Riccardo Paggi1, Francesca Mariotti1, Jessica Mencarini1,2, Silvia Bresci2, Irene Campolmi2, Filippo Bartalesi1,2, Beatrice Borchi2, Luca Nassi3, Benedetta Sordi3,4, Alessandro Maria Vannucchi4 and Alessandro Bartoloni1,2.
1 Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
2 Infectious and Tropical Diseases Unit, Careggi University Hospital, Florence, Italy.
3 Hematology Unit, Careggi University Hospital, Florence, Italy.
4
Center for Innovation and Research in Myeloproliferative Neoplasms,
Hematology Unit, Careggi University Hospital, University of Florence,
Florence, Italy.
Correspondence to:
Riccardo Paggi. Department of Experimental and Clinical Medicine,
University of Florence, Florence, Italy. E-mail:
paggi.riccardo@gmail.com
Published: May 1, 2023
Received: January 31, 2023
Accepted: April 18, 2023
Mediterr J Hematol Infect Dis 2023, 15(1): e2023028 DOI
10.4084/MJHID.2023.028
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
use of specific inhibitory drugs of intracellular signalling pathways
(such as BrutonKinase inhibitors) for the treatment of Waldenström's
macroglobulinaemia (WM) is a recognised risk factor for Aspergillus spp.
infections. The overlapping clinical manifestations of the two
diseases may require the involvement of different medical specialities.
We describe the clinical course of a patient with pulmonary and
encephalic aspergillosis, with concomitant orbital WM involvement,
a rare localisation of the disease: the case required a
multidisciplinary approach to define the ocular lesions and an in-depth
study of the literature, in which approximately twentycases of
lymphoplasmacytic lymphoma with orbital localisation were reported.
|
Introduction
Waldenström
macroglobulinemia (WM) is a rare lymphoplasmacytic lymphoma (LPL)
belonging to the category of Non-Hodgkin B Lymphomas (NHL) with an
indolent course, characterized by monoclonal immunoglobulin M (IgM)
protein hypersecretion.[1] The median age of diagnosis is 70 years,[2] and the disease is much more common in the white population.[3]
Patients with WM can develop systemic symptoms (fever, weight loss,
night sweats), symptoms related to bone marrow infiltration (e.g.,
anemia, leukopenia, thrombocytopenia), lymphoid tissues involvement
(e.g., lymphadenopathy, hepatosplenomegaly) and IgM monoclonal proteins
(e.g., hyperviscosity, peripheral neuropathy, renal disturbances).[1]
Recurrent infections may also occur due to a relative decrease of other
immunoglobulin classes or as a consequence of treatment-induced
immunosuppression. Treatment is indicated in symptomatic patients,
firstly with anti-CD20 agents (e.g., rituximab) and chemotherapy, with
the possible use of drugs such as BTK (Bruton tyrosine kinase) –
inhibitors (e.g., ibrutinib, acalabrutinib or zanubrutinib) or
proteasome inhibitors (e.g. bortezomib).[4]
We briefly present a case description of a patient affected with WM and
treated with multiple lines, including ibrutinib. The clinical history
became particular after the occurrence of intraorbital lesions
requiring the involvement of a multidisciplinary approach in order to
establish a correct diagnosis and treatment.
Case Presentation
Clinical history.
The patient was a 71 years-old male, affected with symptomatic WM since
2000, previously treated with a CHOP-like chemotherapy followed by
rituximab consolidation in 2001. In 2006 he received rituximab and
chlorambucil for a first relapse; in 2011, for a second relapse, he
underwent treatment with rituximab, fludarabine, and cyclophosphamide
and in 2017 with rituximab and bendamustine for a subsequent
recurrence. For chronic obstructive pulmonary disease (COPD)
exacerbations, the patients experienced several hospitalizations since
2018 and started intravenous immunoglobulin support for secondary,
symptomatic hypogammaglobulinemia. The patient was also receiving
entecavir for chronic HBV infection. Due to the increase of IgM
protein, the presence of anemia, and the emergence of abdominal
lymphadenopathies, ibrutinib was started in June 2019: a complete bone
marrow (BM) evaluation was performed before starting the treatment,
showing 80% of clonal lymphoplasmacytic BM infiltration. Multiparameter
flow cytometry demonstrated a characteristic WM phenotype: CD19+,
CD22+, CD79b+, FMC7+, IgM+, monoclonal kappa (k) light chain surface
expression. MYD88 gene mutation was tested as well, resulting positive
for MYD88L265P mutation.
A partial response was then obtained, with the resolution of anemia,
lymphadenopathy reduction, and decreased IgM monoclonal protein. In
October 2020, the patient was admitted for symptomatic COVID-19
pneumonia, treated with remdesivir with rapid improvement.
Diagnosis of invasive aspergillosis. In December 2020, invasive pulmonary aspergillosis (IA) was diagnosed, according to sputum samples positive for Aspergillus flavus and A. fumigatus, bronchoalveolar lavage (BAL) Aspergillus spp. positive polymerase chain reaction (PCR) detected by polymerase chain reaction (PCR), BAL-sample's galactomannan
optical density index of 1.71, and chest high-resolution CT findings
consistent with the disease. Antifungal therapy with isavuconazole was
started in January 2021, improving the pulmonary lesions. Considering
the diagnosis, ibrutinib treatment was briefly interrupted and
restarted at a lower dose, considering the pharmacological interaction
with isavuconazole.
In March 2021, he was admitted to a peripheral hospital for an
epileptic crisis. A brainstem contrast-enhanced (CE) magnetic resonance
(MR) was performed, showing a 12 mm nodular lesion in the left parietal
lobe, weakly enhanced in T1-weighted (T1W) sequences and hypointense in
T2-weighted (T2W) sequences, with ring enhancement, and a similar 4 mm
finding in the right lobe. Additionally, the patient experienced a
likely ischemic stroke.
Ibrutinib was suspended for cerebral IA suspect, a lumbar puncture was
executed (microbiological samples resulted in negatives), and a
cerebral biopsy of the bigger lesion was performed after a month, with
evidence of fungal hyphae and spores and positive Aspergillus spp. PCR, confirming the encephalic fungal localization.
Laboratoristic progression of Waldenström macroglobulinemia.
In May 2021, he was admitted to our hospital for hepatic toxicity
related to isavuconazole, and antifungal therapy was switched firstly
to liposomal B amphotericin, then to voriconazole. Brain C.E. MR in May
2021 was substantially unchanged. In August 2021, the stability of the
radiological brain picture and improving lung imaging were confirmed
with an additional CT examination.
During the same period, considering the evidence of atypical
lymphocytes in blood smear and the serum levels of IgM 29.4 g/L (normal
values 0.4-2.3), BM biopsy (BMB) was performed, with evidence of
lymphoid interstitial infiltrate of 70-80% of cellularity, plasmacytoid
elements and monoclonal expression of k light chain and M heavy chain:
considering the absence of symptoms related to WM and the concomitant
IA, the patient did not start any treatment for WM.
Orbital infiltration - Initial work-up.
In September 2021, the patient was admitted for fever. For the evidence
of a slight lymphocytosis, a peripheral blood flow cytometry was
performed and showed the presence of a mature B lymphocyte population
(CD19+, CD22+, IgM+ CD23 -/+ and clonal expression of k light chain).
During hospitalization, the patient complained of bilateral
conjunctivitis: at the examination, bilateral nodules were palpable
under the eyebrow arch without pain, vision deficit, diplopia, or
corneal involvement. Orbit CT (Figure 1)
evidenced bilateral increased lacrimal glands (20x10 mm). Several (>
10) small and hyperdense nodularities (from 2 to 15 mm) were detected
bilaterally in the eyelid's soft tissues and intra- and extra-conical
endo-orbital areas. Nodular lesions were confirmed with an orbit CE MR (Figure 1),
iso/hypointense in T1W sequences, and hypointense in T2W, with contrast
enhancement. Similar findings were described in both maxillary sinuses
and ethmoidal lamina papyracea. The Serum IgM level was 46 g/L (normal
value: 0.4-2.3).
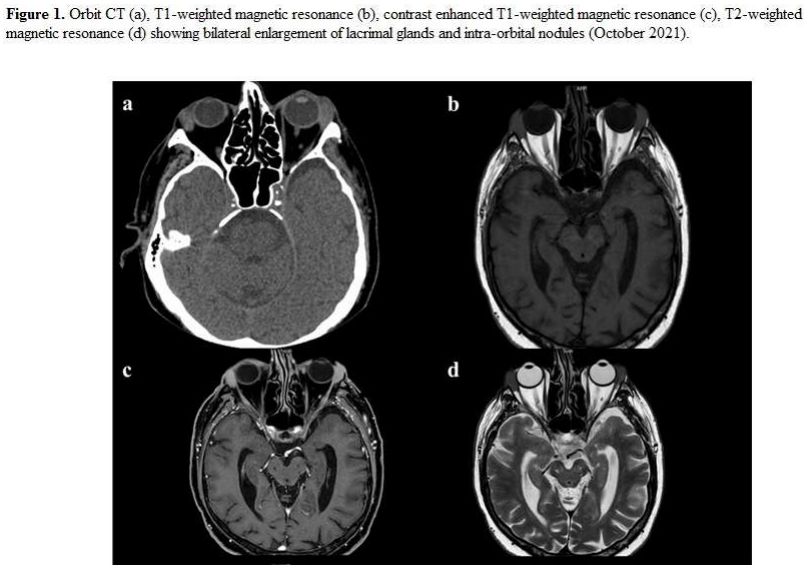 |
- Figure 1. Orbit CT (a), T1-weighted magnetic resonance (b), contrast enhanced T1-weighted magnetic resonance (c), T2-weighted
magnetic resonance (d) showing bilateral enlargement of lacrimal
glands and intra-orbital nodules (October 2021)
|
Differential Diagnosis. Orbital masses in the adult can occur in a wide range of diseases, including infections (e.g., Staphylococcus spp., Mycobacterium tuberculosis, Aspergillus spp.),
inflammatory diseases (e.g., IgG4-related sclerosing disease, systemic
amyloidosis), vascular lesions (e.g., venous and arteriovenous
malformations), benign (e.g. schwannoma, neurofibroma) and malignant
tumors (e.g., B-cell lymphoma, metastasis).[5]
Lacrimal gland lesions account for approximately 10% of all biopsied
orbital masses: the most common causes are inflammatory (as
IgG-4-related disease) or lymphoproliferative disorders, with potential
bilateral involvement.[6,7]
Considering the subacute onset of the manifestation (4 months since the
last brain MR) and that intraorbital lesions are uncommon but possible
in both IA and WM, we focused on the differential diagnosis between
these two pathological entities. Orbital presentation's main
differences are described in Table 1.
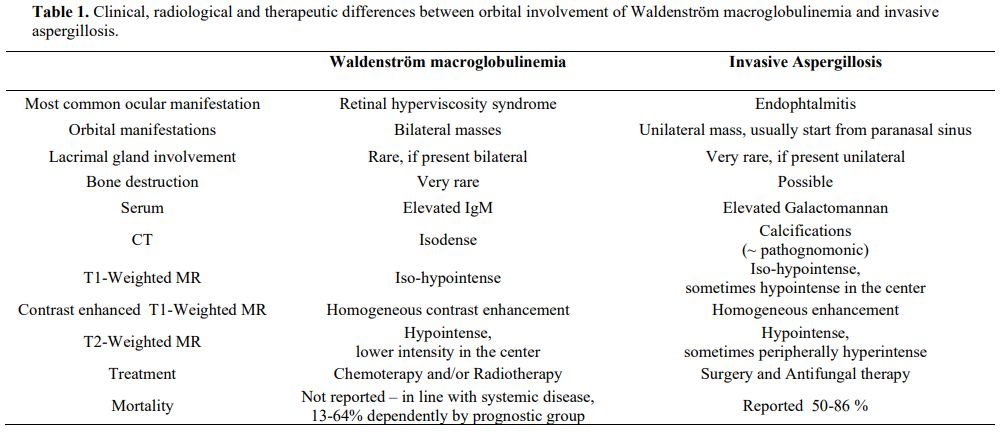 |
- Table
1. Clinical, radiological and therapeutic differences between orbital
involvement of Waldenström macroglobulinemia and invasive
aspergillosis.
|
Further Examinations. Considering IA pulmonary picture improvement and encephalic lesions stability after eight months of antifungal therapy (Figure 2)
and the evidence of WM progression at BMB with peripheral blood
involvement, after a multidisciplinary discussion, treatment with
bortezomib (a proteasome inhibitor) was started in October 2021. At the
same time, voriconazole was suspended, and the patient started
posaconazole prophylaxis. According to ophthalmological and
neuro-radiological evaluation, the patient was discharged with a
scheduled clinical and radiological follow-up. He initially reported
conjunctival chemosis reduction with decreased swelling, especially of
the right eye. Five cycles of bortezomib were administered until
February 2022. The patient was admitted in March 2022 for COPD
exacerbation, presenting worsening bilateral orbital edema (Figure 3):
decreasing pulmonary aspergillosis lesions were confirmed at CT exam,
in line with negative serum beta-D-glucan ad galactomannan, with no
evidence of IA encephalic radiologic worsening. However, MR examination
evidenced increased dimensions of known orbital lesions (Figure 4),
with a worsening picture of erosive foci in subcutaneous, maxillary,
and ethmoidal areas, diffused also in left frontal and sphenoidal
sinuses, mastoid and ethmoidal cells, bilaterally in nasal turbinates.
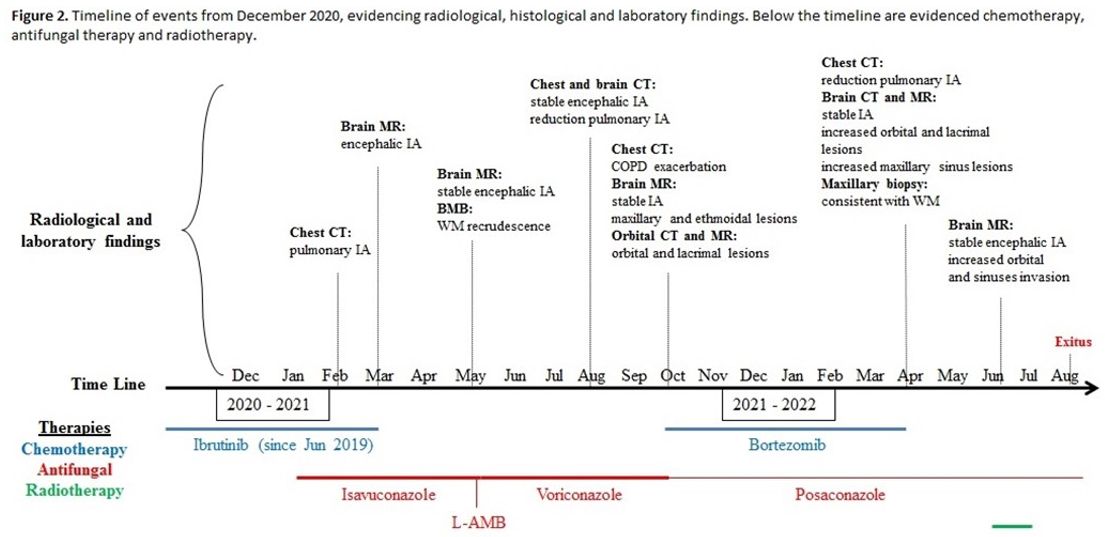 |
Figure 2. Timeline of
events from December 2020, evidencing radiological, histological and
laboratory findings. Below the timeline are evidenced chemotherapy. |
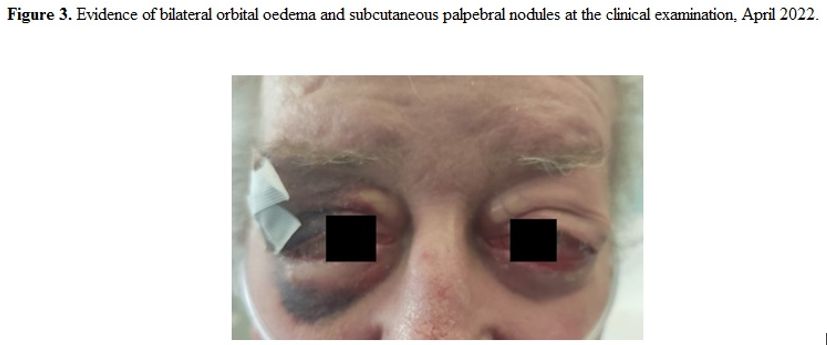 |
Figure 3. Evidence of bilateral orbital oedema and subcutaneus palpebral nodules at the clinical examination, April 2022. |
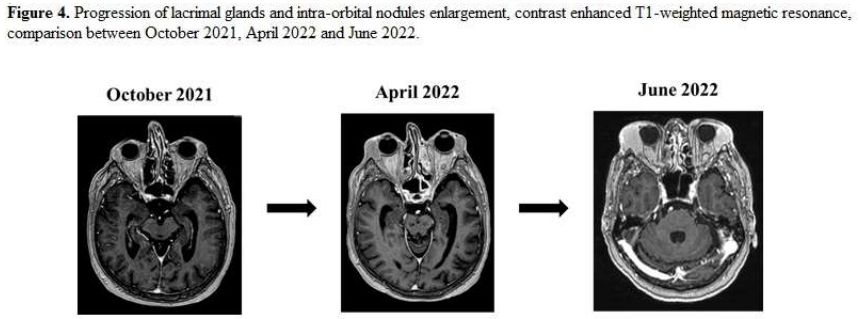 |
Figure 4. Progression of lacrimal glands and intra-orbital nodules enlargement, contrast enhanced T1-weighted magnetic resonance , comparison between October 2021, April 2022 and June 2022.
|
Final Diagnosis.
Considering the worsening clinical picture, according to maxillofacial
surgeons, palpebral and maxillary biopsies were performed. The latter
sample evidenced a picture consistent with LPL (lymphoid proliferation
with plasma cells expressing IgM). Additional therapeutic cycles with
bortezomib were not performed because of the increased infection risk
and the progressively worsening performance status. IgM levels were
reduced to 22.4 g/L (normal values 0.4-2.3).
Orbital infiltration
was confirmed as a progressive WM involvement; interestingly, only
eighteen cases in the literature from 1967 to nowadays report similar
findings (Table 2). Lacrimal gland involvement is reported in only seven cases.[8-14]
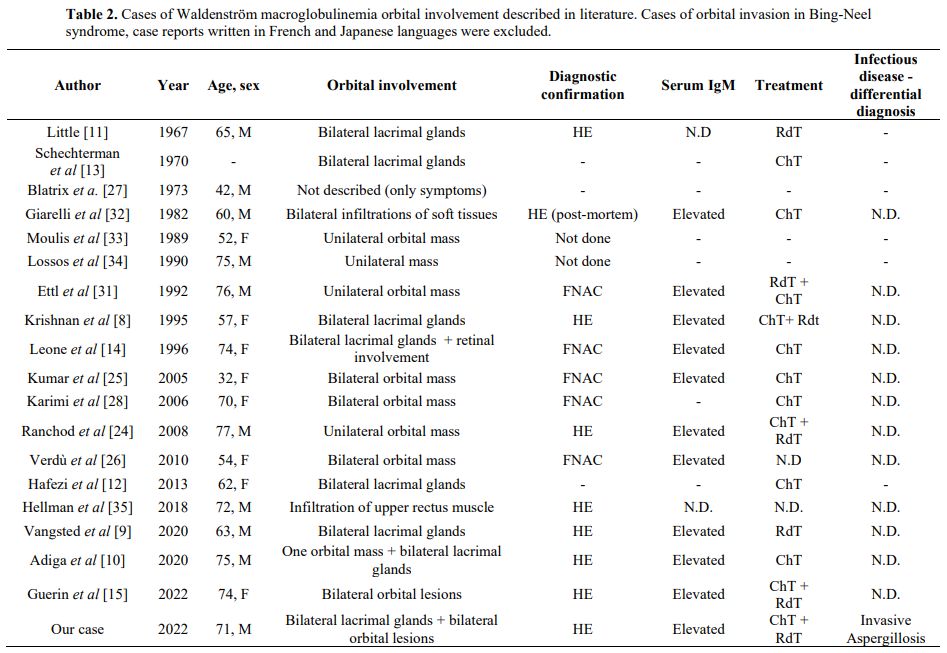 |
- Table 2. Cases of
Waldenström macroglobulinemia orbital involvement described in
literature. Cases of orbital invasion in Bing-Neel syndrome, case
reports written in French and Japanese languages were excluded.
|
In June 2022, intraorbital and maxillofacial diffusion worsened (Figure 4),
involving nasal bones and extending to the left infratemporal fossa and
alveolar processes through the ipsilateral maxillary sinus. Palliative
radiotherapy was performed in June and July 2022. The patient
experienced an additional COPD exacerbation needing hospitalization in
July 2022 with concurrent pancytopenia (red blood cells 3.04x1012/L, white blood cell 1.18x109/L, neutrophils 0.86x109/L, platelets 51x109/L).
Serological markers of fungal infection were persistently negative; an
antimicrobial regimen and recombinant human granulocyte
colony-stimulating factor (Filgrastim) were introduced. The patient was
discharged after one week and died in August 2022.
Discussion
The
case denotes the diagnostic and therapeutic complexity of a patient
affected by WM progression, a pathology requiring a multidisciplinary
approach[4,15,16] in the context of a pulmonary and encephalic IA.
Disseminate or extrapulmonary IA is commonly associated with
hematopoietic cell/solid organ transplantation and hematologic
malignant therapy:[17] in particular, association with Bruton-kinase inhibitor (e.g., ibrutinib) is well described,[18-20] with encephalic involvement commonly reported.[19]
The most common Aspergillus spp. ocular manifestation is
endophthalmitis, while the typical orbital aspergillosis is
characterized by unilateral painful, red eye with proptosis,[21] usually starting from paranasal sinuses.[22] The involvement of lacrimal sack has been rarely described in the literature.[21]
In WM, the most common ocular manifestation (present in up to 34% of cases)[23]
is hyperviscosity syndrome associated with funduscopic abnormalities,
characterized by characteristically tortuous "sausage link"-like
retinal veins. Tumor infiltration of the orbital and periorbital
tissues, involving retro-orbital lymphoid tissue and lacrimal glands,
rarely occurs.[24] If it happens, it is described as bilateral masses [8,15,25,26], in some cases with palpebral edema,[9,26] nodular palpebral involvement,[10] and proptosis.[25]
Bilateral swelling of lacrimal glands is an extremely rare presentation
of WM and has been described only in a few cases in the literature, [8-14,27] as well as bone involvement.[28]
Orbital infiltration can be present also in Bing-Neel syndrome, a WM
malignant form of the central nervous system, but usually with a more
extended involvement.[29,30] Radiologically, LPL lesions are usually mildly hypointense in T1W and T2W MR imaging,[24,28,31] probably for the high density of tumor cells and low interstitial water content.[31] Lesions, shown in T1W sequences, are also characterized by homogeneous CE.[24,28,31]Treatment for WM orbital involvement is not well defined, and in published case reports, chemotherapy alone[10,14,28] or combined with local radiotherapy[8,15,24]
was used, with a reduction of symptoms, intraorbital masses, and blood
IgM levels. In our case, although the reduction of IgM level (22.4 vs.
46 g/L), symptoms persisted, and orbital and maxillofacial foci
gradually increased in size
Conclusion
We
reported a case of orbital WM with lacrimal glands involvement, rarely
described in the available literature. Differential diagnosis between
WM and IA was particularly difficult considering the two pathologies'
systemic nature and overlapping clinical features. A correct diagnosis
was reached only thanks to a multidisciplinary approach.
References
- Mazzucchelli M, Frustaci AM, Deodato M, et al
(2018) Waldenstrom's Macroglobulinemia: An Update. Mediterr J Hematol
Infect Dis 10:e2018004. https://doi.org/10.4084/mjhid.2018.004 PMid:29326801 PMCid:PMC5760071
- Castillo
JJ, Olszewski AJ, Kanan S, et al (2015) Overall survival and competing
risks of death in patients with Waldenström macroglobulinaemia: an
analysis of the Surveillance, Epidemiology and End Results database. Br
J Haematol 169:81-89. https://doi.org/10.1111/bjh.13264 PMid:25521528
- Benjamin M, Reddy S, Brawley OW (2003) Myeloma and race: a review of the literature. Cancer Metastasis Rev 22:87-93. https://doi.org/10.1023/A:1022268103136 PMid:12716040
- Kastritis
E, Leblond V, Dimopoulos MA, et al (2018) Waldenström's
macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol 29:iv41-iv50. https://doi.org/10.1093/annonc/mdy146 PMid:29982402
- Bs
P, Mi V, A A, et al (2016) Orbital tumours and tumour-like lesions:
exploring the armamentarium of multiparametric imaging. Insights
Imaging 7. https://doi.org/10.1007/s13244-015-0443-8 PMid:26518678 PMCid:PMC4729705
- Kim JS, Liss J (2021) Masses of the Lacrimal Gland: Evaluation and Treatment. J Neurol Surg Part B Skull Base 82:100. https://doi.org/10.1055/s-0040-1722700 PMid:33777623 PMCid:PMC7987400
- Teo
L, Seah LL, Choo CT, et al (2013) A survey of the histopathology of
lacrimal gland lesions in a tertiary referral centre. Orbit Amst Neth
32:1-7. https://doi.org/10.3109/01676830.2012.736595 PMid:23387446
- Krishnan
K, Adams PT (2009) Bilateral orbital tumors and lacrimal gland
involvement in Waldenström's macroglobulinemia. Eur J Haematol
55:205-206. https://doi.org/10.1111/j.1600-0609.1995.tb00253.x PMid:7672095
- Vangsted
A, Mikkelsen LH, Jørgensen JS, Heegaard S (2020) Lymphoplasmacytic
lymphoma infiltrating both lacrimal glands in a patient with
Waldenström's macroglobulinemia. Am J Ophthalmol Case Rep 17:100597. https://doi.org/10.1016/j.ajoc.2020.100597 PMid:32016162 PMCid:PMC6992929
- Adiga
S, Mehta A, Singh U, et al (2022) Waldenström Macroglobulinemia of the
orbit: A diagnostic challenge. Eur J Ophthalmol 32:NP246-NP250. https://doi.org/10.1177/1120672120963459 PMid:33183084
- Little
JM (1967) Waldenström's macroglobulinemia in the lacrimal gland. Trans
- Am Acad Ophthalmol Otolaryngol Am Acad Ophthalmol Otolaryngol
71:875-879
- Hafezi
F, Moesen I, Carels G, et al (2010) [Waldenstrom's macroglobulinaemia
of the lacrimal gland in a patient with sarcoidosis]. Ophthalmol Z
Dtsch Ophthalmol Ges 107:60-63. https://doi.org/10.1007/s00347-009-2010-5 PMid:19669149
- Schechterman
L, Tyler SJ (1970) Waldenström's macroglobulinemia. Localization in
ileum and lacrimal glands. N Y State J Med 70:2025-2029
- Leone
G, Parisi V, Rebecchi A, et al (1996) Multiple ocular impairment in a
patient affected by Waldenström's macroglobulinaemia. Graefes Arch Clin
Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol
234:533-535. https://doi.org/10.1007/BF00184864 PMid:8858361
- Guerin
C, Normile C, Quinn J, et al (2022) Orbital involvement in Waldenstrom
macroglobulinaemia: a multidisciplinary approach. Ir J Med Sci 1971 -
191:2229-2230. https://doi.org/10.1007/s11845-021-02846-2 PMid:34762279
- Kapoor
P, Ansell SM, Fonseca R, et al (2017) Diagnosis and Management of
Waldenström Macroglobulinemia: Mayo Stratification of Macroglobulinemia
and Risk-Adapted Therapy (mSMART) Guidelines 2016. JAMA Oncol
3:1257-1265. https://doi.org/10.1001/jamaoncol.2016.5763 PMid:28056114 PMCid:PMC5556979
- Patterson
TF, Thompson GR, Denning DW, et al (2016) Practice Guidelines for the
Diagnosis and Management of Aspergillosis: 2016 Update by the
Infectious Diseases Society of America. Clin Infect Dis 63:e1-e60. https://doi.org/10.1093/cid/ciw326 PMid:27365388 PMCid:PMC4967602
- Fürstenau M, Simon F, Cornely OA, et al (2020) Invasive Aspergillosis in Patients Treated With Ibrutinib. HemaSphere 4:e309. https://doi.org/10.1097/HS9.0000000000000309 PMid:32309779 PMCid:PMC7162080
- Ghez
D, Calleja A, Protin C, et al (2018) Early-onset invasive aspergillosis
and other fungal infections in patients treated with ibrutinib. Blood
131:1955-1959. https://doi.org/10.1182/blood-2017-11-818286 PMid:29437588
- Maus
MV, Lionakis MS (2020) Infections associated with the new "nibs and
mabs" and cellular therapies. Curr Opin Infect Dis 33:281-289. https://doi.org/10.1097/QCO.0000000000000656 PMid:32657964 PMCid:PMC7367497
- Levin
LA, Avery R, Shore JW, et al (1996) The spectrum of orbital
aspergillosis: a clinicopathological review. Surv Ophthalmol
41:142-154. https://doi.org/10.1016/S0039-6257(96)80004-X PMid:8890440
- Khoo
SH, Denning DW (1994) Invasive aspergillosis in patients with AIDS.
Clin Infect Dis Off Publ Infect Dis Soc Am 19 Suppl 1:S41-48. https://doi.org/10.1093/clinids/19.Supplement_1.S41 PMid:7948570
- García-Sanz
R, Montoto S, Torrequebrada A, et al (2001) Waldenström
macroglobulinaemia: presenting features and outcome in a series with
217 cases. Br J Haematol 115:575-582. https://doi.org/10.1046/j.1365-2141.2001.03144.x PMid:11736938
- Ranchod
TM, Mansour TN, Fogt F, Gausas RE (2008) Waldenström macroglobulinemia
of the orbit. Ophthal Plast Reconstr Surg 24:76-77. https://doi.org/10.1097/IOP.0b013e318160dfcc PMid:18209658
- Kumar
S, Das S, Goyal JL, et al (2007) Bilateral orbital tumor formation and
isolated facial palsy in Waldenstrom's macroglobulinemia. Int
Ophthalmol 26:235-237. https://doi.org/10.1007/s10792-007-9037-x PMid:17356930
- Verdú
J, Andrés R, Sánchez-Majano JL, Fernández JA (2011) Bilateral ocular
involvement as a presentation of Waldenström's macroglobulinemia. Med
Oncol Northwood Lond Engl 28:1624-1625. https://doi.org/10.1007/s12032-010-9648-3 PMid:20697839
- Blatrix
C, Fine JM, Yeme D, Lambin P (1973) [Tumoral forms of Waldenstrom's
macroglobulinemia. Apropos of a case involving 2 localisations, orbital
and hepatic]. Sem Hopitaux Organe Fonde Par Assoc Enseign Med Hopitaux
Paris 49:2847-2851
- Karimi
S, Wong RJ, Holodny AI (2006) Skull Base, Orbital, and Perineural
Involvement in Waldenström's Macroglobulinemia. J Otolaryngol 35:68. https://doi.org/10.2310/7070.2005.4112 PMid:16527022
- Varettoni
M, Defrancesco I, Diamanti L, et al (2017) Bing-Neel Syndrome:
Illustrative Cases and Comprehensive Review of the Literature. Mediterr
J Hematol Infect Dis 9:e2017061. https://doi.org/10.4084/MJHID.2017.061 PMid:29181138 PMCid:PMC5667529
- Stacy
RC, Jakobiec FA, Hochberg FH, et al (2010) Orbital involvement in
Bing-Neel syndrome. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol
Soc 30:255-259. https://doi.org/10.1097/WNO.0b013e3181dee96c PMid:20548243
- Ettl
AR, Birbamer GG, Philipp W (1992) Orbital Involvement in Waldenström's
Macroglobulinemia: Ultrasound, Computed Tomography and Magnetic
Resonance Findings. Ophthalmologica 205:40-45. https://doi.org/10.1159/000310309 PMid:1436990
- Giarelli
L, Melato M, Falconieri G (1982) Eye involvement in Waldenströms's
macroglobulinaemia. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z
Augenheilkd 185:214-219. https://doi.org/10.1159/000309245 PMid:6815601
- Moulis
H, Mamus SW (1989) Isolated trochlear nerve palsy in a patient with
Waldenström's macroglobulinemia: complete recovery with combination
therapy. Neurology 39:1399. https://doi.org/10.1212/WNL.39.10.1399 PMid:2507958
- Lossos
A, Averbuch-Heller L, Reches A, Abramsky O (1990) Complete unilateral
ophthalmoplegia as the presenting manifestation of Waldenström's
macroglobulinemia. Neurology 40:1801-1802. https://doi.org/10.1212/WNL.40.11.1801-a PMid:2122277
- Hellman
JB, Harocopos GJ, Lin LK (2018) Waldenstrom macroglobulinemia involving
the superior rectus muscle. Am J Ophthalmol Case Rep 10:304-306. https://doi.org/10.1016/j.ajoc.2018.04.008 PMid:29780960 PMCid:PMC5956744
[TOP]





