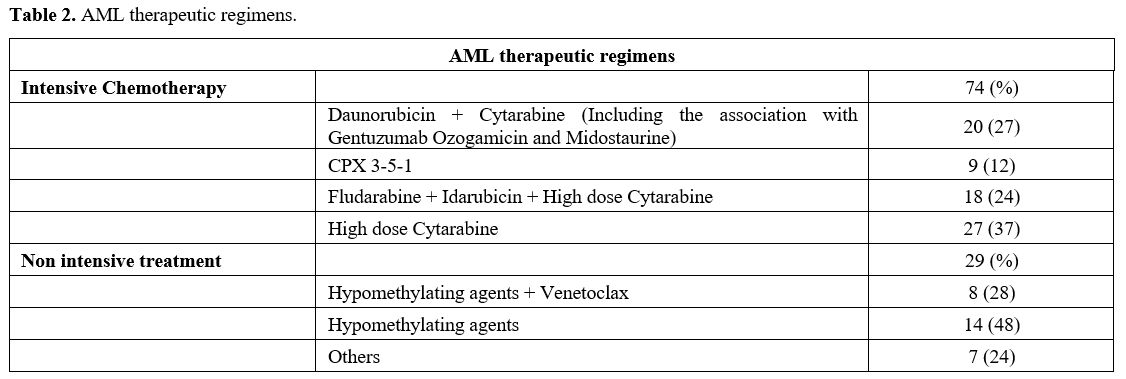 |
Table 2. AML therapeutic regimens.
|
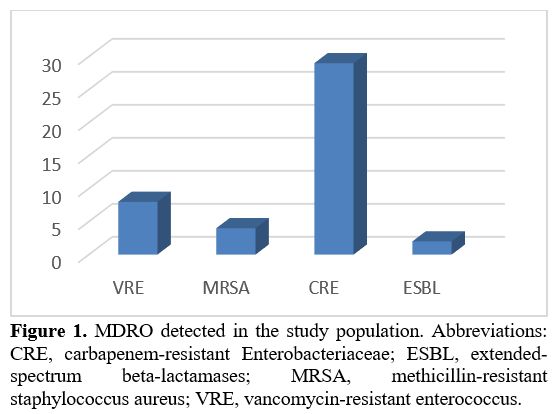 |
Figure 1. MDRO detected
in the study population. Abbreviations: CRE, carbapenem-resistant
Enterobacteriaceae; ESBL, extended-spectrum beta-lactamases; MRSA,
methicillin-resistant staphylococcus aureus; VRE, vancomycin-resistant
enterococcus.
|
BSI
was observed in 33 patients (25%): 8 (24%) had MDROrelBSI (see below),
13 (39%) from GRAM + Vancomycin sensitive bacteria, 3 (9%) from E.
Coli, 3 (9%) from K. Pneumoniae, 1 (3%) from P. Mirabilis, 1 (3%) from
E. Faecium and 2 (6%) from MDRO not detected in culture swabs: E.
Faecium VRE and P. Aeruginosa CRE. Seven patients (21%) required oxygen
therapy, 4 patients (12%) inotropic support; the median length of
hospitalization was 34 days.
BSI was more frequent in colonized
than non-colonized patients [12 (34%) vs. 21 (22%); p=0.1] and
correlated with length of hospitalization (p=0.01).
Eight of 33
patients developed MDROrel BSI (23% of colonized patients; 6 K.
Pneumoniae CRE; 2 E. Faecium VRE); 1 patient required oxygen therapy
(12.5%), and 1 patient required inotropic support (12.5%); the median
length of hospitalization was 48 days. MDROrel BSI correlated with
mucositis (p=0.008) and length of hospitalization (p=0.02).
Patients
presented FUO in 68 admissions (51%); 6 patients (9%) required oxygen
therapy, 2 patients (3%) inotropic support; the median length of
hospitalization was 29 days. We found a correlation with active
treatment (p=0.02), neutropenia (ANC<500μ/L p=0.002, days of
ANC<500μ/L p=0.001, >10 days of ANC <500μ/L p=0.007, ANC
<100μ/L p=0.02) and days of hospitalization (p=0.001); FUO was also
more common in colonized then non-colonized patients, even not reaching
statistical significance [22 (63%) vs. 46 (47%); p=0.1]; in colonized
patients, FUO was not reflected in a worse 60 days outcome (Figure 2). The relations between FUO, BSI and MDRO are shown in figure 3. In multivariate analysis, ANC<500μ/L remained an independent factor significantly associated with FUO (p=0.04).
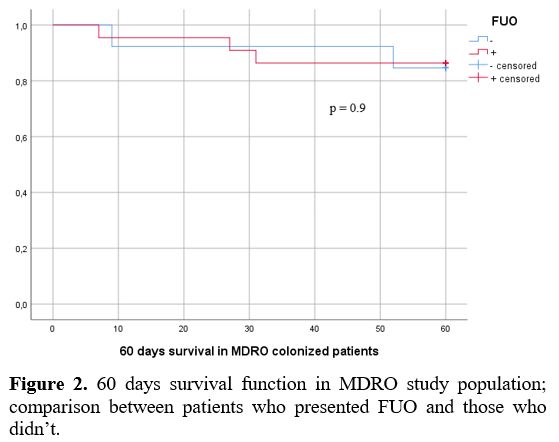 |
Figure 2. 60 days survival
function in MDRO study population; comparison between patients who
presented FUO and those who didn’t. |
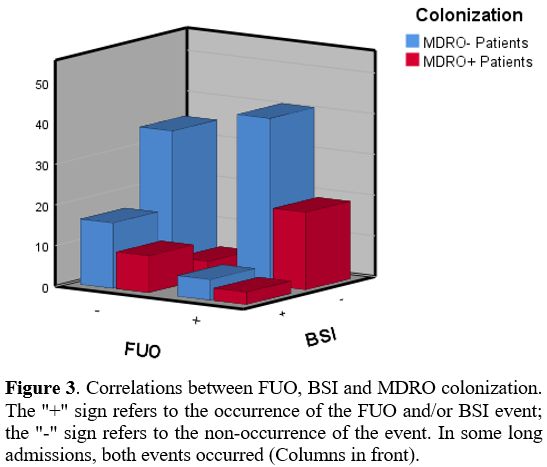 |
Figure 3. Correlations
between FUO, BSI and MDRO colonization. The "+" sign refers to the
occurrence of the FUO and/or BSI event; the "-" sign refers to the
non-occurrence of the event. In some long admissions, both events
occurred (Columns in front).
|
The
severity of the febrile event was higher in BSI than in FUO [in terms
of requirement of oxygen therapy (21% vs. 9%, p= 0.1) and of the
requirement of inotropic support (12% vs. 3%, p=0.08)]. In comparison,
we found no differences between BSI from bacteria not previously
detected in culture swabs and MDROrel BSI [requirement of oxygen
therapy 24% vs. 12.5%, p= 0.6; in terms of requirement of inotropic
support 12% vs. 12.5%, p=1].
Mucositis correlated with MDRO
colonization and MDROrel BSI (see above), LDH (p=0.02), Hb (p=0.03),
days of ANC<500μ/L (p=0.003, >10 days of ANC <500μ/L p=0.01,
days of ANC <100μ/L p=0.003, days of hospitalization (p<0.001),
type of therapy [intensive chemotherapy 20 (27%); non-intensive
treatment 4 (14%); support care 2 (7%); p=0.04); in multivariate
analysis only days of hospitalization remained an independent variable
significantly associated with mucositis (p=0.01).
We then carried
out an outcome analysis: 11/69 patients (16%) died or were referred to
end-of-life care at 30 days from admission, whereas 15/69 patients
(22%) at 60 days. Nine patients died during the admission, 7 of whom
from non-infectious causes (all at 30 days) and 2 because of infections
(both at 60 days, from pneumonia). No patients died because of BSI.
Death
or the referral to end-of-life cares, at 30 and 60 days, correlated
with age (p=0.02 and p=0.006), ECOG (both p<0.001), BSI (p=0.006 and
p=0.003), type of treatment (both p<0.001), LDH (p=0.02 and
p=0.009).
In multivariate analysis, ECOG (p=0.02 and p=0.01) and
BSI (p=0.01 and p=0.005) remained independent significantly associated
factors.
Furthermore, patients who underwent intensive
chemotherapy were categorized as those admitted to receiving induction
(29 patients, 39%), consolidation (29 patients, 39%), or salvage (16
patients, 22%). We detected a lower incidence of mucositis among the
consolidation group (45% vs. 7% vs. 31%, p=0.005) and, although not
reaching the statistical significance, a higher incidence of sepsis in
the salvage group (17% vs. 17% vs. 44%, p=0.08); a higher incidence of
FUO was observed in the induction and salvage group (69% vs. 41% vs.
62%, p=0.09). There were no differences in MDRO colonization across the
3 groups (28% vs. 17% vs. 37%, p=0.3).
Discussion
Given the great impact of nosocomial infections in the management of AML, several studies[10,11] have focused on this topic, whereas only a few authors analyzed the features and role of MDRO colonization.[3,12-14]
Ballo et al. studied a cohort of AML patients undergoing induction
intensive chemotherapy in Frankfurt, Germany; the colonization rate was
41% with a high prevalence of VRE (74%), while CRE colonization
correlated with an inferior outcome.[3] In the same
institution, Scheich et al. found, in a cohort of AML patients
undergoing HSCT, a colonization rate of 54%, mainly from VRE, and a
lower 5-year overall survival in the MDRO-colonized population.[14]
Jaiswal et al. observed, in a cohort of hematological patients in New
Delhi, a high incidence of CRE colonization in those with AML (65%)
and, among colonized patients, the diagnosis of AML resulted in being a
risk factor for infection-related mortality.[13] A
large multicentric Italian study considering a heterogeneous pool of
hematological patients detected, in the AML subgroup, a colonization
rate of 6%, with a large prevalence of CRE and ESBL and lower incidence
of colonization at the onset of disease or during induction than in
consolidation or salvage therapy.[12]
Our
population shares similar characteristics with the previous two
studies, with a high percentage of Colonization by CRE and a low by VRE
(Figure 1). These data are in
accordance with the epidemiological literature, which showed great
variability between geographic areas, and, in recent years, a trend of
increasing GRAM-MDRO and a higher prevalence of CRE in South-East vs.
North-West Europe.[15,16] Furthermore, these
differences may have been exacerbated by the heterogeneity of the
category of patients examined: to the best of our knowledge, the
present study is the first to focus on MDRO colonization in AML
patients, receiving both intensive and non-intensive treatments and in
phases different from induction.
These peculiarities allowed us
to observe a high MDRO colonization persistence rate during
hospitalizations (64%), which could explain a lower survival in the
long term and after HSCT, as highlighted by Ballo et al. and Scheich et
al.[3,14]
No impact on
short-term outcomes was found; the reason is likely ascribed to the
prompt use of targeted antibiotic therapy in case of fever in colonized
patients. BSI, on the other hand, although not a direct cause of
mortality, was found to correlate independently with an early dismal
outcome. This was due to the delay in the resumption of antileukemic
therapy due to the infectious episode and worsening of the patients’
clinical condition.
The evaluation of the impact of MDRO
colonization on mortality cannot be separated from an analysis of FP
(carried out by Ballo et al.[3]). This topic is
central to a long-lasting debate dealing with the risk of the expanding
antibiotic resistance and decreased efficacy of subsequent antibiotic
therapy.[17,18]
Recently, Castanon et al.
published the results of a comparison of two cohorts of AML patients
undergoing intensive chemotherapy. In cohort one, microbiological
screening was not routinely performed, and FP was at the treating
physician's discretion; in cohort two, both FP and microbiological
screening were carried out. No differences were found in the incidence
of infections during the induction phase between the 2 cohorts.
However, during the consolidation phase, there was an increase in
infections of GRAM- bacteria in cohort 1 and of GRAM+ bacteria in the
cohort 2.
Moreover, a significant decrease in deaths secondary to
infections and overall mortality was observed in cohort 2. Of note,
there were no differences in the incidence of FUO between the two
cohorts.[19]
In this study, it is hard to
distinguish the contribution made by bacteriologic screening, which
allowed targeted antibiotic therapy to be instituted, and FP. In the era
of microbiologic surveillance, FP cost-effectiveness, its impact on the
incidence of MDRO colonization, and the occurrence of FP-associated
resistance remain unsolved medical needs.
Although not reaching
statistical significance in multivariate analysis, an association of
MDRO colonization with oral mucositis emerged. This finding, along with
the evidence of a link between alteration of the gastrointestinal
microbiome and infectious complications,[20,21]
suggests that mucositis could promote MDROrel BSI and MDRO
colonization. Such an assumption appears even more realistic based on a
recent meta-analysis showing the protective effect of anti-mucositis
treatment on bacterial colonization in patients developing this
complication after chemo-radiotherapy.[22]
Indeed,
detecting anal abscesses in two patients colonized by CRE made us
hypothesize that MDRO colonization is not only the consequence of an
altered mucosal barrier but also the cause.
In our series, we
found a correlation between mucositis and type of therapy [Intensive
chemotherapy 20 (27%); non-intensive treatment 4 (14%); support care 2
(7%); p=0.04; in line with literature data, indicating a mucositis
incidence of 20-40% in patients receiving standard chemotherapy and
<5% receiving CPX-351[23-25]]. However, this is
not reflected in the correlation between the type of therapy and MDRO
colonization (p=0.2). Therefore, other factors, such as personal
hygiene and previous dental conditions, probably play a role.
From
our analysis, increased length of admission appears to be the common
denominator of MDRO colonization and FUO (both variables independently
correlated with days of hospitalization). In particular, the
relationship between hospitalization and MDRO colonization may reflect
a "chicken-or-the-egg” dilemma. Fever in colonized patients requires
longer therapy and greater precautions than in non-colonized patients;
on the other hand, a longer hospitalization places the patient at risk
of Colonization by MDRO. Curiously, Ballo et al., in a cohort of AML
patients undergoing induction chemotherapy, found no significant
differences between the length of hospitalization in colonized and
non-colonized patients.[3] This discrepancy may be due
to the greater heterogeneity of the population examined in our study
and the different strains of MDROs detected (higher prevalence of CRE
in our population, correlated with a high risk of life-threatening
infections).[3]
The incidence of FUO in AML
patients ranges between 15 and 100% depending on the treatment phase
and type of chemotherapy. Despite improvements in diagnostic
techniques, there is no evidence of a downward trend over the years.[26-30]
The etiology of this phenomenon may be traced back to the inflammatory
state induced by the disease, the precise mechanisms of which are still
partially unknown.[31] It is conceivable that FUO
arises in a condition of bone marrow activation/inflammation sustained
by the chemotherapeutic intervention, with the concomitancy of
neutropenia. In this condition, bone marrow is the target of endogenous
and/or exogenous stimuli that, acting similarly to granulocyte-colony
stimulating factor, can cause fever.[32]
As we
expected, the severity of the febrile event (in terms of the
requirement of oxygen therapy and inotropic support) was higher in BSIs
than in FUO cases; it is also likely that a proportion of the FUO
cases, presumably the most severe ones, were misdiagnosed BSI.
Furthermore, despite the more complex drug-resistance profile of
bacteria, MDROrelBSIs presented a prognosis similar to the BSIs from a
bacteria undetected by culture swabs; this is due to the prompt use of
the correct antibiotic therapy through a de-escalation approach which,
in a fragile population such as AML patients at high risk of infection
(because of the Colonization by MDRO) is the best strategy. At the same
time, no evidence exists for such an approach when no pathogen is
identified.[7]
A useful biomarker in framing the
febrile episode, unfortunately not available in our patients, is
procalcitonin, which accurately identifies infections and correlates
with the severity of BSI.[33-35] The positivity of
this index without any finding on blood cultures could raise suspicion
of a misdiagnosed infection; moreover, procalcitonin-guided management
of febrile patients in intensive care units led to decreased antibiotic
use and reduced mortality.[36,37] The only
prospective trial of a procalcitonin-based decision-making approach
carried out in hematologic patients did not bring the hoped-for
effects, showing any significant differences in antibiotic use.[38]
Of note, the population examined was small (60 patients, randomized
1:1) and included different types of hematologic malignancies.[38]
Larger trials with more stringent selection criteria are needed to
assess the efficacy and safety of this approach in clinical practice.
Conclusions
MDRO
colonization is a frequent and difficult-to-eradicate complication in
AML patients that can arise at all treatment stages, affecting
long-term outcomes. Prompt discharge of patients as soon as clinical
conditions allow may limit the spread of this phenomenon.
FUO
needs to be a better-understood event, with adequate management still
waiting to identify the underlying causes. An in-depth elucidation of
the contributors to FUO occurrence is critical to optimize antibiotic
use and minimize hospitalization length. These achievements are
necessary to tackle antibiotic resistance and limit health costs.[39]
The
retrospective nature of this analysis, the small size of the population
under investigation, and its heterogeneity are the study's main
limitations. Larger studies are needed to confirm these data and put in
place proper measures to reduce the risk of MDRO colonization.
Compliance with Ethical Standards
All
procedures performed in studies involving human participants were in
accordance with the ethical standards of the institutional research
committee and with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum
FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017
ELN recommendations from an international expert panel. Blood. 2017
Jan;129(4):424-47. https://doi.org/10.1182/blood-2016-08-733196 PMid:27895058 PMCid:PMC5291965
- Palmieri
R, Paterno G, De Bellis E, Mercante L, Buzzatti E, Esposito F, et al.
Therapeutic choice in older patients with acute myeloid leukemia: A
matter of fitness. Cancers (Basel). 2020;12(1):1-19. https://doi.org/10.3390/cancers12010120 PMid:31906489 PMCid:PMC7016986
- Ballo
O, Tarazzit I, Stratmann J, Reinheimer C, Hogardt M, Wichelhaus TA, et
al. colonization with multidrug resistant organisms determines the
clinical course of patients with acute myeloid leukemia undergoing
intensive induction chemotherapy. PLoS One. 2019;14(1):e0210991. https://doi.org/10.1371/journal.pone.0210991 PMid:30673776 PMCid:PMC6343922
- Rice
LB. Federal funding for the study of antimicrobial resistance in
nosocomial pathogens: no ESKAPE. Vol. 197, The Journal of Infectious Diseases. United States; 2008. p. 1079-81. https://doi.org/10.1086/533452 PMid:18419525
- Bronzwaer
SLAM, Cars O, Buchholz U, Mölstad S, Goettsch W, Veldhuijzen IK, et al.
A European study on the relationship between antimicrobial use and
antimicrobial resistance. Emerg Infect Dis. 2002 Mar;8(3):278-82. https://doi.org/10.3201/eid0803.010192 PMid:11927025 PMCid:PMC2732471
- Hyde
TB, Gay K, Stephens DS, Vugia DJ, Pass M, Johnson S, et al. Macrolide
resistance among invasive Streptococcus pneumoniae isolates. JAMA. 2001
Oct;286(15):1857-62. https://doi.org/10.1001/jama.286.15.1857 PMid:11597287
- Averbuch
D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, et al.
European guidelines for empirical antibacterial therapy for febrile
neutropenic patients in the era of growing resistance: summary of the
2011 4th European Conference on Infections in Leukemia. Vol. 98,
Haematologica. 2013. p. 1826-35. https://doi.org/10.3324/haematol.2013.091025 PMid:24323983 PMCid:PMC3856957
- Heinz
WJ, Buchheidt D, Christopeit M, von Lilienfeld-Toal M, Cornely OA,
Einsele H, et al. Diagnosis and empirical treatment of fever of unknown
origin (FUO) in adult neutropenic patients: guidelines of the
Infectious Diseases Working Party (AGIHO) of the German Society of
Hematology and Medical Oncology (DGHO). Ann Hematol. 2017
Nov;96(11):1775-92. https://doi.org/10.1007/s00277-017-3098-3 PMid:28856437 PMCid:PMC5645428
- Villa
A, Vollemans M, De Moraes A, Sonis S. Concordance of the WHO, RTOG, and
CTCAE v4.0 grading scales for the evaluation of oral mucositis
associated with chemoradiation therapy for the treatment of oral and
oropharyngeal cancers. Support care cancer Off J Multinatl Assoc
Support Care Cancer. 2021 Oct;29(10):6061-8. https://doi.org/10.1007/s00520-021-06177-x PMid:33788003
- Kolonen
A, Sinisalo M, Huttunen R, Syrjänen J, Aittoniemi J, Huhtala H, et al.
Bloodstream infections in acute myeloid leukemia patients treated
according to the Finnish Leukemia Group AML-2003 protocol - a
prospective nationwide study. Infect Dis (London, England).
2017;49(11-12):799-808. https://doi.org/10.1080/23744235.2017.1347814 PMid:28683646
- Malik
IA, Cardenas-Turanzas M, Gaeta S, Borthakur G, Price K, Cortes J, et
al. Sepsis and Acute Myeloid Leukemia: A Population-Level Study of
Comparative Outcomes of Patients Discharged From Texas Hospitals. Clin
Lymphoma Myeloma Leuk. 2017 Dec;17(12):e27-32. https://doi.org/10.1016/j.clml.2017.07.009 PMid:28844403
- Cattaneo
C, Di Blasi R, Skert C, Candoni A, Martino B, Di Renzo N, et al.
Bloodstream infections in haematological cancer patients colonized by
multidrug-resistant bacteria. Ann Hematol. 2018 Sep;97(9):1717-26. https://doi.org/10.1007/s00277-018-3341-6 PMid:29705860
- Jaiswal
SR, Gupta S, Kumar RS, Sherawat A, Rajoreya A, Dash SK, et al. Gut
Colonization with Carbapenem-resistant Enterobacteriaceae Adversely
Impacts the Outcome in Patients with Hematological Malignancies:
Results of A Prospective Surveillance Study. Mediterr J Hematol Infect
Dis. 2018;10(1):e2018025. https://doi.org/10.4084/mjhid.2018.025 PMid:29755703 PMCid:PMC5937952
- Scheich
S, Lindner S, Koenig R, Reinheimer C, Wichelhaus TA, Hogardt M, et al.
Clinical impact of colonization with multidrug-resistant organisms on
outcome after allogeneic stem cell transplantation in patients with
acute myeloid leukemia. Cancer. 2018 Jan;124(2):286-96. https://doi.org/10.1002/cncr.31045 PMid:28960264
- Tatarelli
P, Mikulska M. Multidrug-resistant bacteria in hematology patients:
emerging threats. Future Microbiol. 2016 Jun;11:767-80. https://doi.org/10.2217/fmb-2015-0014 PMid:27196948
- Mikulska
M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, et al.
Aetiology and resistance in bacteraemias among adult and paediatric
haematology and cancer patients. J Infect. 2014 Apr;68(4):321-31. https://doi.org/10.1016/j.jinf.2013.12.006 PMid:24370562
- Wingard
JR, Eldjerou L, Leather H. Use of antibacterial prophylaxis in patients
with chemotherapy-induced neutropenia. Curr Opin Hematol. 2012
Jan;19(1):21-6. https://doi.org/10.1097/MOH.0b013e32834da9bf PMid:22080847
- Cometta
A, Calandra T, Bille J, Glauser MP. Escherichia coli resistant to
fluoroquinolones in patients with cancer and neutropenia. Vol. 330, The
New England journal of medicine. United States; 1994. p. 1240-1. https://doi.org/10.1056/NEJM199404283301717 PMid:8139646
- Castañón
C, Fernández Moreno A, Fernández Verdugo AM, Fernández J, Martínez
Ortega C, Alaguero M, et al. The Value of Adding Surveillance Cultures
to Fluoroquinolone Prophylaxis in the Management of Multiresistant Gram
Negative Bacterial Infections in Acute Myeloid Leukemia. J Clin Med.
2019 Nov;8(11). https://doi.org/10.3390/jcm8111985 PMid:31731650 PMCid:PMC6912560
- Galloway-Peña
JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG, et
al. The role of the gastrointestinal microbiome in infectious
complications during induction chemotherapy for acute myeloid leukemia.
Cancer. 2016 Jul;122(14):2186-96. https://doi.org/10.1002/cncr.30039 PMid:27142181 PMCid:PMC5574182
- Galloway-Peña
JR, Shi Y, Peterson CB, Sahasrabhojane P, Gopalakrishnan V, Brumlow CE,
et al. Gut Microbiome Signatures Are Predictive of Infectious Risk
Following Induction Therapy for Acute Myeloid Leukemia. Clin Infect Dis
an Off Publ Infect Dis Soc Am. 2020 Jun;71(1):63-71. https://doi.org/10.1093/cid/ciz777 PMid:31436833 PMCid:PMC7312220
- Yang
C, Gong G, Jin E, Han X, Zhuo Y, Yang S, et al. Topical application of
honey in the management of chemo/radiotherapy-induced oral mucositis: A
systematic review and network meta-analysis. Int J Nurs Stud. 2019
Jan;89:80-7. https://doi.org/10.1016/j.ijnurstu.2018.08.007 PMid:30352321
- Dreizen
S, McCredie KB, Keating MJ. Chemotherapy-induced oral mucositis in
adult leukemia. Postgrad Med. 1981 Feb;69(2):103-108,111-112. https://doi.org/10.1080/00325481.1981.11715676 PMid:7454641
- Lee
Y-H, Hong J, Kim I, Choi Y, Park H-K. Prospective evaluation of
clinical symptoms of chemotherapy-induced oral mucositis in adult
patients with acute leukemia: A preliminary study. Clin Exp Dent Res.
2020 Feb;6(1):90-9. https://doi.org/10.1002/cre2.253 PMid:32067405 PMCid:PMC7025998
- Chiche
E, Rahmé R, Bertoli S, Dumas P-Y, Micol J-B, Hicheri Y, et al.
Real-life experience with CPX-351 and impact on the outcome of
high-risk AML patients: a multicentric French cohort. Blood Adv. 2021
Jan;5(1):176-84. https://doi.org/10.1182/bloodadvances.2020003159 PMid:33570629 PMCid:PMC7805314
- Kern
W, Behre G, Rudolf T, Kerkhoff A, Grote-Metke A, Eimermacher H, et al.
Failure of fluconazole prophylaxis to reduce mortality or the
requirement of systemic amphotericin B therapy during treatment for
refractory acute myeloid leukemia: results of a prospective randomized
phase III study. German AML Cooperative Group. Cancer. 1998
Jul;83(2):291-301. https://doi.org/10.1002/(SICI)1097-0142(19980715)83:2<291::AID-CNCR13>3.0.CO;2-O
- Link
H, Freund M, Diedrich H, Wilke H, Austein J, Henke M, et al.
Mitoxantrone, cytosine arabinoside, and VP-16 in 36 patients with
relapsed and refractory acute myeloid leukemia. Haematol Blood
Transfus. 1990;33:322-5. https://doi.org/10.1007/978-3-642-74643-7_60 PMid:2182426
- Palmieri
S, Sebastio L, Mele G, Annunziata M, Annunziata S, Copia C, et al.
High-dose cytarabine as consolidation treatment for patients with acute
myeloid leukemia with t(8;21). Leuk Res. 2002 Jun;26(6):539-43. https://doi.org/10.1016/S0145-2126(01)00177-1 PMid:12007501
- Gil
L, Styczynski J, Komarnicki M. Infectious complication in 314 patients
after high-dose therapy and autologous hematopoietic stem cell
transplantation: risk factors analysis and outcome. Infection. 2007
Dec;35(6):421-7. https://doi.org/10.1007/s15010-007-6350-2 PMid:17926001
- Hämäläinen
S, Kuittinen T, Matinlauri I, Nousiainen T, Koivula I, Jantunen E.
Neutropenic fever and severe sepsis in adult acute myeloid leukemia
(AML) patients receiving intensive chemotherapy: Causes and
consequences. Leuk Lymphoma. 2008 Mar;49(3):495-501. https://doi.org/10.1080/10428190701809172 PMid:18297526
- Loizidou A, Aoun M, Klastersky J. Fever of unknown origin in cancer patients. Crit Rev Oncol Hematol. 2016 May;101:125-30. https://doi.org/10.1016/j.critrevonc.2016.02.015 PMid:26995082
- Kawano
Y, Fukui C, Shinohara M, Wakahashi K, Ishii S, Suzuki T, et al.
G-CSF-induced sympathetic tone provokes fever and primes antimobilizing
functions of neutrophils via PGE2. Blood. 2017 Feb;129(5):587-97. https://doi.org/10.1182/blood-2016-07-725754 PMid:27827823
- Yang
M, Choi SJ, Lee J, Lee DG, Kim Y-J, Park Y-J, et al. Serum
procalcitonin as an independent diagnostic marker of bacteremia in
febrile patients with hematologic malignancies. PLoS One.
2019;14(12):e0225765. https://doi.org/10.1371/journal.pone.0225765 PMid:31821331 PMCid:PMC6903763
- Gac
A-C, Parienti J-J, Chantepie S, Fradin S, Le Coutour X, Leclercq R, et
al. Dynamics of procalcitonin and bacteremia in neutropenic adults with
acute myeloid leukemia. Leuk Res. 2011 Oct;35(10):1294-6. https://doi.org/10.1016/j.leukres.2011.05.035 PMid:21831426
- Moustafa
R, Albouni T, Aziz G. The role of procalcitonin and presepsin in the
septic febrile neutropenia in acute leukemia patients. PLoS One.
2021;16(7):e0253842. https://doi.org/10.1371/journal.pone.0253842 PMid:34324506 PMCid:PMC8321513
- Bouadma
L, Luyt C-E, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of
procalcitonin to reduce patients' exposure to antibiotics in intensive
care units (PRORATA trial): a multicentre randomised controlled trial.
Lancet (London, England). 2010 Feb;375(9713):463-74. https://doi.org/10.1016/S0140-6736(09)61879-1 PMid:20097417
- de
Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et
al. efficacy and safety of procalcitonin guidance in reducing the
duration of antibiotic treatment in critically ill patients: a
randomised, controlled, open-label trial. Lancet Infect Dis. 2016
Jul;16(7):819-27. https://doi.org/10.1016/S1473-3099(16)00053-0 PMid:26947523
- Lima
SSS, Nobre V, de Castro Romanelli RM, Clemente WT, da Silva Bittencourt
HN, Melo ACM, et al. Procalcitonin-guided protocol is not useful to
manage antibiotic therapy in febrile neutropenia: a randomized
controlled trial. Ann Hematol. 2016 Jun;95(7):1169-76. https://doi.org/10.1007/s00277-016-2639-5 PMid:27118539
- Macedo-Viñas
M, De Angelis G, Rohner P, Safran E, Stewardson A, Fankhauser C, et al.
Burden of meticillin-resistant Staphylococcus aureus infections at a
Swiss University hospital: excess length of stay and costs. J Hosp
Infect. 2013 Jun;84(2):132-7. https://doi.org/10.1016/j.jhin.2013.02.015 PMid:23608003
[TOP]




