Pulsoni A1, Assanto GM1, Salvatori M1, Musiu P1, Luise C1, Passucci M1, Lapietra G1, Pappalardo L.1, Annechini G1, D’Elia GM1, Martelli M1, Del Giudice I1.
Hematology, Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
Correspondence to:
Prof. Prof. Alessandro Pulsoni, Hematology, Sapienza University of
Rome, via Benevento 6, 00161 Italy. Email:
alessandro.pulsoni@uniroma1.it
Published: March 1, 2023
Received: December 14, 2022
Accepted: February 19, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023018 DOI
10.4084/MJHID.2023.018
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Although
exhibiting high response rates and prolonged survival, follicular
lymphoma (FL) is still considered an incurable disease.[1]
Therefore, the conventional approach currently relies on delaying
therapy until necessary, avoiding side effects and long-term
toxicities.[2-4] Indeed, treatment is limited to symptomatic patients fulfilling the GELF criteria.[5] Furthermore, conventional chemo-immunotherapy does not allow disease eradication.[1] Therefore, new targeted therapies have attempted to change the disease course in the current era.
Several large cohort studies report a minority (from 20% to 40%) of
long-term surviving FL patients who maintain clinical response for 10
or 15 years and may be considered “cured”.[2-3,7]
We conducted a retrospective single-institution analysis on FL patients
with progression-free survival (PFS) longer than ten years from the
last treatment (very long responders - VLR), describing the clinical
and histological characteristics at onset, front-line treatment, and
treatment response. We included in the study also a subgroup of FL
patients who achieved a prolonged PFS even after one or more relapses,
analyzing the treatment strategies which allowed such an outcome.
Our purpose was to identify possible distinctive clinical features or
treatment approaches of FL patients who turned out to be cured,
defining a subset of patients who could be candidates for disease
eradication.
Across twenty years (1992-2012), we identified 99 patients affected by
FL that achieved a PFS of at least ten years after the first (n= 71) or
subsequent treatment lines (n= 28), representing about 12% of the newly
diagnosed patients, estimated about 800 in the same time frame.
Data were collected from the retrospective revision of clinical files.
The median follow-up was 169 months (range 120-303). The median age at
diagnosis was 53 years (range 25-75). Patients’ baseline
characteristics are reported in table 1. The distribution of clinical features in this cohort of VLRs was not different from large cohorts reported in the literature.[2,7,9]
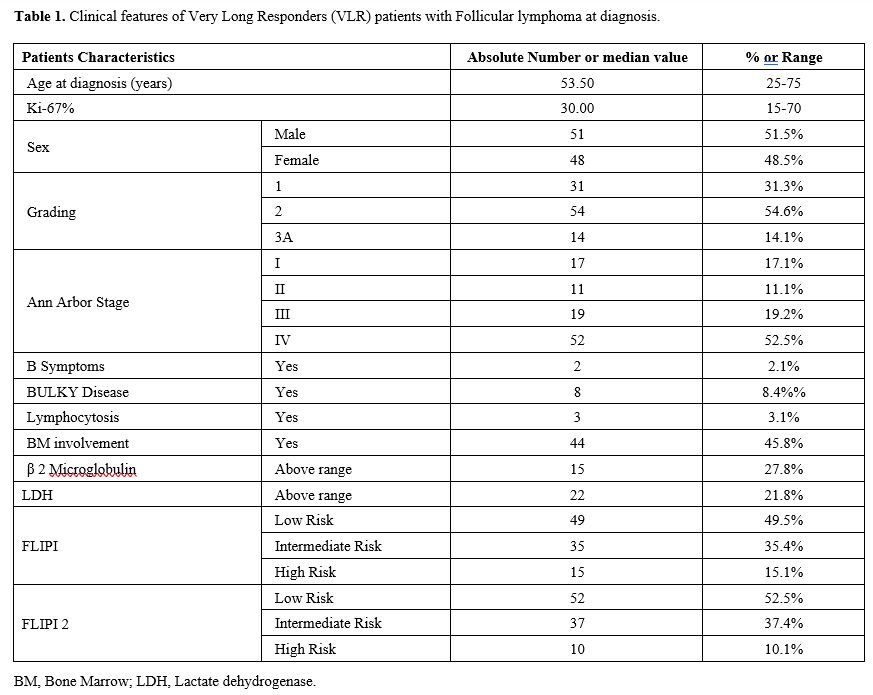 |
- Table
1. Clinical features of Very Long Responders (VLR) patients with Follicular lymphoma at diagnosis.
|
Among
VLRs after front-line treatment (n=71), 31 patients (43.6%) received
immune-chemotherapy (mostly R-CHOP), 22 (31%) chemotherapy alone, 2
(2.8%) rituximab alone, and 16 (22.5%) received radiotherapy alone.
Twenty-eight patients became VLRs after the second or subsequent lines
of treatment: 19 achieved a long-lasting response after the second line
of therapy, 4 after the third line, 3 after the fourth line, including
allogeneic stem cell transplantation, and 2 after the fifth line.
Overall, consolidation/maintenance was administered to a minority of
patients, given the time frame of data collection. Treatment
characteristics are reported in table 2.
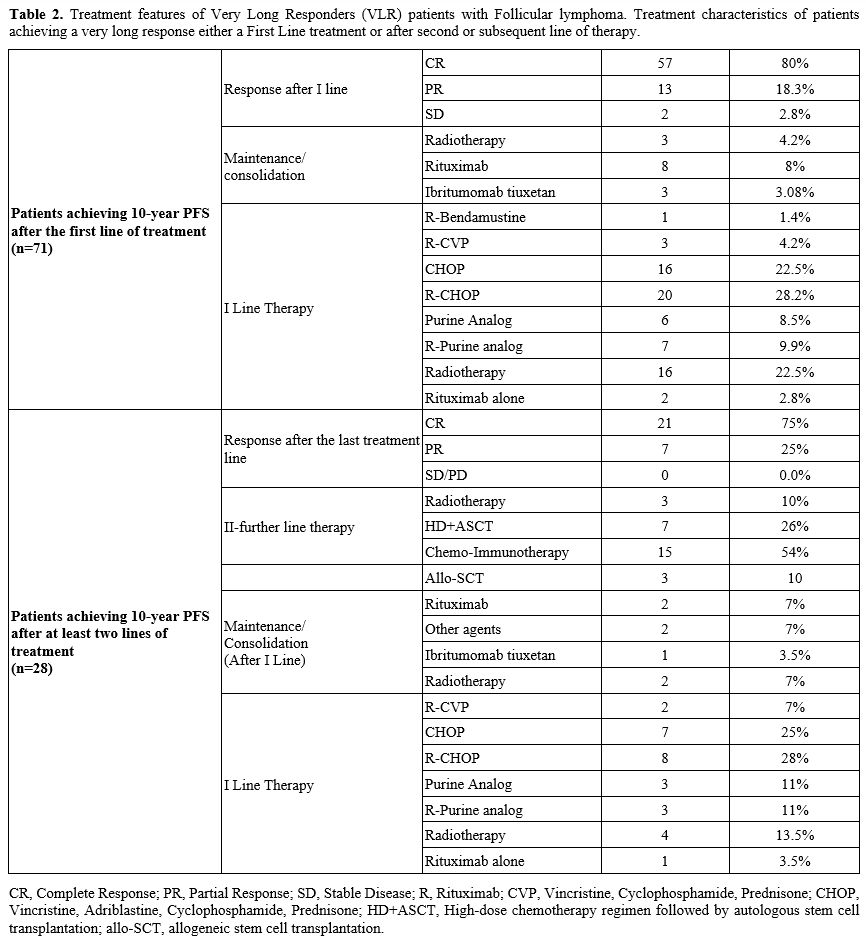 |
- Table 2. Treatment
features of Very Long Responders (VLR) patients with Follicular
lymphoma. Treatment characteristics of patients achieving a very long
response either a First Line treatment or after second or subsequent
line of therapy..
|
Considering
the type of response achieved after the first line or subsequent lines
of treatment that lasted at least 10 years, a CR was documented in
79.7% of patients (79/99) and a PR in 20.2% (20/99). Interestingly,
13.1% (13 out of 99) considered in PR after first-line were found to be
VLRs without further treatment. Given the extended follow-up of this
cohort, the metabolic response was assessed only in 13 patients (9 CR
and 4 PR).
At the time of writing, all patients are disease-free after more than
ten years from the last treatment. Seventy-three percent of patients
(71/99) still maintain a response after the first line.
As expected, a higher proportion of FL achieved the “cure” after
chemo-immunotherapy received in the first line (82% vs. 57%, p 0.036);
similarly, patients obtaining CR exhibit a higher probability of being
VLR (77% vs. 65% p 0.001).
In our cohort, the VLR outcome was not strictly treatment-dependent and
was achievable within any stage, histology subtype, and FLIPI risk
category. Unfortunately, it was impossible to determine the clinical
characteristics of a control cohort, including all other patients
treated in the same time frame.
Long-term follow-up of large trials confirmed the favorable outcome of
patients with advanced-stage FL treated with chemo-immunotherapy[3-4] and have shown the possibility of achieving PFS>10 years up to 40% of FL patients.[2]
Moreover, in the post-rituximab era, several new therapeutic agents
have been developed to further improve the prognosis of FL patients,
leading to better overall response (ORR) and CR rates and longer PFS.
Therefore, the philosophical approach of physicians towards FL might be
changed, as addressed by Jonathan Friedberg with the biggest question:
can we cure this disease?[10]
The recently published 13-year update of the multicenter randomized
GITMO-IIL trial compared the outcome of 134 high-risk FL patients aged
<60 years after first-line therapy with high-dose chemotherapy with
rituximab and autograft (R-HDS) versus conventional chemotherapy with
rituximab (CHOP-R). The study showed significant superiority of PFS in
the R-HDS arm (59.1% and 28.8%, respectively), while no statistical
differences in OS were found (64.5% vs. 68.5%, respectively).[2]
Nevertheless, the higher eradicative potential of R-HDS + ASCT could be
considered aiming at disease eradication. A portion of our VLR patients
after the second or subsequent treatment line achieved this result
after ASCT (7/28).
Allogeneic SCT is rarely employed in FL; nevertheless, in our
experience, it allowed disease eradication in 3 patients after three
treatment lines.
Long-term follow-ups of trials, including novel agents, are awaited.
Surely, the landscape of disease monitoring has changed; we have
potential new tools to monitor the quality of response, predict
patients’ outcomes and possibly support the clinician in identifying
candidates to be long-term survival. Recent studies demonstrated a high
prognostic value of the end-induction PET scan, a predictor of better
survival and superior PFS.[6-9,11,14]
Unfortunately, in this series, only a few patients underwent the
metabolic assessment of disease response. The proportion of
false-positive PR observed by CT in our series would probably have been
reduced.
Basal biological and clinical features can predict a favorable outcome:
many studies showed the prognostic role of total metabolic tumor volume
(TMTV) and total-lesion-glycolysis in patients with FL as strong
predictors of PFS and OS.[13,14] Even the role of baseline SUVmax as an independent PFS predictor is debated.[7,10] In the experience of Rossi et al., the rate of POD24 events was related to different SUVmax and mutational burden.[6-7]
Minimal residual disease (MRD)-driven treatment approaches are
currently under investigation in FL. In the MIRO phase II trial for
early-stage FL, the first results showed that the addition of
immunotherapy (with anti-CD20 MoAb) to RT induced a negative MRD in
more than 90% of the patients with BCL2/IGH rearrangement at baseline
who remain MRD+ after RT.[13] In the FOLL12 study,
for advanced FL, MRD positivity after induction treatment and over the
follow-up was associated with a higher risk of relapse, suggesting the
importance of a combined PET/MRD-based approach.[12]
Long-term follow-up of trials combining PET assessment, reliable MRD
monitoring, and lymphoma-associated mutations could allow defining a
better risk stratification for outcome prediction, which could also
identify candidates to be “cured”. Moreover, we must mention the new
agents, such as bispecific monoclonal antibodies and chimeric antigen
receptor T-cells (CAR-T), which could furtherly be included in
treatment plans aiming at disease eradication.[15-16]
The suggestion in 2023 is that in a subset of young and fit patients
with FL, the treatment strategy could move from disease control and
treatment of symptomatic disease to a curative approach. To achieve
this goal, some suggestions could be:
- To treat patients with low tumor burden disease,
giving preference to new agents as an alternative to chemotherapy for
specific subgroups.[12-16]
- To monitor treatment response with metabolic and MRD assessment.[12-14,17]
- To evaluate an MRD-driven consolidation, including
new agents, in patients considered eligible for disease eradication.[13-16]
We think it is time to design a prospective clinical trial with long-term follow-up, which could consider these points.
References
- Bachy E, Seymour JF, Feugier P, Offner F, López-Guillermo A, Belada
D, Xerri L, Catalano JV, Brice P, Lemonnier F, Martin A, Casasnovas O,
Pedersen LM, Dorvaux V, Simpson D, Leppa S, Gabarre J, da Silva MG,
Glaisner S, Ysebaert L, Vekhoff A, Intragumtornchai T, Le Gouill S,
Lister A, Estell JA, Milone G, Sonet A, Farhi J, Zeuner H, Tilly H,
Salles G. Sustained Progression-Free Survival Benefit of Rituximab
Maintenance in Patients With Follicular Lymphoma: Long-Term Results of
the PRIMA Study. J Clin Oncol. 2019 Nov 1;37(31):2815-2824. doi:
10.1200/JCO.19.01073. Epub 2019 Jul 24.
https://doi.org/10.1200/JCO.19.01073 PMid:31339826
PMCid:PMC6823890
- Bruna R, Benedetti F, Boccomini C, Patti C,
Barbui AM, Pulsoni A, Musso M, Liberati AM, Gini G, Castellino C,
Rossini F, Ciceri F, Rota-Scalabrini D, Stelitano C, Di Raimondo F,
Tucci A, Devizzi L, Zoli V, Zallio F, Narni F, Dondi A, Parvis G,
Semenzato G, Lanza F, Perrone T, Angrilli F, Billio A, Gueli A, Mantoan
B, Rambaldi A, Gianni AM, Corradini P, Passera R, Ladetto M, Tarella C.
Prolonged survival in the absence of disease-recurrence in
advanced-stage follicular lymphoma following chemo-immunotherapy:
13-year update of the prospective, multicenter randomized GITMO-IIL
trial. Haematologica. 2019 Nov;104(11):2241-2248.
https://doi.org/10.3324/haematol.2018.209932 PMid:31666344
PMCid:PMC6821615
- Rummel MJ, Niederle N, Maschmeyer G, Banat
GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M,
Balser C, Kaiser U, Weidmann E, Dürk H, Ballo H, Stauch M, Roller F,
Barth J, Hoelzer D, Hinke A, Brugger W; Study group indolent Lymphomas
(StiL). Bendamustine plus rituximab versus CHOP plus rituximab as
first-line treatment for patients with indolent and mantle-cell
lymphomas: an open-label, multicentre, randomised, phase 3
non-inferiority trial. Lancet. 2013 Apr 6;381(9873):1203-10.
https://doi.org/10.1016/S0140-6736(12)61763-2 PMid:23433739
- Luminari
S, Ferrari A, Manni M, Dondi A, Chiarenza A, Merli F, Rusconi C,
Tarantino V, Tucci A, Vitolo U, Kovalchuk S, Angelucci E, Pulsoni A,
Arcaini L, Angrilli F, Gaidano G, Stelitano C, Bertoldero G, Cascavilla
N, Salvi F, Ferreri AJM, Vallisa D, Marcheselli L, Federico M.
Long-Term Results of the FOLL05 Trial Comparing R-CVP Versus R-CHOP
Versus R-FM for the Initial Treatment of Patients With Advanced-Stage
Symptomatic Follicular Lymphoma. J Clin Oncol. 2018 Mar
1;36(7):689-696. https://doi.org/10.1200/JCO.2017.74.1652
PMid:29095677
- Brice P., Bastion Y., Lepage E., Brousse N.,
Haioun C., Moreau P., Straetmans N., Tilly H., Tabah I., Solal-Céligny
P. Comparison in low-tumor-burden follicular lymphomas between an
initial no-treatment policy, prednimustine, or interferon alfa: A
randomized study from the Groupe d'Etude des Lymphomes Folliculaires.
Groupe d'Etude des Lymphomes de l'Adulte. J. Clin. Oncol.
1997;15:1110-1117. doi: 10.1200/JCO.1997.15.3.1110.
https://doi.org/10.1200/JCO.1997.15.3.1110 PMid:9060552
- Rossi
C, Tosolini M, Gravelle P, Pericart S, Kanoun S, Evrard S, Gilhodes J,
Franchini DM, Amara N, Syrykh C, Bories P, Oberic L, Ysebaert L, Martin
L, Ramla S, Robert P, Tabouret-Viaud C, Casasnovas RO, Fournié JJ,
Bezombes C, Laurent C. Baseline SUVmax is related to tumor cell
proliferation and patient outcome in follicular lymphoma.
Haematologica. 2022 Jan 1;107(1):221-230. doi:
10.3324/haematol.2020.263194.
https://doi.org/10.3324/haematol.2020.263194 PMid:33327711
PMCid:PMC8719066
- Hiddemann W, Barbui AM, Canales MA, Cannell
PK, Collins GP, Dürig J, Forstpointner R, Herold M, Hertzberg M,
Klanova M, Radford J, Seymour JF, Tobinai K, Trotman J, Burciu A,
Fingerle-Rowson G, Wolbers M, Nielsen T, Marcus RE. Immunochemotherapy
With Obinutuzumab or Rituximab for Previously Untreated Follicular
Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy
and Safety. J Clin Oncol. 2018 Aug 10;36(23):2395-2404. doi:
10.1200/JCO.2017.76.8960. Epub 2018 Jun 1. https://doi.org/10.1200/JCO.2017.76.8960 PMid:29856692
- Armitage
JO, Weisenburger DD. New approach to classifying non-Hodgkin's
lymphomas: clinical features of the major histologic subtypes.
Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998
Aug;16(8):2780-95. doi:
10.1200/JCO.1998.16.8.2780. https://doi.org/10.1200/JCO.1998.16.8.2780
PMid:9704731
- Federico M, Bellei M, Marcheselli L, Luminari
S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran
P, Cortelazzo S, Martinelli G, Martelli M, Rigacci L, Arcaini L, Di
Raimondo F, Merli F, Sabattini E, McLaughlin P, Solal-Céligny P.
Follicular lymphoma international prognostic index 2: a new prognostic
index for follicular lymphoma developed by the international follicular
lymphoma prognostic factor project. J Clin Oncol. 2009 Sep
20;27(27):4555-62. doi: 10.1200/JCO.2008.21.3991. Epub 2009 Aug 3.
https://doi.org/10.1200/JCO.2008.21.3991 PMid:19652063
- Friedberg
JW. Toward a Cure for Follicular Lymphoma. Hematol Oncol Clin North Am.
2020 Aug;34(4):xiii-xiv. doi: 10.1016/j.hoc.2020.05.001. Epub 2020 May
20. https://doi.org/10.1016/j.hoc.2020.05.001 PMid:32586582
- Assanto
GM, Ciotti G, Brescini M, et al. High Basal Maximal Standardized Uptake
Value (SUVmax) in Follicular Lymphoma Identifies Patients with a Low
Risk of Long-Term Relapse. Cancers (Basel). 2021;13(12):2876. Published
2021 Jun 9. doi:10.3390/cancers13122876
https://doi.org/10.3390/cancers13122876 PMid:34207518
PMCid:PMC8227030
- Pulsoni A., Tosti ME, Ferrero S, Luminari
S, Liberati AM at al; EARLY STAGE Follicular Lymphoma: First Results of
the FIL "Miro" Study, a Multicenter Phase II Trial Combining Local
Radiotherapy and MRD-Driven Immunotherapy. Blood 2019; 134
(Supplement_1): 124.
- Ladetto M, Ferrero S, Del Giudice I,
Galimberti S, et al; A Comprehensive and Systematic Analysis of Minimal
Residual Disease (MRD) Monitoring in Follicular Lymphoma: Results from
the Fondazione Italiana Linfomi (FIL) FOLL12 Trial. Blood 2021; 138
(Supplement 1) https://doi.org/10.1182/blood-2021-146773
- Major
A, Hammes A, Schmidt MQ, Morgan R, Abbott D, Kamdar M. Evaluating Novel
PET-CT Functional Parameters TLG and TMTV in Differentiating Low-grade
Versus Grade 3A Follicular Lymphoma. Clin Lymphoma Myeloma Leuk. 2020
Jan;20(1):39-46. doi: 10.1016/j.clml.2019.09.609. Epub 2019 Sep
28. https://doi.org/10.1016/j.clml.2019.09.609 PMid:31761712
PMCid:PMC9040515
- Jacobson CA, Chavez JC, Sehgal AR, William
BM, Munoz J, Salles G, Munshi PN, Casulo C, Maloney DG, de Vos S,
Reshef R, Leslie LA, Yakoub-Agha I, Oluwole OO, Fung HCH, Rosenblatt J,
Rossi JM, Goyal L, Plaks V, Yang Y, Vezan R, Avanzi MP, Neelapu SS.
Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin
lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet
Oncol. 2022 Jan;23(1):91-103. doi: 10.1016/S1470-2045(21)00591-X. Epub
2021 Dec 8. https://doi.org/10.1016/S1470-2045(21)00591-X
PMid:34895487
- Budde LE, Assouline S, Sehn LH, Schuster SJ,
Yoon SS, Yoon DH, Matasar MJ, Bosch F, Kim WS, Nastoupil LJ, Flinn IW,
Shadman M, Diefenbach C, O'Hear C, Huang H, Kwan A, Li CC, Piccione EC,
Wei MC, Yin S, Bartlett NL. Single-Agent Mosunetuzumab Shows Durable
Complete Responses in Patients With Relapsed or Refractory B-Cell
Lymphomas: Phase I Dose-Escalation Study. J Clin Oncol. 2022 Feb
10;40(5):481-491. doi: 10.1200/JCO.21.00931. Epub 2021 Dec
16. https://doi.org/10.1200/JCO.21.00931 PMid:34914545 PMCID: PMC8824395.
- Giudice
ID, Starza ID, Foà R. Does MRD have a role in the management of iNHL?
Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):320-330.
doi: 10.1182/hematology.2021000312. https://doi.org/10.1182/hematology.2021000312
PMid:34889425 PMCid:PMC8791119
[TOP]

