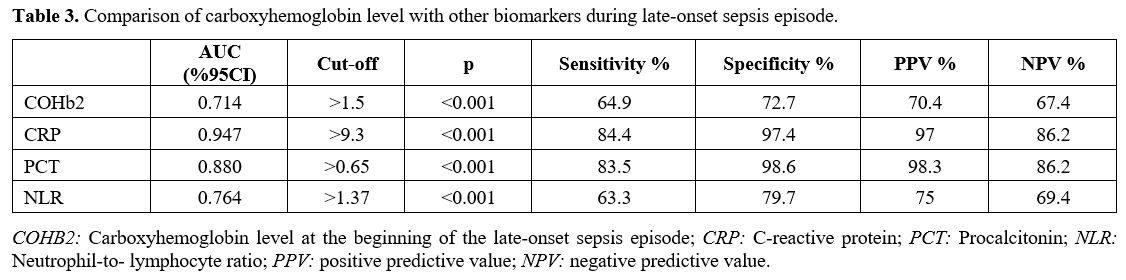
Participants. All infants that were <37 weeks of gestational age born during the study period required NICU admission and were evaluated for inclusion in the study. The LOS group included neonates with culture-proven sepsis after 72h of life. The control group included preterm infants born in our hospital during the same period who met the inclusion criteria and were infection free. In addition, neonates with any of the following were excluded: (1) proven or suspected hemolytic disease, (2) pulmonary hypertension requiring NO therapy, (3) use of therapeutic cooling, (4) presence of major congenital and chromosomal anomalies, (5) incomplete laboratory data or any episode of early-onset sepsis (Figure 1).
 |
|
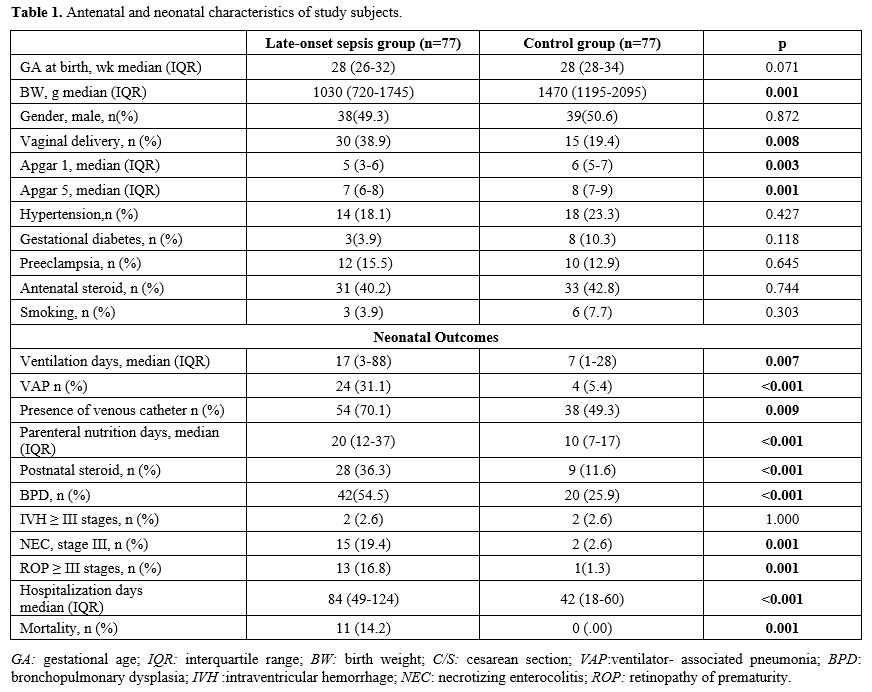
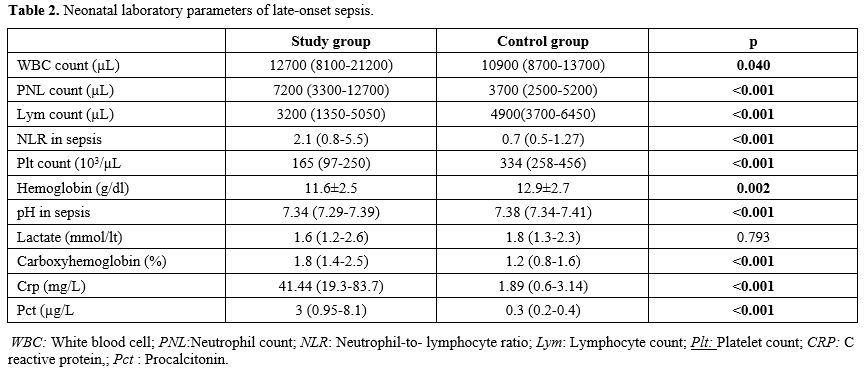
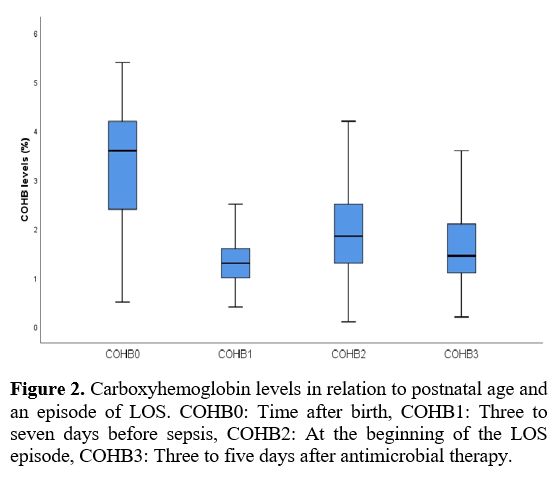
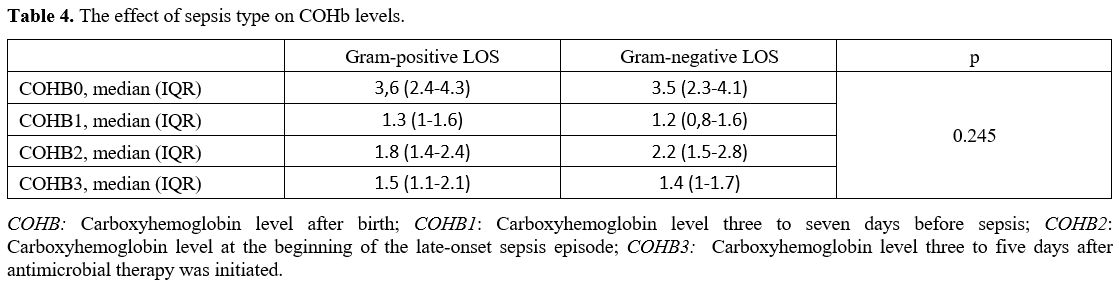
Data collection and definitions. Data were extracted from patients' records. Demographic, perinatal, and neonatal characteristics were recorded and analyzed. Positive blood culture was a prerequisite for LOS. Two positive blood cultures were required for the diagnosis of coagulase-negative staphylococci sepsis. A fully automated BACTEC method (BACT/ALERT 3D system, bioMerieux, SA, France) was used to analyze blood culture isolates. The blood gas analyzer (ABL 800 FLEX, Radiometer, Copenhagen, Denmark) used for the measurement of COHb was calibrated daily. COHb levels were determined from blood sampling that was performed using custom-prepared heparinized containers (Clinitubes, Radiometer, Copenhagen, Denmark), as only a small amount of blood was required (35-55µl). COHb levels are a percentage (%) of total hemoglobin levels. Four-time points were used for assessing COHb levels in the LOS group: one during NICU admission after birth (COHB0); one three to seven days before sepsis (COHB1); one at the beginning of the LOS episode (6h before or 24h after blood culture samples were obtained in the presence of clinical signs) (COHB2); and one three to five days after antimicrobial therapy was initiated (COHB3) (Treatment group). Since the median day for the onset of sepsis was 19 in the LOS group, the COHb level in the control group was assessed to coincide with the first day of the septic attack.
Neonatal morbidities included: intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), and retinopathy of prematurity (ROP). Severe IVH included stages ≥ III, classified according to Volpe's cranial ultrasound classification.[8] BPD was defined according to oxygen requirement and ventilator support at 36 weeks of gestation.[9] Severe ROP included stages ≥3 or any stage requiring cryotherapy or laser photocoagulation as classified by standardized international criteria.[10] Bell criteria were used to define severe NEC.[11]
Outcome Measures. The primary outcome measure was the change in COHb levels. Secondary outcome measurements included the presence of a venous catheter, ventilator-associated pneumonia (VAP), postnatal steroids, parenteral nutrition days, duration of ventilation, BPD, NEC, ROP, IVH, hospitalization days, and mortality.
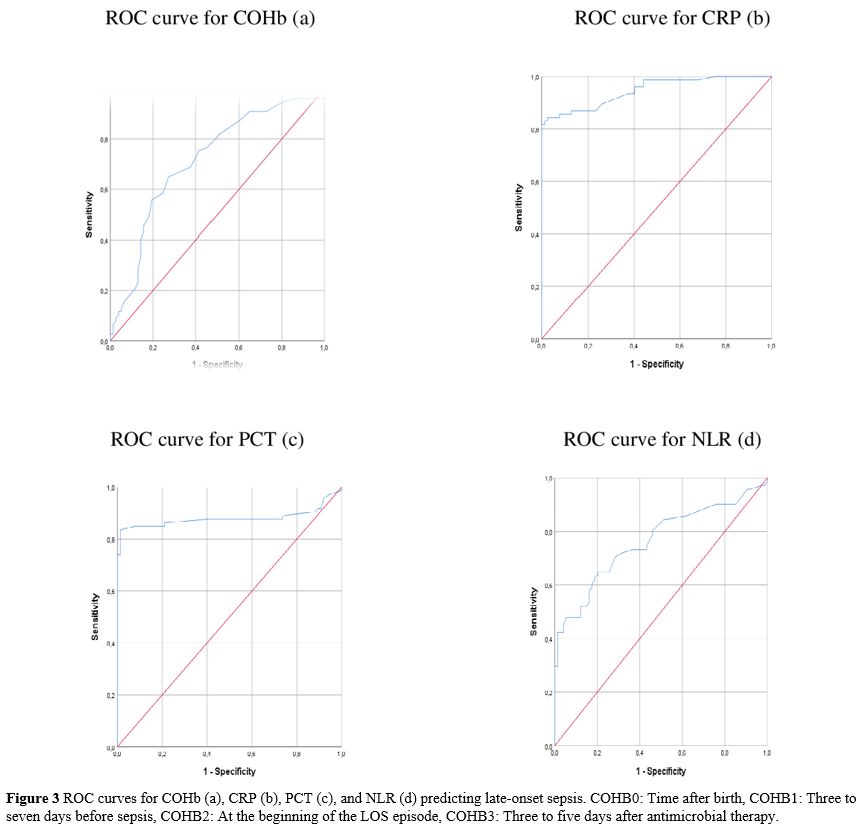
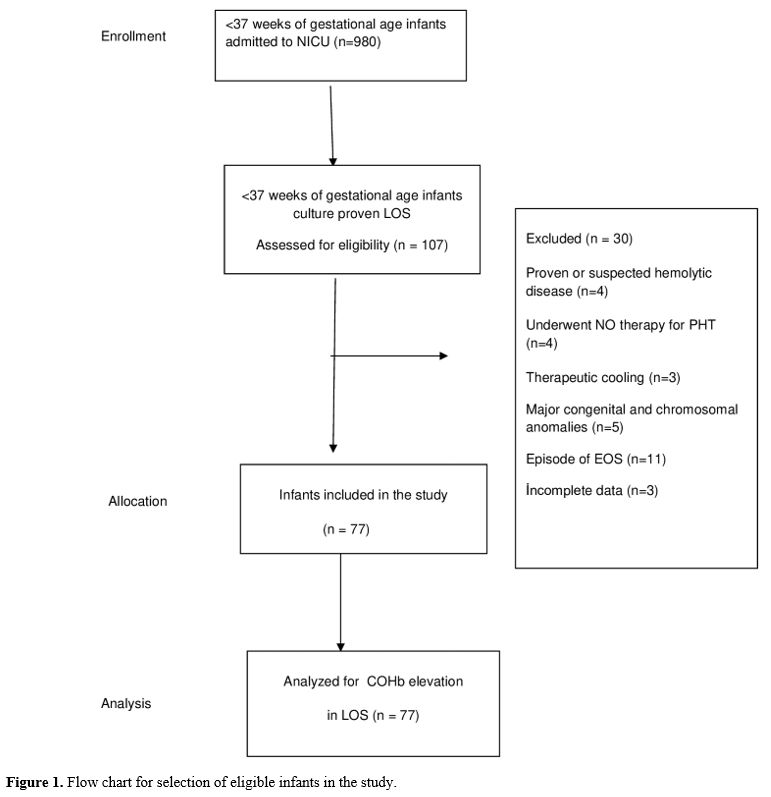
Statistical analysis. "IBM SPSS Statistics for Windows" (IBM Corp. Released 2017, Version 25.0. Armonk, NY, USA) was used for statistical analysis, and "G*Power" version 3.1.9.4 was used to compute the sample size. The sample size was calculated as 142 when an alpha of 0.1, an effect size of 0.432, and a power of 0.90 were used. The Pearson chi-square test was used to compare categorical variables reported as n (%). Continuous variables were expressed using the mean, standard deviation (SD), or medians and ranges (min-max). The normality of data for continuous variables was tested with the Kolmogorov-Smirnov test and compared with either unpaired Student's t-test or the Mann-Whitney U test. The Spearman analysis evaluated the correlation between continuous variables. Repeated Measures Analysis was used to compare the variation in multiple measured values between groups. Receiver operating characteristic (ROC) analysis was used to investigate the COHb values in predicting LOS. The sensitivity, specificity, positive likelihood ratio (+LR), and negative likelihood ratio (-LR) ratio were calculated for the ideal cut-off value. Cut-off values showed the highest sensitivity. Statistical significance was considered when p<0.05 for all tests.