 |
|
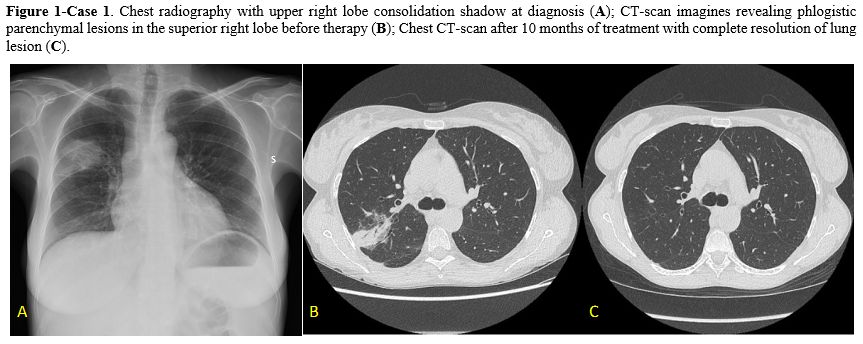
Case 1. A 44-year-old woman with acquired bone marrow aplasia (with CTLA4 mutation) and concomitant systemic erythematosus lupus presented with right scapular pain during deep breathing. She had no history of smoke or lung disease. The patient was in chronic immunosuppressive therapy with prednisone and cyclosporin. Physical examination revealed no apparent abnormalities. Laboratory tests showed a white blood cell count of 1.100/uL (517/uL neutrophils), hemoglobin levels of 10,8 g/dL, and a platelet count of 33.000/uL. The C-reactive protein levels were 7.16 mg/dL (range 0,00-5,00 mg/dL), and the lactate dehydrogenase level was 657 UI/L (range 240-480 UI/L). She underwent a chest radiography, which revealed an upper right lobe consolidation shadow and a chest computed tomography (CT) confirming the presence of a parenchymal lesion (5x4 cm) in the posterior segment of the superior right lobe Figure 1. The serum (1,3)-beta-D-glucan (BDG) test and the galactomannan (GM) test were negative. The QuantiFERON and the urinary antigen for Legionella pneumophila and Streptococcus pneumoniae were negative. A bronchoscopy was performed, and bronchoalveolar (BAL) fluid samples were collected for cytological and microbiological examination. An Actinomyces graevenitzii (10^4 CFU/mL) without evidence of antibiotic resistance was the only isolated pathogen from the BAL specimens.
Antibiotic therapy with ceftriaxone 2 g/daily was started. After 4 weeks of treatment, a CT scan showed no more lesions at the right upper lobe. Considering the persistence of cytopenia due to underlying hematological disease and the control of infection, the patient started anti-TNF alpha therapy (Abatacept) with strict radiological follow-up. After 2 weeks from the beginning of abatacept (6 weeks from the beginning of antibiotic therapy), the patient presented with fever and chills but no respiratory symptoms. Antibiotic therapy was switched to amoxicillin-clavulanate 1 g orally every 8 hours combined with antifungal therapy with isavuconazole 200 mg/daily due to the high risk of fungal infection. Considering clinical signs and symptoms, a new thoracic CT scan was performed, and multiple ground-glass lesions were found in both lobes. Another bronchoscopy with BAL was conducted, but it resulted in non-diagnostic. Since the resolution of symptoms and the persistence of negativity of GM and BDG serological tests, she discontinued isavuconazole while continuing amoxicillin-clavulanate therapy. After other 3 months a new chest CT scan was performed with evidence of complete resolution of pulmonary lesions. Considering clinical and radiological resolution of the infection, treatment was discontinued. At the last follow-up, the patient was alive with no abnormal clinical findings.
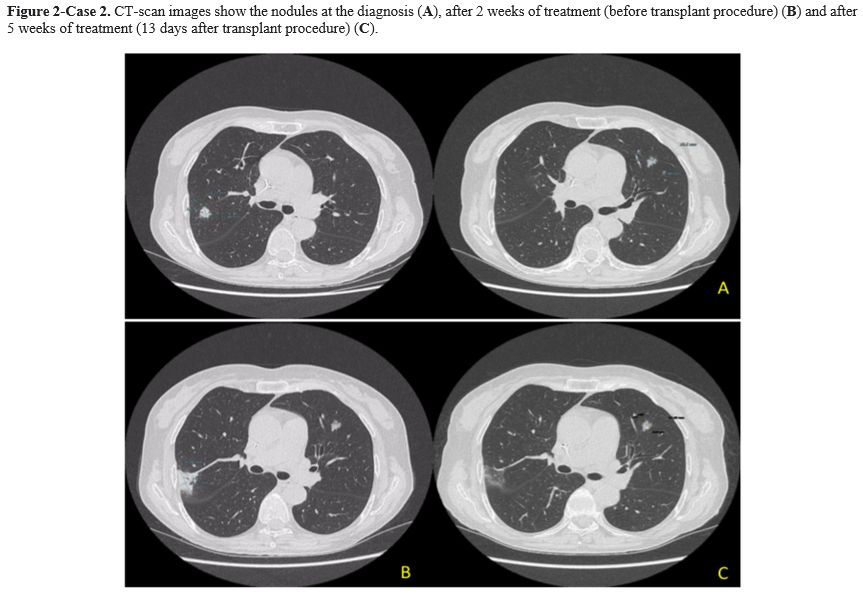
Case 2. A 64-year-old woman with Acute Myeloid Leukemia (AML), ELN high risk, received consolidation chemotherapy with high dose Cytosine Arabinoside and was scheduled to receive a matched unrelated donor allogeneic transplant (MUD-SCT). A pulmonary nodule on the upper posterior right lobe (15x13 mm) and a smaller one on the left lobe lesion were detected during pre-transplant screening with a CT scan. The patient was asymptomatic with normal vital parameters and without abnormalities on physical examination. The QuantiFERON test, the serum GM and BDG test were all negative. A bronchoscopy with BAL was performed, and only an Actinomyces graevenitzii (>10^5 CFU/mL) was isolated. Drug-susceptibility testing revealed that it was sensitive to ampicillin (minimum inhibitory concentration-MIC- <0.25), amoxicillin (MIC<0,25), and clindamycin (MIC<0,5). Antibiotic therapy with amoxicillin-clavulanate 1g orally every 8 hours was started. A follow-up CT scan was performed after 2 weeks with the evidence of a dimensional increase of the right lobe nodule (from 15x13 mm to 35x25 mm) Figure 2. There was no evidence of new symptoms, and physical examination did not show clinical modifications. The amoxicillin-clavulanate dosage was increased to 2,2 g intravenous every 8 hours due to hospitalization for a transplant procedure. MUD-SCT was performed, and no fever or pulmonary symptoms were reported during hospitalization. Acute graft versus host disease (GVHD) 16 days from HSCT, requiring a high dose of steroid treatment, was the only complication after the transplant procedure. A follow-up CT scan, performed after five weeks of antibiotic treatment (13 days from MUD-SCT), showed no further evidence of the right lobe nodule but the persistence of the small left lobe lesion without changes. Upon discharge from the hospital, the patient continued treatment with amoxicillin-clavulanate 1g orally every 8 hours for 4,5 months without any new signs or symptoms of infection. Unfortunately, the patient died of relapsed AML refractory to salvage chemotherapy.
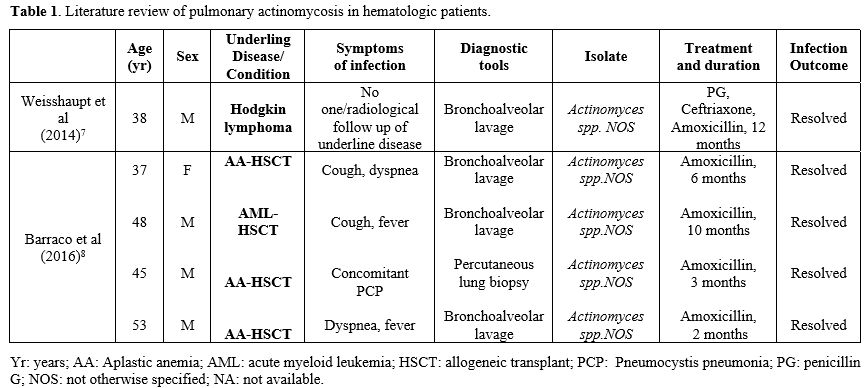
As highlighted in these two cases, diagnosing pulmonary actinomycosis is quite difficult because it could be confused with other pulmonary infections or lung malignancies.[1] The most common differential diagnosis, particularly in hematologic patients, is with fungal infections. While GM and BDG tests are useful for differential diagnosis, BAL and/or histologic tests remain the gold standard for diagnosing pulmonary actinomycosis. Unfortunately, invasive diagnostic procedures could be difficult in hematological patients due to low platelet levels resulting in an elevated risk of bleeding. In our two cases, infection was diagnosed by BAL culture with molecular identification by Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF/MS) method. Antibiotic susceptibility was evaluated with the epsilometer quantitative method (ETEST-Biomerieux, France). In both cases, the isolated was A. graevenitzii without evidence of antibiotic resistance. Actinomyces spp. are usually very susceptible to beta-lactams and high doses of penicillin and amoxicillin, while cephalosporins are less used.[1] The duration of treatment is still a matter of debate. In our literature review, the duration of antibiotic therapy ranged from 2 to 12 months (Table 1), and our 2 cases were in line with these data. First signs of radiological improvement are expected within 4-6 weeks of treatment.[6] In case 2, after the first 2 weeks of treatment, the CT scan showed a radiological progression, whereas after 6 weeks, the CT scan documented a full resolution of the infection. This finding confirmed the slow response of this infection to antibiotic therapy, suggesting that radiological monitoring should not be planned too early. The outcome of pulmonary actinomycosis is usually favorable also in highly immunocompromised patients.[5,6] Pierre et al reported an overall mortality of 21% (7/20) in this context, with only one death directly related to actinomycosis, while all other deaths were attributable to the underlying disease.[9] All cases reported in Table 1 resolved the infection after treatment. In conclusion, pulmonary actinomycosis is a rare complication in hematological patients, but it must be considered in the differential diagnosis of pulmonary lesions. Bronchoscopy, if possible, is recommended for appropriate diagnosis due to nonspecific clinical and radiological signs of this infection.