 |
|
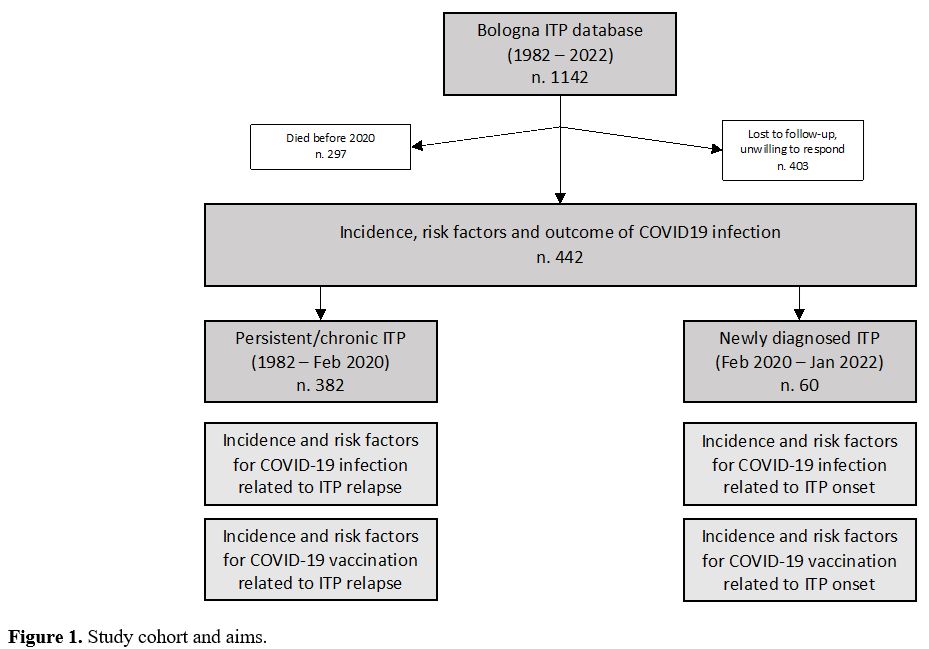
Incidence and risk factors of newly diagnosed ITP during the SARS-CoV-2 pandemic. From February 2020 to January 2022, 60 patients received a diagnosis of ITP (28, 46.7%, during the first year of the pandemic). All these 60 patients were followed for at least 6 months after ITP diagnosis.
Five of these diagnoses (8.3%) were attributed to SARS-CoV-2 infection (inf-ITP) and 13 (21.7%) to vaccination (vax-ITP): 7 after mRNA-BNT162b2, 3 after mRNA-1273 and 3 after ChAdOx1-S. Four patients were diagnosed with vax-ITP after their first dose of vaccine, 4 after the second and 5 after the first booster dose.
In univariate analysis, only a younger age was associated with a higher risk of inf-ITP, with a median age of 41.9 years versus 70.2 years in patients with ITP unrelated to Sars-CoV-2 infection (p=0.02). The remaining risk factors were not associated to inf-ITP, including male sex (40% of inf-ITP patients versus 45.5%, p=0.6), CCI ≥1 (20% of inf-ITP patients versus 25.5%, p=0.63), other autoimmune diseases (0% of inf-ITP patients versus 12.7%, p=0.52).
Conversely, only an older age was associated with a higher risk of vax-ITP (median age 74 versus 55.9, p=0.04). Male sex (OR: 3.48 [0. 85-14.27], p=0.08), CCI ≥1 (OR: 2.3 [0.62-8.64], p=0.21), and presence of additional autoimmune diseases (OR: 3.2 [0.62-16.75], p=0.16) were not associated to vax-ITP.
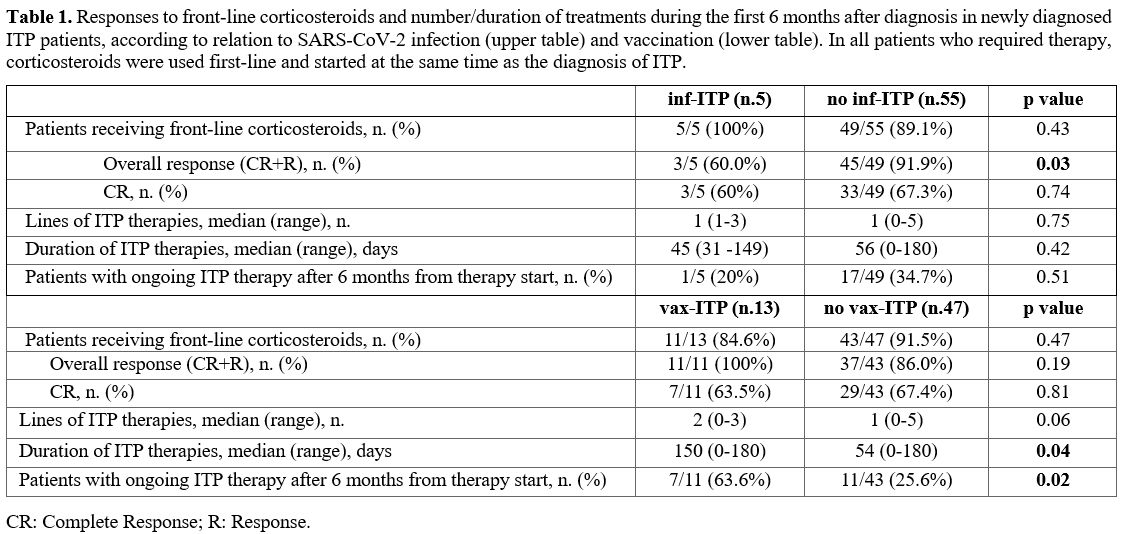
Response to therapy of newly diagnosed ITP according to relation to SARS-CoV-2 infection/vaccination. Among the 60 newly diagnoses ITP patients, 54 (90%) received ITP therapy in the first 6 months from diagnosis. First-line therapy was prednisone in all patients (dosage of 0.5-1 mg/kg for 21 days, with tapering and discontinuation in about 2-4 weeks).
Overall, 36 patients (66.7%) had a complete response, 12 patients (22.2%) a response, and 6 patients (11.1%) had no response.
A second-line therapy was required in 24 (66.6%) patients, namely TPO-RAs (79.2%) and corticosteroids (20.8%). Seven patients (29.1%) required a third line therapy (71.4% TPO-RAs and 28.6% corticosteroids). A fourth line therapy was administered to 3 patients (42.8%). The median duration of days spent on therapy was 55.5 days (range 0-180). Six months after diagnosis, 18 patients were still on therapy.
All five inf-ITP patients received first line therapy with steroids. Compared with other patients diagnosed during the pandemic, inf-ITP patient achieved less frequently a platelet response to the first line (p= 0.03), with comparable rates of complete responses (p=0.74), median number of lines of therapy (p=0.75), duration of therapy (p=0.42), patients on therapy after 6 months of disease (p = 0.51).
Out of 13 vax-ITP patients, 11 (84.6%) required corticosteroids. Compared with other patients diagnosed during the pandemic, no differences in terms of response to the first line (p= 0.19), complete response to the first line (p= 0.81), and number of lines of therapy (p=0.06) were observed. However, vax-ITP patients required more prolonged therapy (p= 0.04). Also, 63.6% of vax-ITP patients were still on therapy at 6 months after diagnosis (p= 0.02) (Table 1).
Incidence of relapsed ITP and correlation with SARS-CoV-2 infection and vaccine. Overall, 382 patients with an ITP diagnosed before the pandemic start were evaluable for this analysis and 69 (18.1%) had at least one relapse between February 2020 and January 2022.
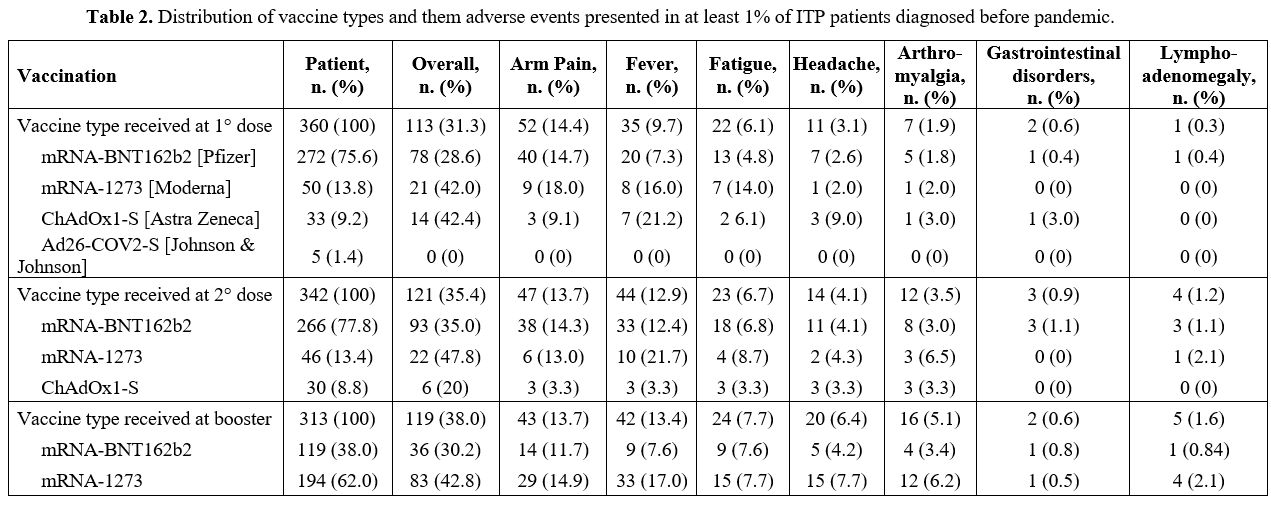
A total of 360 (94.2%), 342 and 313 patients received the first, second and the booster dose of the vaccine, respectively. The distribution of vaccine types and tolerability is described in Table 2.
 |
|
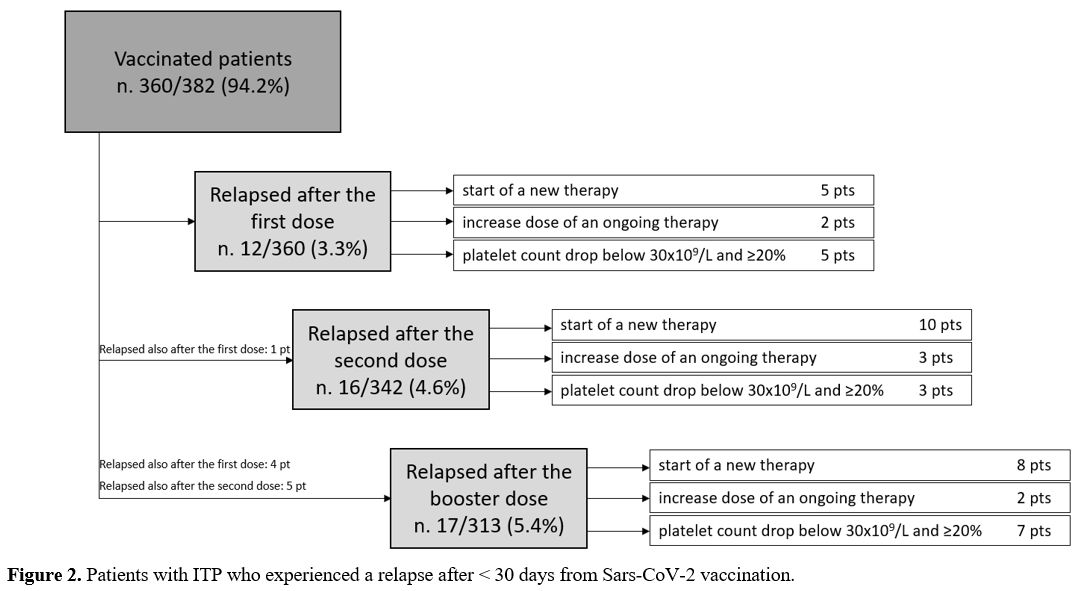
Relapse was attributed to SARS-CoV-2 vaccine in 35 patients (50.7%) and to SARS-CoV-2 infection in 1 patient (1.5%). In 33 patients (47.8%), ITP relapse was independent from both conditions.
Out of 35 patients with a vaccine-related relapse, 10 had a second ITP relapse attributed to the vaccine, for a total of 45 observed relapses. Relapses were defined as reduced platelet count requiring the start of a new therapy (n. 23) or a dose increase of an ongoing therapy (n. 7), or platelet count drop below 30x109/L and ≥20% from baseline in absence of treatment start/modification (n. 15). Only one patient experienced a grade 3 bleeding requiring hospitalization.
Vaccine-related relapses occurred in 12 (3.3%) cases after the first dose, in 16 cases (4.7%) after the second dose and in 17 cases (5.4%) after the booster (p=0.3) (Figure 2).
 |
|
After the first and the second dose, the only risk factor associated with relapse was an active disease (first dose: OR 10.81, CI 2.33-16.13, p 0.002; second dose: OR 6.99, CI 2.16-12.85, p=0.001). After the booster dose, the risk factors associated with relapse were active disease (OR 9.9, CI 2.90-16.88, p< 0.001) and ITP relapse after a previous dose (OR 5.33, CI 1.84-17.87, p=0.007). These two factors remained associated with relapse in multivariable analysis (OR 17.42, CI 3.8-39.2, p<0.001 and OR 5.9, CI 1.7-21.3, p=0.006).
Incidence and risk factor for COVID-19 in ITP patients. Between February 2020 and July 2022, 81 out of the total cohort of 442 (18.3%) patients contracted the SARS-CoV-2 infection (asymptomatic in 16, 19.8%: mild in 47 patients, 58.0%; moderate in 10 patients, 12.3%; severe in 8 patients, 9.9%; no fatal cases).
Infections occurred during the first wave (3 patients, 3.7%; severe in one case), second wave (25 patients, 30.9%; sever in 5 patients) and third wave (37 patients, 45.7%; severe in two patients) and fourth wave (16 patients, 19.7%; no severe cases).
Among the patients who received at least one dose of vaccine, none had a severe infection. Moreover, as the number of doses received increased, the frequency of infections decreased, with an incidence rate of infection of 0.55 per 100 patient-years in patients who did not receive any vaccine dose, 0.25 per 100 patient-years in patients who completed the first vaccine cycle, 0.04 per 100 patient-years in patients who received the booster dose (p<0.001).
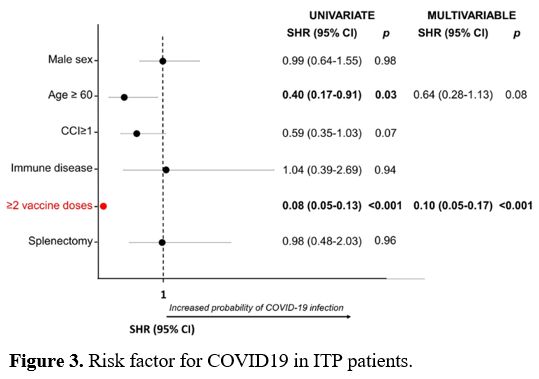
In univariate analysis, age ≥60 years and the completion of the vaccine cycle were protective factors against the SARS-CoV-2 infection (SHR 0.4, p=0.03 and SHR 0.08, p<0.001, respectively). In multivariate analysis, only vaccine cycle completion was confirmed as a protective factor (SHR 0.1, p< 0.001) (Figure 3).
 |
|