We conducted a retrospective study of adult patients undergoing HSCT (allogeneic and autologous) during the year 2021 at the Hospital Universitario de la Princesa and who received at least one dose of the SARS-CoV2 vaccine. According to our service protocol, patients received vaccination in the third-month post hematologic transplantation. An analysis was made of the main demographic characteristics of the transplanted patients, as well as the type of transplant and its indication. Regarding vaccination data, the type of vaccine received, the time elapsed since HSCT to vaccination, and the time of development and degree of neutropenia were included. Early vaccination was defined as vaccination before 100 days post-transplant.
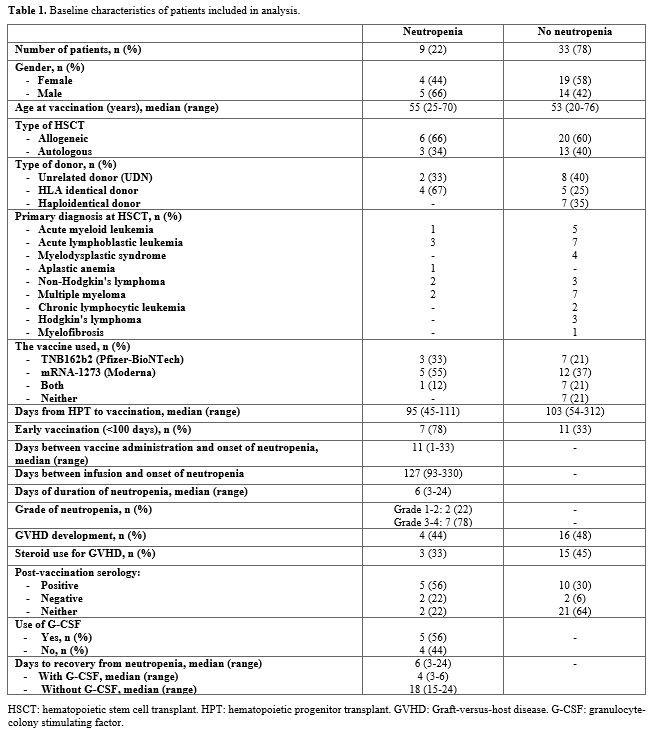
Forty-two patients who underwent autologous (n=16) or allogeneic (n=26) HSCT were analyzed. 22% presented an episode of neutropenia after vaccination for SARS-CoV2. We analyzed the group of patients who presented neutropenia compared with the rest of the patients (Table 1). The median age of both groups was similar (55 vs 53 years). In both groups, the most commonly used vaccine was mRNA-1273 (Moderna), followed by BNT162b2 (Pfizer). In our work we reviewed the post-vaccination serology of all post-HPT patients, a total of 35.7% of them presented positive serologies. Of the group that developed neutropenia, 7 patients had positive serology and 2 had negative serology. Of the 7 who presented positive serology, 6 were vaccinated early (before 100 days) post-HPT. The median number of days between HSCT and vaccination was similar between both groups (95 days vs. 103 days), with a higher percentage of neutropenia (78%) in the early vaccinated population (before 100 days post-HSCT).
 |
|
The highest percentage of cases of neutropenia developed among patients undergoing allogeneic HSCT, particularly in those with HLA-identical donors. Among the group that presented neutropenia, the median number of days between vaccination and the appearance of neutropenia was 11 days, with a median duration of 6 days. Most patients presented grade 3-4 neutropenia. 9 patients (56%) received treatment with granulocyte-colony stimulating factor (G-CSF), with early recovery of neutrophil numbers. Of the total of patients who developed post-vaccination neutropenia, 3 patients were receiving low-dose corticosteroids for the treatment of mild GVHD. None of the cases that developed neutropenia had CMV infection.
In our series of patients, early vaccination (administration before 100 days post-HSCT) seems to be related to the appearance of neutropenia in a proportion of cases, although of short duration. The percentage of patients with neutropenia represented about 21% of the cases, of which 78% were grade 3-4.
Neutropenia after SARS-CoV-2 vaccination in HSCT has been reported in a few previous studies. Ali et al (2021), published the results of a study of 113 allogeneic hematopoietic progenitor transplant recipients, 13.3% of the total cases presented neutropenia after vaccination.[6] Similarly, the study published by Ram et al in 2021 in which they included 80 patients, described that 12% of patients developed some cytopenia after the administration of the first dose and 10% developed cytopenia after the second dose.[7] Of the total number of patients, 4 (5%) developed neutropenia, 1 of grade 3-4. None of the studies reported whether cytopenias were related to early administration of the vaccines.
Although rare, cytopenias were also reported in healthy individuals, and are thought to be related to the presence of preformed antibodies against certain vaccine components or to altered hematologic cell production as part of the systemic inflammatory response following vaccination. The latter explanation seems the most consistent, given that most of the patients presented a spontaneous recovery.[4,5] As early post-HSCT vaccination (≤3 months) seems to be associated with a poor response, and it might be complicated by transient although severe neutropenia, it seems reasonable to postpone SARS-CoV-2 to later times of HSCT.