Gene Therapy in Thalassemia and Hemoglobinopathies
Laura Breda1, Roberto Gambari2 and Stefano Rivella1
1 Weill College Medical Center, Department of Pediatrics, Division of Hematology-Oncology, , NY, USA.
2 Department of Biochemistry and Molecular Biology, Section of Molecular Biology, University of Ferrara, Italy
Correspondence
to: Prof Stefano Rivella, Weill College Medical Center,
Department of Pediatrics – Division of Hematology-Oncology, 515 E 71st
street, S702, 10021 New York, NY, USA. Tel: +212 746 4941, Fax: +212
746 8423, e-mail: str2010@med.cornell.edu
Published: November 13, 2009
Received: October 12, 2009
Accepted: November 12, 2009
Medit J of Hemat Infect Dis 2009, 1(1): e2009008 DOI 10.4084/MJHID.2009.008
This article is available from: http://www.mjhid.org/article/view/5089
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Sickle
cell disease (SCD) and ▀-thalassemia represent the most common
hemoglobinopathies caused, respectively, by the alteration of
structural features or deficient production of the ▀-chain of the Hb
molecule. Other hemoglobinopathies are characterized by different
mutations in the α- or ▀-globin genes and are associated with anemia
and might require periodic or chronic blood transfusions. Therefore,
▀-thalassemia, SCD and other hemoglobinopathies are excellent
candidates for genetic approaches since they are monogenic disorders
and, potentially, could be cured by introducing or correcting a single
gene into the hematopoietic compartment or a single stem cell. Initial
attempts at gene transfer of these hemoglobinopathies have proved
unsuccessful due to limitations of available gene transfer vectors.
With the advent of lentiviral vectors many of the initial limitations
have been overcame. New approaches have also focused on targeting the
specific mutation in the ▀-globin genes, correcting the DNA sequence or
manipulating the fate of RNA translation and splicing to restore
▀-globin chain synthesis. These techniques have the potential to
correct the defect into hematopoietic stem cells or be utilized to
modify stem cells generated from patients affected by these disorders.
This review discusses gene therapy strategies for the
hemoglobinopathies, including the use of lentiviral vectors, generation
of induced pluripotent stem cells (iPS) cells, gene targeting,
splice-switching and stop codon readthrough.
▀-Thalassemia, Sickle Cell Anemia and Other
Hemoglobinopathies:
Hematopoietic Stem Cell Transplantation:
Gene Transfer Using Oncoretroviral Vectors:
Gene Transfer Using Lentiviral Vectors:
Gene Correction and Ips Cells:
Splice-Switching and Stop Codon Readthrough:
The thalassemias are a group of
disorders due to a large number of
heterogeneous mutations causing abnormal globin gene expression
resulting in the total absence or quantitative reduction of globin
chain synthesis [1]. Mutations in the α- or ▀-globin
gene lead to α- and
▀-thalassemia, respectively [1]. α-Thalassemia is
usually due to deletions
within the α-globin gene cluster, leading to loss of function of one or
both α-globin genes in each locus [2]. However,
non-deletion mutations
have been described, although they are much less frequent [1].
Depending
on the number of genes that are unable to synthesize the α-globin
protein, different clinical manifestations can be observed. If one or
two α-globin genes are mutated (in cis or trans), normally no or
minimal hematological effects are seen, and individuals are normally
silent thalassemia carriers or show α-thalassemia trait [1].
If three out
of four genes are mutated, the condition is called hemoglobin H (HbH)
disease, resulting in a hemolytic anemia that can worsen with febrile
illness or exposure to certain drugs, chemicals, or infectious agents.
Hemoglobin H disease is characterized by moderate to severe anemia,
hepatosplenomegaly, and jaundice. Transfusion may occasionally be
required and, if provided frequently, can lead to iron overload. If all
four α-globin genes are deleted, the resulting condition is called
α-thalassemia major, which is so severe that death occurs in utero.
Children rescued through intrauterine transfusions remain dependent on
red blood cell transfusions for survival [3].
The thalassemias are
characterized by their clinical severity and
genetic mutations. Patients with Cooley’s anemia, also known as
▀-thalassemia major, which is the most severe form of this disease,
require many blood transfusions per year and is characterized by
ineffective erythropoiesis and extra medullary hematopoiesis (EMH)[1]. If
untreated, ▀-thalassemia major is fatal in the first few years of
life [1]. In ▀-thalassemia intermedia, where a
greater number of ▀-globin
chains are synthesized, the clinical picture is milder, and the
patients require only infrequent or no transfusions [4,1]. In both
thalassemias, with time the spleen is enlarged, the hemoglobin level
decreases, and progressive iron overload occurs from increased GI iron
absorption in addition to transfusions [1]. The vast
majority of
▀-thalassemias are caused by point mutations within the gene or its
immediate flanking sequences and are classified according to the
mechanism by which they affect gene regulation: transcription, RNA
processing and mRNA translation [1]. These mutations
are also classified
as ▀0 and ▀+ according to the quantity of ▀-globin chains synthesized.
Mutations that lead to alternative splicing are associated with reduced
synthesis of normal ▀-globin mRNA and protein and are defined ▀+. In
contrast, mutations that completely impair ▀-globin synthesis (for
instance premature termination codons or PTCs) are defined ▀0.
Depending on the association of these different mutations, patients are
classified into three principal groups with none, very low or low
▀-globin production (▀0/0, 0/+, +/+ respectively). The levels of fetal
hemoglobin (HbF) account for a large part of the clinical heterogeneity
observed in patients with ▀-thalassemia. Variation in HbF expression
among individuals is an
inheritable disease modifier and high HbF (composed from 2 α- and 2
γ-chains) levels generally correlate with reduced morbidity and
mortality in this disorder, since the γ-globin chains combine with the
excess α-chains.
A single mutation leads to SCD,
causing an adenine (A) to thymidine (T)
substitution in codon [6] (GAG-GTG), which leads to
insertion of valine
in place of glutamic acid in the ▀-globin chain. The resulting Hb (HbS)
has the unique property of polymerizing when deoxygenated [1].
When the
polymer becomes abundant, the red cells “sickle”, stiff rods form that
stretch and distort the red cells. These distorted cells can obstruct
blood flow through the small vessels, and the restricted oxygen
delivery to the tissues damages cells, injures organs, and produces
pain. Similarly to SCD, other hemoglobinopathies can be triggered by
the substitution of one amino acid (HbE [5,6,2]), deletion of a portion of
the amino acid sequence (Hb Gun Hill7), abnormal hybridization between
two chains (Hb Lepore [8,9]), or abnormal elongation
of the globin chain
(Hb Constant Spring [10]). These abnormal Hbs can
have a variety of
pathophysiologically significant effects, including ineffective
erythropoiesis and anemia [1].
SCD and the thalassemias are quite common among Asian, African,
African-American and Mediterranean populations1. It has been estimated
that approximately 7% of the world population are carriers of such
disorders, and that 300,000–400,000 children with severe forms of these
diseases are born each year [117]. Hematopoietic Stem Cell Transplantation:
Current disease management of
▀-thalassemia consists of prenatal
diagnosis, transfusion therapy, or allogeneic BMT [11-13].
Only the latter
is potentially curative14. The first successful BMT of ▀-thalassemia
was reported in 198215. Consequently, several centers have utilized
this approach as definitive therapy [16-18]. The
most extensive experience
in treating ▀-thalassemia patients with BMT is that of Lucarelli and
coworkers in Italy [18]. Established protocols can
lead to a high success
of thalassemia-free survival, although the transplant-related mortality
is still significant and the chronic graft-versus-host disease is still
a potential long-term complication of allogeneic HSCs
transplantation [17,19]. In addition, availability
of allogeneic bone
marrow is limited by finding an identical human leucocyte antigen (HLA)
matched bone marrow donor. However, development of new techniques to
improve the management of graft-versus-host disease, to perform BMT
from unrelated donors and cord blood stem cells may expand the pool of
potential donors in the near future [20].
In addition, patients with severe ▀-thalassemia and SCD might benefit from new genetic and cellular approaches. From this prospective, ▀-thalassemia and SCD are excellent candidate diseases for genetically based therapies in autologous hematopoietic stem cells (HSCs)[21-23]. Alternatively, somatic cells reprogrammed to induced pluripotent stem cells might also provide a possible new approach to treat ▀-thalassemia [24,25].
In addition, patients with severe ▀-thalassemia and SCD might benefit from new genetic and cellular approaches. From this prospective, ▀-thalassemia and SCD are excellent candidate diseases for genetically based therapies in autologous hematopoietic stem cells (HSCs)[21-23]. Alternatively, somatic cells reprogrammed to induced pluripotent stem cells might also provide a possible new approach to treat ▀-thalassemia [24,25].
Gene Transfer Using Oncoretroviral Vectors:
Gene addition mediated by
retroviral vectors is an attractive approach
for monogenic disorder, However, when applied to hemoglobinopathies,
this strategy raises major challenges in terms of controlling transgene
expression, which should be erythroid-specific, elevated, position
independent and sustained over time. In fact, many studies were
performed before positive preclinical data were generated. The first
attempts were done using oncoviruses. These viruses belong to the large
family of Retroviridae and are characterized by a genome that encodes
the genes gag-pol and env [26]. Onco-retroviral
vectors, such as those
derived from Moloney murine leukemia virus, efficiently transfer
therapeutic genes into murine hematopoietic stem cells (HSC) without
transferring any viral gene [27]. Recombinant
oncoretroviruses were the
first viral vectors used to transfer the human ▀-globin gene in mouse
HSCs [28,29]. These experiments resulted in
tissue-specific but low and
variable (position-dependent) human ▀-globin expression in bone marrow
chimeras, usually varying between 0 and 2% of endogenous mouse ▀-globin
mRNA levels [29,30-33]. Studies
aimed at increasing expression levels of
transferred ▀-globin genes have focused on including locus control
region (LCR) elements of the human ▀-globin gene locus into
oncoretroviral vectors. The LCR contains cis-acting DNase I
hypersensitivity sites (HS) that are critical for high-level,
long-term, position-independent, and erythroid-specific
expression [34,35]. These HS elements contain
several DNA-binding motifs
for transcriptional and chromatin remodeling factors that facilitates
chromatin opening. Also, thes genomic regions allow for binding of
other regulatory elements required for high-level expression of the
▀-globin gene36. Incorporation of the core elements of HS2, HS3, and
HS4 of the human ▀-globin LCR significantly increased expression levels
in murine erythroleukemia (MEL) cells but failed to abolish positional
variability of expression [37,35].
Additional efforts aimed to include
larger elements resulted in the inability of the vector to incorporate
large quantities of genetic material, as shown by the rearrangements of
the transferred sequences [38-41]. Since these
rearrangements frequently
occur because of activation of splicing sites of the LCR sequence
contained in the retroviral RNA, additional attempts were done to
eliminate these sites. However, even these new vectors failed to
include HS elements sufficient large to considerably increase
expression of the ▀-globin gene [37,35].
Additional erythroid-specific transcriptional elements were investigated within oncoretroviral vectors, including the HS40 regulatory region from the human α-locus [42-44] and alternative promoters. The promoter of ankyrin, a red cell membrane protein, has shown some promise in transgenic mice and in transduced MEL cells [45]. In mice, the ankyrin promoter has been used to drive expression of the human γ-globin gene resulting, at double copy, in an average expression of 8% of that of the endogenous α-globin genes [46]. To overcome transcriptional silencing of the γ-globin promoter in hematopoietic chimeras, mutant γ-globin promoters from patients with hereditary persistence of fetal hemoglobin (HPFH) were also investigated [118,47]. The Greek mutation at position −117 thus appeared to substantially increase γ-globin expression in MEL cells [47]. However, even these vectors failed to increase the level of the ▀-globin gene to therapeutic levels.
Although oncoretrovirus vectors integrate into the genome, many integrants undergo transcriptional silencing, posing an additional challenge to the success of gene therapy using these vectors. Kalberer and co-workers attempted to avoid gene silencing by preselecting ex vivo retrovirally transduced hematopoietic stem cells on the basis of expression of the green fluorescent protein (GFP). In this vector the GFP gene was driven by the phosphoglycerate kinase promoter, while the human ▀-globin gene by its own promoter and small elements from the LCR [48]. Using this approach, in vivo hematopoietic stem cell gene silencing and age-dependent extinction of expression were avoided, although suboptimal expression levels and heterocellular position effects persisted.
Another major limitation is that oncoretroviral vectors need to infect cells before and close to their division, otherwise the viral RNA cannot migrate into the nucleus due to the presence of a nuclear membrane [49]. Since most hematopoietic stem cells are in a quiescent state, they must be induced with cytokines to divide in order to achieve higher transduction efficiencies and overall expression levels. Stimulation of quiescent hematopoietic stem cells, however, impairs or halts their long-term repopulating capacities [49].
Additional erythroid-specific transcriptional elements were investigated within oncoretroviral vectors, including the HS40 regulatory region from the human α-locus [42-44] and alternative promoters. The promoter of ankyrin, a red cell membrane protein, has shown some promise in transgenic mice and in transduced MEL cells [45]. In mice, the ankyrin promoter has been used to drive expression of the human γ-globin gene resulting, at double copy, in an average expression of 8% of that of the endogenous α-globin genes [46]. To overcome transcriptional silencing of the γ-globin promoter in hematopoietic chimeras, mutant γ-globin promoters from patients with hereditary persistence of fetal hemoglobin (HPFH) were also investigated [118,47]. The Greek mutation at position −117 thus appeared to substantially increase γ-globin expression in MEL cells [47]. However, even these vectors failed to increase the level of the ▀-globin gene to therapeutic levels.
Although oncoretrovirus vectors integrate into the genome, many integrants undergo transcriptional silencing, posing an additional challenge to the success of gene therapy using these vectors. Kalberer and co-workers attempted to avoid gene silencing by preselecting ex vivo retrovirally transduced hematopoietic stem cells on the basis of expression of the green fluorescent protein (GFP). In this vector the GFP gene was driven by the phosphoglycerate kinase promoter, while the human ▀-globin gene by its own promoter and small elements from the LCR [48]. Using this approach, in vivo hematopoietic stem cell gene silencing and age-dependent extinction of expression were avoided, although suboptimal expression levels and heterocellular position effects persisted.
Another major limitation is that oncoretroviral vectors need to infect cells before and close to their division, otherwise the viral RNA cannot migrate into the nucleus due to the presence of a nuclear membrane [49]. Since most hematopoietic stem cells are in a quiescent state, they must be induced with cytokines to divide in order to achieve higher transduction efficiencies and overall expression levels. Stimulation of quiescent hematopoietic stem cells, however, impairs or halts their long-term repopulating capacities [49].
Gene Transfer Using Lentiviral Vectors:
With the extensive research on
human immunodeficiency virus-1, it has
been realized that lentivirus, engineered to be devoid of any
pathogenic elements, can become efficient gene transfer vectors.
Lentiviruses are characterized by a complex genome that encodes a
number of accessory proteins besides the canonical retroviral genes
gag-pol and env. They share all the common characteristic of retroviral
replication including receptor-mediated entry, capsid uncoating,
reverse transcription of the viral RNA, and integration into the host
cell genome [26]. In addition, they are able to
transduce non-replicating
cells, which confers to these viruses a special value for the
development of clinically functional gene vectors. Moreover, compared
to oncoretroviral vectors, the stabilization of the proviral mRNA
genome by the interaction of the accessory protein Rev with its cognate
motif Rev-responsive element (RRE), increases their range of
application, since larger genomic elements can be introduced in their
genome with limited or no sequence rearrangement [50].
Therefore,
lentiviral vectors are thus likely to be selected as vectors of choice
for the stable delivery of regulated transgenes in stem cell–based gene
therapy. The use of lentiviral vectors has allowed the introduction of
large genomic elements from the ▀-globin locus, different promoters,
enhancers, and chromatin structure determinants that led to
lineage-specific and elevated of ▀-, γ- and α-globin expression in vivo.
This resulted, in the amelioration or correction of anemia and
secondary organ damage in several murine models of hemoglobinopathies,
making the recombinant lentiviruses the most effective vector system to
date for gene therapy of these disorders.
α-Thalassemia could potentially be a target for fetal gene therapy since fetuses with this disorder usually die between the third trimester of pregnancy and soon after birth. The potential use of lentiviral vectors to treat α-thalassemia was investigated a vector containing the HS2, 3, and 4 of the LCR from the human ▀-globin locus, and the human α-globin gene promoter directing the human α-globin gene. Using this vector, Han and colleagues performed gene delivery in utero during midgestation targeting embryos affected by a lethal form of α-thalassemia. They showed that in newborn mice, the human α-globin gene expression was detected in the liver, spleen, and peripheral blood [51]. The human α-globin gene expression was at the peak at 3–4 months, when it reached 20% in some recipients. However, the expression declined at 7 months. Colony-forming assays in these mice showed low levels of transduction and lack of human α-globin transcript. Thus, lentiviral vectors can be an effective vehicle for delivering the human α-globin gene into erythroid cells in utero, but, in the mouse model, delivery at late midgestation could not transduce hematopoietic stem cells adequately to sustain gene expression.
Treatment of ▀-thalassemia, SCD and other disorders through lentiviral mediated gene transfer is studied in murine and primate models [52-60]. The original studies in mice showed that lentiviral mediated human ▀-globin gene transfer can rescue mice affected by ▀-thalassemia intermedia and ▀-thalassemia major [61,62,59]. The mouse ▀-globin cluster has two adult ▀-globin genes, ▀minor- and ▀major-globin. Thalassemic mice were generated with deletion of both the ▀minor- and ▀major-globin on one allele, designated th3/+ mice (63; 64). Also adult th3/+ mice have a degree of disease severity (hepatosplenomegaly, anemia, aberrant erythrocyte morphology) comparable to that of patients affected by ▀-TI. May and colleagues tested two lentiviral vectors termed RNS1 (carrying minimal core LCR elements) and TNS9 (with large LCR fragments encompassing HS2, HS3 and HS4; approximately 3.2 kb in size) on th3/+ mice. Compared to RNS1, mice recipient of the larger TNS9 vector maintained higher human ▀-globin transcript levels over time showing amelioration of red cell pathology (anisocytosis and poikilocytosis) and significantly increased hemoglobin levels (from 8-9 g/dL to 11-13 g/dL). The massive splenomegaly found in chimeras engrafted with control th3/+ bone marrow was not observed in TNS9-treated animals [61]. This correction was sustained in secondary mice [62].
Mice completely lacking adult ▀-globin genes (th3/th3) die late in gestation, limiting their utilization as a model for Cooley's anemia [64]. For this reason, adult animals affected by Cooley’s anemia were generated by transplantation of hematopoietic fetal liver cells harvested from th3/th3 embryos at E14.5 into lethally irradiated syngeneic adult recipients [59]. Hematological analyses of engrafted mice performed 6 to 8 weeks post-transplant revealed severe anemia due not to pancytopenia but rather to low red blood cell and reticulocyte counts together with massive splenomegaly and extensive EMH [62,59]. These animals could be rescued using TNS9 or by blood transfusions, supporting the notion that their phenotype is due specifically to erythroid impairment [65,59].
Pawliuk and colleagues investigated the efficacy of a lentiviral vector harboring the ▀-globin promoter, LCR elements and a mutated human ▀-globin gene with enhanced anti-sickling activity (▀87) in two different transgenic mouse models for SCD: SAD and BERK [66,67]. Mice transplanted with BERK and SAD bone marrow cells transduced with this modified ▀-globin gene exhibited corrected reticulocyte counts and amelioration of Hemoglobin concentration, anisocytosis, and poikilocytosis. Moreover, the proportion of irreversibly sickled cells, SCD-associated splenomegaly, and characteristic urine concentration defect in SAD and BERK mice were vastly improved or corrected by ▀87. Using a similar vector, Levasseur and colleagues obtained equivalent results. They transduced Sca1+c-Kit+Lin− cells rather than unselected bone marrow cells and achieved durable therapeutic results (5–7 months) following transplantation of 100 cells in lethally irradiated C57BL/6 mice[113,114].
Samakoglu and coworkers applied the principle of RNA interference (RNAi) to down-regulate the ▀-globin mRNA in CD34(+) cells from patients affected by SCD [116]. They utilized a lentiviral vector harboring a promoterless small-hairpin RNA (shRNA) within the intron of a recombinant γ-globin gene. Expression of both γ-globin and the lariat-embedded small interfering RNA (siRNA) was induced upon erythroid differentiation, specifically downregulating the targeted gene in tissue and differentiation stage-specific fashion. The position of the shRNA within the intron was critical to concurrently achieve high transgene expression, effective siRNA generation and minimal interferon induction.
Miccio and colleagues also utilized an erythroid-specific lentiviral vector driving the expression of the human ▀-globin gene from a minimal promoter/enhancer element containing two hypersensitive sites from the ▀-globin locus control region in mouse models of ▀-thalassemia [68]. They showed that genetically corrected erythroblasts underwent in vivo selection. The selected erythroblast that derived from progenitors harboring proviral integrations in genome sites and were more favorable to high levels of vector expression. These data suggested that a regimen of partially myeloablative transplantation might be sufficient to achieve a chimerism that would therapeutic in ▀-thalassemic patients.
While correction of murine models of ▀-thalassemia has been achieved through lentiviral-mediated high levels of globin gene transfer into mouse HSCs, transduction of human HSCs is less robust and may be inadequate to achieve therapeutic levels of genetically modified erythroid cells. Zhao and coworkers therefore developed a double gene lentiviral vector encoding both human γ-globin under the transcriptional control of erythroid regulatory elements and methylguanine methyltransferase (MGMT), driven by a constitutive cellular promoter [60]. MGMT is an alkyltransferase that normally functions to repair cellular DNA damage at the O6 position of guanine [69,70]. The cytotoxic effects of alkylating agents, such as temozolomide and 1,3-bis-chloroethyl-1-nitrosourea (BCNU), can be prevented if there is adequate expression of MGMT, which removes the O6 adduct from the modified DNA. Variant MGMT proteins with specific amino acid changes retain significant activity while possessing the useful property of resistance to inactivation by O6-benzylguanine (BG) [71]. BG can be used to inactivate endogenous MGMT to enhance the specificity of alkylator-mediated cell death to cells not expressing the variant form. Therefore, expression of these variant forms of MGMT provides cellular resistance to alkylator drugs, which can be administered to kill residual untransduced HSCs, whereas transduced cells are protected. To test this hypothesis, mice transplanted with ▀-thalassemic HSCs cells transduced with a lentiviral γ-globin/MGMT vector were treated with BCNU [60]. This led to significant increas in the number of γ-globin–expressing red cells, the amount of fetal hemoglobin and resolution of anemia. One important advantage of using the γ-globin gene, normally expressed exclusively during fetal life, is that high level γ-globin expression would be therapeutic not only for ▀-thalassemia, but also SCD. Interestingly, selection of transduced HSCs was also obtained when cells were drug-treated before transplantation. These data suggest that coexpression of MGMT allowed autologous, γ-globin vector-transduced ▀-thalassemic HSCs to be enriched to therapeutic levels through either pre or post-transplantation selection.
Imren and colleagues engrafted immunodeficient mice with human cord blood cells infected with a lentiviral vector encoding an anti-sickling ▀-globin transgene [35,72]. After 6-months, half of the human erythroid and myeloid progenitors regenerated in the mice containing the transgene, and erythroid cells derived in vitro from these cells produced high levels of the ▀-globin protein. In addition, these authors investigated the integrated proviral copies showing that 86% of the proviral inserts had occurred within genes, including several genes implicated in human leukemia. These findings indicate effective transduction of very primitive human cord blood cells achieving robust and erythroid-specific production of therapeutically relevant levels of ▀-globin protein. The frequency of proviral integration within genes observed in this study and the data from Miccio and coworkers that indicate that selected erythroblasts were derived from progenitors harboring proviral integrations more favorable to high levels of vector expression, indicate that regulated hematopoiesis might require additional safety modifications to prevent potential genotoxic effects [35,72,68]. This risk is inherent to the integration of foreign genetic material and the risk of insertional oncogenesis has been established both in mice and humans [73-78].
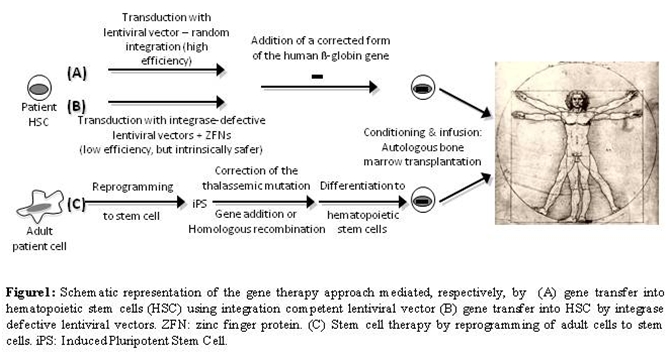
In light of these results, genetic elements with enhancer-blocking properties, such as insulators, could increase the safety of the clinical trails. These elements have been investigated to shelter the vector from the repressive influence of flanking chromatin by blocking interactions between regulatory elements within the vector and chromosomal elements at the site of integration [79-81]. This property of insulators can also be harnessed to diminish the risk that the vector will activate a neighboring oncogene [82,83]. The initial studies indicated that inclusion of the cHS4 insulator element into the 3′ LTR of recombinant murine leukemia virus increases the probability that randomly integrated proviruses will express the transgene [46,84-86]. Puthenveetil and coworkers tested a lentiviral vector carrying the human ▀-globin expression cassette flanked by a chromatin insulator in transfusion-dependent human ▀-thalassemia major cells [87]. Using this vector, they demonstrated normal expression of human ▀-globin in erythroid cells produced in vitro. They also observed restoration of effective erythropoiesis and reversal of the abnormally elevated apoptosis that characterizes ▀-thalassemia. The gene-corrected human ▀-thalassemia progenitor cells were also transplanted into immune-deficient mice, where they underwent normal erythroid differentiation, expressed normal levels of human ▀-globin, and displayed normal effective erythropoiesis 3 to 4 months after xenotransplantation. Based on all these preclinical studies on mouse models of ▀-thalassemia and SCD, clinical trials have been proposed or are underway [53]. Figure 1A depicts this approach.
Alternatively, the homologous recom-bination pathway can be harnessed to avoid random integration. Zinc-finger nucleases (ZFNs) can been used to enhance the frequency of gene correction [88,89]. However, achieving the full potential of ZFNs for genome engineering in human cells requires their efficient delivery to the relevant cell types. Lombardo and colleagues exploited the infectivity of integrase-defective lentiviral vectors (IDLV) to express ZFNs and provide the template DNA for ge ne correction in different cell types [90]. IDLV-mediated delivery supported high rates (13–39%) of editing at the IL-2 receptor common γ-chain gene (IL2RG) across different cell types as well as human embryonic stem cells (5%), allowing selection-free isolation of clonogenic cells with the desired genetic modification. Therefore, this technique opens new and exciting possibilities. By modifying the ZFN binding specificity and selecting an appropriate donor sequence, one could target the IDLV-ZFN system to any individual site in the human genome avoiding random integration (Figure 1B) and, potentially, genome toxicity [88-91].
α-Thalassemia could potentially be a target for fetal gene therapy since fetuses with this disorder usually die between the third trimester of pregnancy and soon after birth. The potential use of lentiviral vectors to treat α-thalassemia was investigated a vector containing the HS2, 3, and 4 of the LCR from the human ▀-globin locus, and the human α-globin gene promoter directing the human α-globin gene. Using this vector, Han and colleagues performed gene delivery in utero during midgestation targeting embryos affected by a lethal form of α-thalassemia. They showed that in newborn mice, the human α-globin gene expression was detected in the liver, spleen, and peripheral blood [51]. The human α-globin gene expression was at the peak at 3–4 months, when it reached 20% in some recipients. However, the expression declined at 7 months. Colony-forming assays in these mice showed low levels of transduction and lack of human α-globin transcript. Thus, lentiviral vectors can be an effective vehicle for delivering the human α-globin gene into erythroid cells in utero, but, in the mouse model, delivery at late midgestation could not transduce hematopoietic stem cells adequately to sustain gene expression.
Treatment of ▀-thalassemia, SCD and other disorders through lentiviral mediated gene transfer is studied in murine and primate models [52-60]. The original studies in mice showed that lentiviral mediated human ▀-globin gene transfer can rescue mice affected by ▀-thalassemia intermedia and ▀-thalassemia major [61,62,59]. The mouse ▀-globin cluster has two adult ▀-globin genes, ▀minor- and ▀major-globin. Thalassemic mice were generated with deletion of both the ▀minor- and ▀major-globin on one allele, designated th3/+ mice (63; 64). Also adult th3/+ mice have a degree of disease severity (hepatosplenomegaly, anemia, aberrant erythrocyte morphology) comparable to that of patients affected by ▀-TI. May and colleagues tested two lentiviral vectors termed RNS1 (carrying minimal core LCR elements) and TNS9 (with large LCR fragments encompassing HS2, HS3 and HS4; approximately 3.2 kb in size) on th3/+ mice. Compared to RNS1, mice recipient of the larger TNS9 vector maintained higher human ▀-globin transcript levels over time showing amelioration of red cell pathology (anisocytosis and poikilocytosis) and significantly increased hemoglobin levels (from 8-9 g/dL to 11-13 g/dL). The massive splenomegaly found in chimeras engrafted with control th3/+ bone marrow was not observed in TNS9-treated animals [61]. This correction was sustained in secondary mice [62].
Mice completely lacking adult ▀-globin genes (th3/th3) die late in gestation, limiting their utilization as a model for Cooley's anemia [64]. For this reason, adult animals affected by Cooley’s anemia were generated by transplantation of hematopoietic fetal liver cells harvested from th3/th3 embryos at E14.5 into lethally irradiated syngeneic adult recipients [59]. Hematological analyses of engrafted mice performed 6 to 8 weeks post-transplant revealed severe anemia due not to pancytopenia but rather to low red blood cell and reticulocyte counts together with massive splenomegaly and extensive EMH [62,59]. These animals could be rescued using TNS9 or by blood transfusions, supporting the notion that their phenotype is due specifically to erythroid impairment [65,59].
Pawliuk and colleagues investigated the efficacy of a lentiviral vector harboring the ▀-globin promoter, LCR elements and a mutated human ▀-globin gene with enhanced anti-sickling activity (▀87) in two different transgenic mouse models for SCD: SAD and BERK [66,67]. Mice transplanted with BERK and SAD bone marrow cells transduced with this modified ▀-globin gene exhibited corrected reticulocyte counts and amelioration of Hemoglobin concentration, anisocytosis, and poikilocytosis. Moreover, the proportion of irreversibly sickled cells, SCD-associated splenomegaly, and characteristic urine concentration defect in SAD and BERK mice were vastly improved or corrected by ▀87. Using a similar vector, Levasseur and colleagues obtained equivalent results. They transduced Sca1+c-Kit+Lin− cells rather than unselected bone marrow cells and achieved durable therapeutic results (5–7 months) following transplantation of 100 cells in lethally irradiated C57BL/6 mice[113,114].
Samakoglu and coworkers applied the principle of RNA interference (RNAi) to down-regulate the ▀-globin mRNA in CD34(+) cells from patients affected by SCD [116]. They utilized a lentiviral vector harboring a promoterless small-hairpin RNA (shRNA) within the intron of a recombinant γ-globin gene. Expression of both γ-globin and the lariat-embedded small interfering RNA (siRNA) was induced upon erythroid differentiation, specifically downregulating the targeted gene in tissue and differentiation stage-specific fashion. The position of the shRNA within the intron was critical to concurrently achieve high transgene expression, effective siRNA generation and minimal interferon induction.
Miccio and colleagues also utilized an erythroid-specific lentiviral vector driving the expression of the human ▀-globin gene from a minimal promoter/enhancer element containing two hypersensitive sites from the ▀-globin locus control region in mouse models of ▀-thalassemia [68]. They showed that genetically corrected erythroblasts underwent in vivo selection. The selected erythroblast that derived from progenitors harboring proviral integrations in genome sites and were more favorable to high levels of vector expression. These data suggested that a regimen of partially myeloablative transplantation might be sufficient to achieve a chimerism that would therapeutic in ▀-thalassemic patients.
While correction of murine models of ▀-thalassemia has been achieved through lentiviral-mediated high levels of globin gene transfer into mouse HSCs, transduction of human HSCs is less robust and may be inadequate to achieve therapeutic levels of genetically modified erythroid cells. Zhao and coworkers therefore developed a double gene lentiviral vector encoding both human γ-globin under the transcriptional control of erythroid regulatory elements and methylguanine methyltransferase (MGMT), driven by a constitutive cellular promoter [60]. MGMT is an alkyltransferase that normally functions to repair cellular DNA damage at the O6 position of guanine [69,70]. The cytotoxic effects of alkylating agents, such as temozolomide and 1,3-bis-chloroethyl-1-nitrosourea (BCNU), can be prevented if there is adequate expression of MGMT, which removes the O6 adduct from the modified DNA. Variant MGMT proteins with specific amino acid changes retain significant activity while possessing the useful property of resistance to inactivation by O6-benzylguanine (BG) [71]. BG can be used to inactivate endogenous MGMT to enhance the specificity of alkylator-mediated cell death to cells not expressing the variant form. Therefore, expression of these variant forms of MGMT provides cellular resistance to alkylator drugs, which can be administered to kill residual untransduced HSCs, whereas transduced cells are protected. To test this hypothesis, mice transplanted with ▀-thalassemic HSCs cells transduced with a lentiviral γ-globin/MGMT vector were treated with BCNU [60]. This led to significant increas in the number of γ-globin–expressing red cells, the amount of fetal hemoglobin and resolution of anemia. One important advantage of using the γ-globin gene, normally expressed exclusively during fetal life, is that high level γ-globin expression would be therapeutic not only for ▀-thalassemia, but also SCD. Interestingly, selection of transduced HSCs was also obtained when cells were drug-treated before transplantation. These data suggest that coexpression of MGMT allowed autologous, γ-globin vector-transduced ▀-thalassemic HSCs to be enriched to therapeutic levels through either pre or post-transplantation selection.
Imren and colleagues engrafted immunodeficient mice with human cord blood cells infected with a lentiviral vector encoding an anti-sickling ▀-globin transgene [35,72]. After 6-months, half of the human erythroid and myeloid progenitors regenerated in the mice containing the transgene, and erythroid cells derived in vitro from these cells produced high levels of the ▀-globin protein. In addition, these authors investigated the integrated proviral copies showing that 86% of the proviral inserts had occurred within genes, including several genes implicated in human leukemia. These findings indicate effective transduction of very primitive human cord blood cells achieving robust and erythroid-specific production of therapeutically relevant levels of ▀-globin protein. The frequency of proviral integration within genes observed in this study and the data from Miccio and coworkers that indicate that selected erythroblasts were derived from progenitors harboring proviral integrations more favorable to high levels of vector expression, indicate that regulated hematopoiesis might require additional safety modifications to prevent potential genotoxic effects [35,72,68]. This risk is inherent to the integration of foreign genetic material and the risk of insertional oncogenesis has been established both in mice and humans [73-78].
In light of these results, genetic elements with enhancer-blocking properties, such as insulators, could increase the safety of the clinical trails. These elements have been investigated to shelter the vector from the repressive influence of flanking chromatin by blocking interactions between regulatory elements within the vector and chromosomal elements at the site of integration [79-81]. This property of insulators can also be harnessed to diminish the risk that the vector will activate a neighboring oncogene [82,83]. The initial studies indicated that inclusion of the cHS4 insulator element into the 3′ LTR of recombinant murine leukemia virus increases the probability that randomly integrated proviruses will express the transgene [46,84-86]. Puthenveetil and coworkers tested a lentiviral vector carrying the human ▀-globin expression cassette flanked by a chromatin insulator in transfusion-dependent human ▀-thalassemia major cells [87]. Using this vector, they demonstrated normal expression of human ▀-globin in erythroid cells produced in vitro. They also observed restoration of effective erythropoiesis and reversal of the abnormally elevated apoptosis that characterizes ▀-thalassemia. The gene-corrected human ▀-thalassemia progenitor cells were also transplanted into immune-deficient mice, where they underwent normal erythroid differentiation, expressed normal levels of human ▀-globin, and displayed normal effective erythropoiesis 3 to 4 months after xenotransplantation. Based on all these preclinical studies on mouse models of ▀-thalassemia and SCD, clinical trials have been proposed or are underway [53]. Figure 1A depicts this approach.
Alternatively, the homologous recom-bination pathway can be harnessed to avoid random integration. Zinc-finger nucleases (ZFNs) can been used to enhance the frequency of gene correction [88,89]. However, achieving the full potential of ZFNs for genome engineering in human cells requires their efficient delivery to the relevant cell types. Lombardo and colleagues exploited the infectivity of integrase-defective lentiviral vectors (IDLV) to express ZFNs and provide the template DNA for ge ne correction in different cell types [90]. IDLV-mediated delivery supported high rates (13–39%) of editing at the IL-2 receptor common γ-chain gene (IL2RG) across different cell types as well as human embryonic stem cells (5%), allowing selection-free isolation of clonogenic cells with the desired genetic modification. Therefore, this technique opens new and exciting possibilities. By modifying the ZFN binding specificity and selecting an appropriate donor sequence, one could target the IDLV-ZFN system to any individual site in the human genome avoiding random integration (Figure 1B) and, potentially, genome toxicity [88-91].

However, there are current
obstacles to successfully apply this
therapeutic approach to humans. Some of them include the need for
improved efficiency of gene delivery, insertion of the gene into
non-oncogenic sites and the potential negative or positive
contributions of the ▀-thalassemic genotype and potential modifiers to
the effectiveness of the gene transfer [1]. Original
studies in animal
models utilized mice with deletions of the ▀-globin genes. These
mutations do not reflect the phenotypic variability observed in
▀-thalassemic patients. Thus, there is a gap in knowledge between our
understanding of the primary mutation, the corresponding phenotype, and
the approach to cure an individual patient based on his/her genotype
(i.e. understanding of the disease and its treatment by genetic
modalities). To date this variability has not been addressed and no
studies have focused on the efficacy of gene therapy in relation to the
different genotypes of the patients. Although gene therapy is an area
of active clinical investigation, the aforementioned obstacles limit
its use in the management of thalassemia. Nonetheless, as we showed in
our review the successful transfer of globin genes into hematopoietic
cells of humans has been demonstrated and is encouraging.
Gene Correction and Ips Cells:
Triplex-forming
oligonucleotides and triplex-forming peptide nucleic
acids (PNAs) have been shown to stimulate recombination in mammalian
cells via site-specific binding and creation of altered helical
structures that provoke DNA repair [92,93].
Cotransfection of PNAs and
recombinatory donor DNA fragments, Chin and co-workers demonstrated
that these complexes can promote single base-pair modification at the
start of the second intron of the beta-globin gene, the site of a
common thalassemia-associated mutation [94]. This
single base pair change
was detected by the restoration of proper splicing of transcripts
produced from a green fluorescent protein-beta-globin fusion gene. The
ability of these PNAs to induce recombination was dependent on dose,
sequence, cell-cycle stage, and the presence of a homologous donor DNA
molecule. They also showed that these PNAs were effective in
stimulating the modification of the endogenous beta-globin locus in
human cells, including primary hematopoietic progenitor cells. Enhanced
recombination, however, did not exhibit frequencies superior to 0.4% [94].
However, this technology could be a powerful tool in combination with
the generation of stem cells. In particular, introduction of the genes
Oct3/4, Sox2 with either Klf4 and c-Myc or Nanog and Lin28 genes can
induced pluripotent stem (iPS) cells [95,115,24,96]. Ye and
coworkers
shown that iPS cells can be generated from cells derived from skin
fibroblasts, amniotic fluid or chorionic villus sampling of patients
with ▀-thalassemia [97]. Subsequently, the iPS cells
were differentiated
into hematopoietic cells that synthesized hemoglobin. Therefore, in the
future the mutation in the ▀-globin gene of these iPS cells could be
corrected by gene targeting and the cells differentiated into HSCs to
be returned to the patient [94]. Figure 1C depicts this approach. In
fact, mice affected by SCD were cured using this strategy [98].
However,
there are some obstacles that need to be overcome before iPS treatment
of ▀-thalassemia will be utilized. One of the most pressing problems is
elimination of the transcription factors when they are no longer
needed. Second, it is necessary to reestablish the correct
re-programming so that the iPS cells do not develop into tumors.
Splice-Switching and Stop Codon Readthrough:
Defective ▀-globin gene
expression and ▀-globin deficiency can be
attributed to almost 200 thalassemic mutations. However, only 10
mutations are responsible for the majority of cases worldwide and some
of the most frequent cause aberrant splicing of intron 1 (IVS1-110,
IVS1-6, IVS1-5) or intron 2 (IVS2-654, IVS2-745) [99,112]. These mutations
lead to incorrectly spliced mRNAs, even though the correct splice sites
remain undamaged and potentially functional. Use of small nuclear RNA
(snRNA) and splice-switching oligo-nucleotides represents a promising
approach since these molecules can restore the corrected splicing
re-establishing the synthesis of the normal protein [94,100-108].
Therefore blocking the aberrant splice sites with antisense
oligonucleotides forces the splicing machinery to reselect the existing
correct splice sites. Expression of antisense sequences targeted to the
aberrant splice sites in thalassemic pre-mRNA has been successful,
restoring the correct splicing pattern and ultimately restoring
hemoglobin synthesis [102,93].
This was demonstrated in HSCs and erythroid
progenitor cells from a patient with IVS2-745/IVS2-1 thalassemia. After
transduction of the patient cells with a lentiviral vector that express
a snRNA targeting the mutant RNA, the levels of correctly spliced
▀-globin mRNA and adult hemoglobin were approximately 25-fold over
baseline [108]. Similarly, the correct splicing
pattern was restored in a
mouse model of IVS2-654 thalassemia. This was achieved by delivery in
vivo of a splice-switching oligonucleotide, a morpholino oligomer
conjugated with an arginine-rich peptide. Repaired ▀-globin mRNA
restored significant amounts of hemoglobin in the peripheral blood of
the IVS2-654 mouse, improving the number and quality of erythroid
cells [107].
Another approach showing a great potential for the treatment of genetic disorders characterized by to premature termination codons (PTCs) is the use of drugs to induce stop codon readthrough. These modified RNA would protected against non-sense mediated mRNA decay (NMD) and allow production of a protein [109]. Aminoglycoside antibiotics can decrease the accuracy in the codon-anticodon base pairing, inducing a ribosomal read through of premature termination codon. These findings have led to the development of a pharmacologic approach to treat thalassemic patients carrying the ▀0-39 mutation, which introduces a PTC in codon 39 of the ▀-globin gene and is one of the most frequent thalassemic mutations in the Mediterranean littoral1. Aminoglycosides and analogous molecules were tested in their ability to restore ▀-globin protein synthesis on human erythroid cells (K562) carrying a lentiviral construct containing the ▀0-39 globin-gene[110]. Treatment of these cells with geneticin (G418) and other aminoglycosides restored the production of ▀-globin [110]. Moreover, after FACS and high performance liquid chromatography (HPLC) analyses, G418 was also demonstrated to partially correct the biological function of the ▀0-39 globin mRNA in erythroid precursor cells from ▀0-39 homozygous thalassemia patients [111]. This study strongly suggests that ribosomal read-through should be considered a novel approach for treatment of ▀0 thalassemia caused by premature stop codon mutations and NMD.
Another approach showing a great potential for the treatment of genetic disorders characterized by to premature termination codons (PTCs) is the use of drugs to induce stop codon readthrough. These modified RNA would protected against non-sense mediated mRNA decay (NMD) and allow production of a protein [109]. Aminoglycoside antibiotics can decrease the accuracy in the codon-anticodon base pairing, inducing a ribosomal read through of premature termination codon. These findings have led to the development of a pharmacologic approach to treat thalassemic patients carrying the ▀0-39 mutation, which introduces a PTC in codon 39 of the ▀-globin gene and is one of the most frequent thalassemic mutations in the Mediterranean littoral1. Aminoglycosides and analogous molecules were tested in their ability to restore ▀-globin protein synthesis on human erythroid cells (K562) carrying a lentiviral construct containing the ▀0-39 globin-gene[110]. Treatment of these cells with geneticin (G418) and other aminoglycosides restored the production of ▀-globin [110]. Moreover, after FACS and high performance liquid chromatography (HPLC) analyses, G418 was also demonstrated to partially correct the biological function of the ▀0-39 globin mRNA in erythroid precursor cells from ▀0-39 homozygous thalassemia patients [111]. This study strongly suggests that ribosomal read-through should be considered a novel approach for treatment of ▀0 thalassemia caused by premature stop codon mutations and NMD.
References
- Adamkiewicz TV, Szabolcs P, Haight A, Baker
KS, Staba S, Kedar A, Chiang KY, Krishnamurti L, Boyer MW, Kurtzberg J,
Wagner JE, Wingard JR, Yeager AM. Unrelated cord blood
transplantation in children with sickle cell disease: review of
four-center experience. Pediatr Transplant 2007;11: 641-644.

- Arumugam PI, Scholes J, Perelman N, Xia P,
Yee JK, Malik PImproved human beta-globin expression from
self-inactivating lentiviral vectors carrying the chicken
hypersensitive site-4 (cHS4) insulator element. Mol Ther 2007;15:
1863-1871.

- Bank A, Dorazio R, Leboulch P A
phase I/II clinical trial of beta-globin gene therapy for
beta-thalassemia. Ann N Y Acad Sci 2005; 1054: 308-316.

- Bell AC, West AG, Felsenfeld G. The
protein CTCF is required for the enhancer blocking activity of
vertebrate insulators. Cell 1999; 98: 387-396.

- Bell AC, West AG, Felsenfeld G. Insulators
and boundaries: versatile regulatory elements in the
eukaryotic. Science 2001; 291: 447-450.

- Bender MA, Gelinas RE, Miller AD. A
majority of mice show long-term expression of a human beta-globin gene
after retrovirus transfer into hematopoietic stem cells. Molecular and
cellular biology 1989;9(4): 1426-1434.

- Beutel G, Meyer J, Ma L, Yin S, Eder M, von
Neuhoff N, Wilkens L, Wei J, Hertenstein B, Heil G, Schlegelberger B,
Ganser A, Li Z, Baum C. Expression of the p75 neurotrophin
receptor in acute leukaemia. Br J Haematol 2005;131: 67-70.

- Bibikova M, Carroll D, Segal DJ, Trautman
JK, Smith J, Kim YG, Chandrasegaran S. Stimulation of homologous
recombination through targeted cleavage by chimeric nucleases.
Molecular and cellular biology 2001;21: 289-297.

- Bodine DM, Karlsson S, Nienhuis AW.
Combination of interleukins 3 and 6 preserves stem cell function in
culture and enhances retrovirus-mediated gene transfer into
hematopoietic stem cells. Proceedings of the National Academy of
Sciences of the United States of America 1989; 86: 8897-8901.

- Boulad F, Giardina P, Gillio A, Kernan N,
Small T, Brochstein J, Van Syckle K, George D, Szabolcs P, O'Reilly RJ
.Bone marrow transplantation for homozygous beta-thalassemia. The
Memorial Sloan-Kettering Cancer Center experience. Ann N Y Acad Sci
1998;850: 498-502.

- Burgess-Beusse
B, Farrell C, Gaszner M,
Litt M, Mutskov V, Recillas-Targa F, Simpson M, West A, Felsenfeld
G.The insulation of genes from external enhancers and silencing
chromatin. Proc Natl Acad Sci U S A. 2002 Dec 10;99 Suppl
4:16433-7. Epub 2002 Aug 1.

- Bush S, Mandel FS, Giardina PJ .
Future orientation and life expectations of adolescents and young
adults with thalassemia major. Ann N Y Acad Sci 1998; 850: 361-369.

- Calmels B, Ferguson C, Laukkanen MO, Adler
R, Faulhaber M, Kim HJ, Sellers S, Hematti P, Schmidt M, von Kalle C,
Akagi K, Donahue RE, Dunbar CE. Recurrent retroviral vector
integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic
cells. Blood 2005;106: 2530-2533.

- Carey BW, Markoulaki S, Hanna J, Saha K,
Gao Q, Mitalipova M, Jaenisch R. Reprogramming of murine and
human somatic cells using a single polycistronic vector. Proceedings of
the National Academy of Sciences of the United States of America
2009;106: 157-162.

- Case SS, Price MA, Jordan CT, Yu XJ, Wang
L, Bauer G, Haas DL, Xu D, Stripecke R, Naldini L, Kohn DB, Crooks
GM.Stable transduction of quiescent CD34(+)CD38(-) human
hematopoietic cells by HIV-1-based lentiviral vectors. Proceedings of
the National Academy of Sciences of the United States of America
1999;96:
2988-2993.

- Chang JC, Liu D, Kan YW .A
36-base-pair core sequence of locus control region enhances
retrovirally transferred human beta-globin gene expression. Proceedings
of the National Academy of Sciences of the United States of America
1992;89: 3107-3110.

- Chin JY, Kuan JY, Lonkar PS, Krause DS,
Seidman MM, Peterson KR, Nielsen PE, Kole R, Glazer PM .
Correction of a splice-site mutation in the beta-globin gene stimulated
by triplex-forming peptide nucleic acids. Proceedings of the National
Academy of Sciences of the United States of America 2008;105:
13514-13519.

- Chui DH, Hardison R, Riemer C, Miller W,
Carver MF, Molchanova TP, Efremov GD, Huisman TH . An electronic
database of human hemoglobin variants on the World Wide Web. Blood
1998;9: 2643-2644.

- Ciavatta DJ, Ryan TM, Farmer SC, Townes TM
.Mouse model of human beta zero thalassemia: targeted deletion of
the mouse beta maj- and beta min-globin genes in embryonic stem cells.
Proceedings of the National Academy of Sciences of the United States of
America 1995;92: 9259-9263.

- Coffin JM, Hughes SH, Varmus HE (1997) Retrovirus: Cold Spring Harbor Laboratory Press.
- Cone RD, Weber-Benarous A, Baorto D,
Mulligan RC. Regulated expression of a complete human beta-globin
gene encoded by a transmissible retrovirus vector. Molecular and
cellular biology 1987; 7: 887-897.

- Crone TM, Pegg AE. A single amino
acid change in human O6-alkylguanine-DNA alkyltransferase decreasing
sensitivity to inactivation by O6-benzylguanine. Cancer Res 1993;53:
4750-4753.

- Cunningham MJ, Macklin EA, Neufeld EJ,
Cohen AR. Complications of beta-thalassemia major in North
America. Blood 2004; 10: 34-39.

- Dave UP, Akagi K, Tripathi R, Cleveland
SM, Thompson MA, Yi M, Stephens R, Downing JR, Jenkins NA, Copeland
NG.Murine leukemias with retroviral insertions at Lmo2 are
predictive of the leukemias induced in SCID-X1 patients following
retroviral gene therapy. PLoS
Genet. 2009 May;5:e1000491.

- Dominski Z, Kole R. Restoration of
correct splicing in thalassemic pre-mRNA by antisense oligonucleotides.
Proc Natl Acad Sci U S A 1993;90: 8673-8677.

- Dzierzak EA, Papayannopoulou T, Mulligan
RC. Lineage-specific expression of a human beta-globin gene in
murine bone marrow transplant recipients reconstituted with
retrovirus-transduced stem cells. Nature 1988;331: 35-41.

- Emery DW, Morrish F, Li Q,
Stamatoyannopoulos G .Analysis of gamma-globin expression
cassettes in retrovirus vectors. Hum Gene Ther 1999;1: 877-888.

- Emery DW, Yannaki E, Tubb J, Nishino T, Li
Q, Stamatoyannopoulos G .Development of virus vectors for gene
therapy of beta chain hemoglobinopathies: flanking with a chromatin
insulator reduces gamma-globin gene silencing in vivo. Blood 2002;100:
2012-2019.

- Emery DW, Yannaki E, Tubb J,
Stamatoyannopoulos G. A chromatin insulator protects retrovirus
vectors from chromosomal position effects. Proceedings of the National
Academy of Sciences of the United States of America 2000;97: 9150-9155.

- Evans-Galea MV, Wielgosz MM, Hanawa H,
Srivastava DK, Nienhuis AW . Suppression of clonal dominance in
cultured human lymphoid cells by addition of the cHS4 insulator to a
lentiviral vector. Mol Ther 2007;15: 801-809.

- Fragkos M, Anagnou NP, Tubb J, Emery DW.
Use of the hereditary persistence of fetal hemoglobin 2 enhancer
to increase the expression of oncoretrovirus vectors for human
gamma-globin. Gene therapy 2005;12: 1591-1600.

- Fu XH, Liu DP, Liang CC. Chromatin
structure and transcriptional regulation of the beta-globin locus.
Experimental cell research 2002;278: 1-11.

- Fucharoen S, Winichagoon P.Clinical
and hematologic aspects of hemoglobin E beta-thalassemia. Curr Opin
Hematol 2000;7: 106-112.

- Gardenghi S, Marongiu MF, Ramos P, Guy E,
Breda L, Chadburn A, Liu Y, Amariglio N, Rechavi G, Rachmilewitz EA,
Breuer W, Cabantchik ZI, Wrighting DM, Andrews NC, de Sousa M, Giardina
PJ, Grady RW, Rivella S . Ineffective erythropoiesis in
{beta}-thalassemia is characterized by increased iron absorption
mediated by down-regulation of hepcidin and up-regulation of
ferroportin. Blood 2007; 109: 5027-5035.

- Gerson SL (2000) Drug resistance gene
transfer: Stem cell protection and therapeutic efficacy. Exp Hematol
28(12): 1315-1324.

- Giardina PJ, Grady RW. Chelation therapy
in beta-thalassemia: an optimistic update. Semin Hematol 2001;38:
360-366.

- Giardine B, van Baal S, Kaimakis P, Riemer
C, Miller W, Samara M, Kollia P, Anagnou NP, Chui DH, Wajcman H,
Hardison RC, Patrinos GP. HbVar database of human hemoglobin
variants and thalassemia mutations: 2007 update. Hum Mutat 2007;
28: 206.

- Giardini C, Lucarelli G . Bone marrow
transplantation in the treatment of thalassemia. Curr Opin Hematol
1994;1: 170-176.

- Gorman L, Suter D, Emerick V, Schumperli
D, Kole R . Stable alteration of pre-mRNA splicing patterns by
modified U7 small nuclear RNAs. Proc Natl Acad Sci U S A 1998; 95:
4929-4934.

- Greaves DR, Fraser P, Vidal MA, Hedges MJ,
Ropers D, Luzzatto L, Grosveld F.A transgenic mouse model of
sickle cell disorder [see comments]. Nature 1990;343: 183-185.

- Grosveld F, van Assendelft GB, Greaves DR,
Kollias G. Position-independent, high-level expression of the
human beta-globin gene in transgenic mice. Cell 1987;51: 975-985.

- Hacein-Bey-Abina S, Von Kalle C, Schmidt
M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R,
Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile
G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D,
Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre
E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le
Deist F, Fischer A, Cavazzana-Calvo M . LMO2-associated clonal T
cell proliferation in two patients after gene therapy for SCID-X1.
Science 2003; 302: 415-419.

- Han XD, Lin C, Chang J, Sadelain M, Kan YW
. Fetal gene therapy of alpha-thalassemia in a mouse model.
Proceedings of the National Academy of Sciences of the United States of
America 2007; 104: 9007-9011.

- Hanawa H, Yamamoto M, Zhao H, Shimada T,
Persons DA .Optimized lentiviral vector design improves titer and
transgene expression of vectors containing the chicken beta-globin
locus HS4 insulator element. Mol Ther 2009; 17: 667-674.

- Hanna J, Wernig M, Markoulaki S, Sun CW,
Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM,
Jaenisch R.Treatment of sickle cell anemia mouse model with iPS
cells generated from autologous skin. Science 2007;318: 1920-1923.

- Hargrove PW, Kepes S, Hanawa H, Obenauer
JC, Pei D, Cheng C, Gray JT, Neale G. Persons DA. Globin
lentiviral vector insertions can perturb the expression of endogenous
genes in beta-thalassemic hematopoietic cells. Mol Ther 2008;16:
525-533.

- Hongeng S, Pakakasama S, Chuansumrit A,
Sirachainan N, Sura T, Ungkanont A, Chuncharunee S, Jootar S,
Issaragisil S. Reduced intensity stem cell transplantation for
treatment of class 3 Lucarelli severe thalassemia patients. Am J
Hematol 2007; 82: 1095-1098.

- Imren S, Fabry ME, Westerman KA, Pawliuk
R, Tang P, Rosten PM, Nagel RL, Leboulch P, Eaves CJ, Humphries RK.
High-level beta-globin expression and preferred intragenic
integration after lentiviral transduction of human cord blood stem
cells. J Clin Invest 2004; 114: 953-962.

- Imren S, Payen E, Westerman KA, Pawliuk R,
Fabry ME, Eaves CJ, Cavilla B, Wadsworth LD, Beuzard Y, Bouhassira EE,
Russell R, London IM, Nagel RL, Leboulch P, Humphries RK.
Permanent and panerythroid correction of murine beta thalassemia by
multiple lentiviral integration in hematopoietic stem cells.
Proceedings of the National Academy of Sciences of the United States of
America 2002;99: 14380-14385.

- Kalberer
CP, Pawliuk R, Imren S, Bachelot
T, Takekoshi KJ, Fabry M, Eaves CJ, London IM, Humphries RK, Leboulch P
. Preselection of retrovirally transduced bone marrow avoids
subsequent stem cell gene silencing and age-dependent extinction of
expression of human beta-globin in engrafted mice.PG - 5411-5.
Proceedings of the National Academy of Sciences of the United States of
America 2000; 97: 5411-5415.

- Karlsson S, Bodine DM, Perry L,
Papayannopoulou T, Nienhuis AW. Expression of the human
beta-globin gene following retroviral-mediated transfer into
multipotential hematopoietic progenitors of mice. Proceedings of the
National Academy of Sciences of the United States of America 1988;85:
6062-6066.

- Karlsson S, Papayannopoulou T, Schweiger
SG, Stamatoyannopoulos G, Nienhuis AW. Retroviral-mediated
transfer of genomic globin genes leads to regulated production of RNA
and protein. Proceedings of the National Academy of Sciences of the
United States of America 1987;84: 2411-2415.

- Kim YJ, Kim YS, Larochelle A, Renaud G,
Wolfsberg TG, Adler R, Donahue RE, Hematti P, Hong BK, Roayaei J, Akagi
K, Riberdy JM, Nienhuis AW, Dunbar CE, Persons DA. Sustained
high-level polyclonal hematopoietic marking and transgene expression 4
years after autologous transplantation of rhesus macaques with SIV
lentiviral vector-transduced CD34+ cells. Blood 2009; 113:
5434-5443.

- La Nasa G, Argiolu F, Giardini C, Pession
A, Fagioli F, Caocci G, Vacca A, De Stefano P, Piras E, Ledda A,
Piroddi A, Littera R, Nesci S, Locatelli F. Unrelated bone marrow
transplantation for beta-thalassemia patients: The experience of the
Italian Bone Marrow Transplant Group. Ann N Y Acad Sci 2005;1054:
186-195.

- Lacerra G, Sierakowska H, Carestia C,
Fucharoen S, Summerton J, Weller D, Kole R . Restoration of
hemoglobin A synthesis in erythroid cells from peripheral blood of
thalassemic patients. Proceedings of the National Academy of Sciences
of the United States of America 2000;97: 9591-9596.

- Leboulch P, Huang GM, Humphries RK, Oh YH,
Eaves CJ, Tuan DY, London IM. Mutagenesis of retroviral vectors
transducing human beta-globin gene and beta-globin locus control region
derivatives results in stable transmission of an active transcriptional
structure. Embo J 1994;13: 3065-3076

- Levasseur DN, Ryan TM, Pawlik KM, Townes
TM. Correction of a mouse model of sickle cell disease:
lentiviral/antisickling beta-globin gene transduction of unmobilized,
purified hematopoietic stem cells. Blood 2003;102: 4312-4319.

- Levasseur DN, Ryan TM, Reilly MP, McCune
SL, Asakura T, Townes TM. A recombinant human hemoglobin with
anti-sickling properties greater than fetal hemoglobin. J Biol Chem
2004;279: 27518-27524.

- Li CL, Emery DW. The cHS4 chromatin
insulator reduces gammaretroviral vector silencing by epigenetic
modifications of integrated provirus. Gene therapy 2008; 15:
49-53.

- Li CL, Xiong D, Stamatoyannopoulos G,
Emery DW. Genomic and functional assays demonstrate reduced
gammaretroviral vector genotoxicity associated with use of the cHS4
chromatin insulator. Mol Ther 2009;17: 716-724.

- Lombardo A, Genovese P, Beausejour CM,
Colleoni S, Lee YL, Kim KA, Ando D, Urnov FD, Galli C, Gregory PD,
Holmes MC, Naldini L. Gene editing in human stem cells using zinc
finger nucleases and integrase-defective lentiviral vector delivery.
Nature biotechnology 2007;25: 1298-1306.

- Lucarelli G, Galimberti M, Polchi P,
Angelucci E, Baronciani D, Giardini C, Politi P, Durazzi SM, Muretto P,
Albertini F. Bone marrow transplantation in patients with
thalassemia. N Engl J Med 1990;322: 417-421.

- Lung HY, Meeus IS, Weinberg RS, Atweh GF.
In vivo silencing of the human gamma-globin gene in murine
erythroid cells following retroviral transduction. Blood Cells Mol
Dis 2000;
26: 613-619.

- Luzzatto L. Genetics of red cells and
susceptibility to malaria. Blood 1979;54: 961-976.

- Luzzatto L, Goodfellow P . Sickle cell
anaemia. A simple disease with no cure [news]. Nature 337: 17-18.

- Maherali N, Sridharan R, Xie W, Utikal J,
Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R,
Plath K, Hochedlinger K . Directly reprogrammed fibroblasts show
global epigenetic remodeling and widespread tissue contribution. Cell
Stem Cell 2007; 1: 55-70.

- Malik P, Arumugam PI, Yee JK, Puthenveetil
G. Successful correction of the human Cooley's anemia
beta-thalassemia major phenotype using a lentiviral vector flanked by
the chicken hypersensitive site 4 chromatin insulator. Ann N Y Acad
Sci 2005;1054: 238-249.

- Maquat LE. Nonsense-mediated mRNA decay in
mammals. J Cell Sci 2005;118: 1773-1776.

- May C, Rivella S, Callegari J, Heller G,
Gaensler KM, Luzzatto L, Sadelain M. Therapeutic haemoglobin
synthesis in beta-thalassaemic mice expressing lentivirus-encoded human
beta-globin. Nature 2000; 406: 82-86.

- May C, Rivella S, Chadburn A, Sadelain M .
Successful treatment of murine beta-thalassemia intermedia by
transfer of the human beta-globin gene. Blood 2002; 99: 1902-1908.

- Miccio A, Cesari R, Lotti F, Rossi C,
Sanvito F, Ponzoni M, Routledge SJ, Chow CM, Antoniou MN, Ferrari G .
In vivo selection of genetically modified erythroblastic
progenitors leads to long-term correction of beta-thalassemia.
Proceedings of the National Academy of Sciences of the United States of
America 2008;105: 10547-10552.

- Miller JL, Walsh CE, Ney PA, Samulski RJ,
Nienhuis AW. Single-copy transduction and expression of human
gamma-globin in K562 erythroleukemia cells using recombinant
adeno-associated virus vectors: the effect of mutations in NF-E2 and
GATA-1 binding motifs within the hypersensitivity site 2 enhancer
[published erratum appears in Blood 1995 Feb 1;85(3):862]. Blood
1993;82:
1900-1906.

- Milner PF, Clegg JB, Weatherall DJ .
Haemoglobin-H disease due to a unique haemoglobin variant with an
elongated alpha-chain. Lancet 1971;1: 729-732.

- Murari J, Smith LL, Wilson JB, Schneider
RG, Huisman TH. Some properties of hemoglobin Gun Hill. Hemoglobin 1:
267-282.

- Nishino T, Tubb J, Emery DW. Partial
correction of murine beta-thalassemia with a gammaretrovirus vector for
human gamma-globin. Blood Cells Mol Dis 2006; 37: 1-7.

- Novak U, Harris EA, Forrester W, Groudine
M, Gelinas R. High-level beta-globin expression after retroviral
transfer of locus activation region-containing human beta-globin gene
derivatives into murine erythroleukemia cells. Proceedings of the
National Academy of Sciences of the United States of America 1990;87:
3386-3390.

- Pabo CO, Peisach E, Grant RA. Design
and selection of novel Cys2His2 zinc finger proteins. Annual review of
biochemistry 2001;70: 313-340.

- Pawliuk R, Westerman KA, Fabry ME, Payen
E, Tighe R, Bouhassira EE, Acharya SA, Ellis J, London IM, Eaves CJ,
Humphries RK, Beuzard Y, Nagel RL, Leboulch P. Correction of
sickle cell disease in transgenic mouse models by gene therapy. Science
2001;
294: 2368-2371.

- Pike-Overzet K, van der Burg M, Wagemaker
G, van Dongen JJ, Staal FJ. New insights and unresolved issues
regarding insertional mutagenesis in X-linked SCID gene therapy. Mol
Ther 2007;15: 1910-1916.

- Plavec I, Papayannopoulou T, Maury C,
Meyer F. A human beta-globin gene fused to the human beta-globin
locus control region is expressed at high levels in erythroid cells of
mice engrafted with retrovirus-transduced hematopoietic stem cells.
Blood 1993;81: 1384-1392.

- Porteus MH, Carroll D . Gene targeting
using zinc finger nucleases. Nature biotechnology 2005; 23: 967-973.

- Puthenveetil
G, Scholes J, Carbonell D,
Qureshi N, Xia P, Zeng L, Li S, Yu Y, Hiti AL, Yee JK, Malik P
Successful correction of the human beta-thalassemia major phenotype
using a lentiviral vector. Blood2004;104: 3445-3453.

- Raftopoulos H, Ward M, Leboulch P, Bank A.
Long-term transfer and expression of the human beta-globin gene
in a mouse transplant model. Blood 1997;90: 3414-3422.

- Ragg S, Xu-Welliver M, Bailey J, D'Souza
M, Cooper R, Chandra S, Seshadri R, Pegg AE, Williams DA Direct
reversal of DNA damage by mutant methyltransferase protein protects
mice against dose-intensified chemotherapy and leads to in vivo
selection of hematopoietic stem cells. Cancer Res 2000;60: 5187-5195.

- Rechavi G, Rivella S.Regulation of iron
absorption in hemoglobinopathies. Curr Mol Med 2008;8: 646-662.

- Ren S, Wong BY, Li J, Luo XN, Wong PM,
Atweh GF. Production of genetically stable high-titer retroviral
vectors that carry a human gamma-globin gene under the control of the
alpha-globin locus control region. Blood 1996;87: 2518-2524.

- Rivella S, Callegari JA, May C, Tan CW,
Sadelain M .The cHS4 insulator increases the probability of
retroviral expression at random chromosomal integration sites. J Virol
2000;74: 4679-4687.

- Rivella S, May C, Chadburn A, Riviere I,
Sadelain M . A novel murine model of Cooley anemia and its rescue
by lentiviral-mediated human beta -globin gene transfer. Blood 2003;
101:
2932-2939.

- Rogers FA, Vasquez KM, Egholm M, Glazer
PM. Site-directed recombination via bifunctional PNA-DNA conjugates.
Proceedings of the National Academy of Sciences of the United States of
America 2002;99: 16695-16700.

- Sabatino DE, Seidel NE, Aviles-Mendoza GJ,
Cline AP, Anderson SM, Gallagher PG, Bodine DM Long-term
expression of gamma-globin mRNA in mouse erythrocytes from retrovirus
vectors containing the human gamma-globin gene fused to the ankyrin-1
promoter. Proceedings of the National Academy of Sciences of the United
States of America 2000a;97: 13294-13299.

- Sabatino DE, Wong C, Cline AP, Pyle L,
Garrett LJ, Gallagher PG, Bodine DM (2000b) A minimal ankyrin promoter
linked to a human gamma-globin gene demonstrates erythroid specific
copy number dependent expression with minimal position or enhancer
dependence in transgenic mice. J Biol Chem 275(37): 28549-28554.

- Sadelain M, Boulad F, Galanello R,
Giardina P, Locatelli F, Maggio A, Rivella S, Riviere I, Tisdale J
Therapeutic options for patients with severe beta-thalassemia:
the need for globin gene therapy. Hum Gene 2007;Ther 18: 1-9.

- Sadelain M, Wang CH, Antoniou M, Grosveld
F, Mulligan RC.Generation of a high-titer retroviral vector
capable of expressing high levels of the human beta-globin gene.
Proceedings of the National Academy of Sciences of the United States of
America 1995;92: 6728-6732.

- Salvatori F, Breveglieri G, Zuccato C,
Finotti A, Bianchi N, Borgatti M, Feriotto G, Destro F, Canella A,
Brognara E, Lampronti I, Breda L, Rivella S, Gambari R. Production of
beta-globin and adult hemoglobin following G418 treatment
of erythroid precursor cells from homozygous beta(0)39 thalassemia
patients. Am
J Hematol. 2009 Nov;84:720-8.

- Salvatori F, Cantale V, Breveglieri G,
Zuccato C, Finotti A, Bianchi N, Borgatti M, Feriotto G, Destro F,
Canella A, Breda L, Rivella S, Gambari R. Development of K562
cell clones expressing beta-globin mRNA carrying the beta039
thalassaemia mutation for the screening of correctors of stop-codon
mutations. Biotechnology and applied biochemistry 2009b;54: 41-52.

- Samakoglu S, Lisowski L, Budak-Alpdogan T,
Usachenko Y, Acuto S, Di Marzo R, Maggio A, Zhu P, Tisdale JF, Riviere
I, Sadelain M .A genetic strategy to treat sickle cell anemia by
coregulating globin transgene expression and RNA interference. Nature
biotechnology 2006;24: 89-94.

- Sazani P, Kole R. Therapeutic
potential of antisense oligonucleotides as modulators of alternative
splicing. J Clin Invest 2003;112: 481-486.

- Schambach A, Baum C .Clinical application
of lentiviral vectors - concepts and practice. Curr Gene Ther 2008;8:
474-482.

- Sierakowska H, Sambade MJ, Agrawal S, Kole
R. Repair of thalassemic human beta-globin mRNA in mammalian
cells by antisense oligonucleotides. Proceedings of the National
Academy of Sciences of the United States of America 1996; 93:
12840-12844.

- Silvestroni E, Bianco I. [a New
Kind of Drepanocytic Anemia: Hemoglobin a-Hemoglobin Lepore Disease.].
Progr Med (Napoli)1963a; 19: 545-548.

- Silvestroni E, Bianco I (1963b) [First
Case of the Hb Lepore Disease with Microcythemia Observed in Italy.].
Policlinico [Prat] 70: 1513-1517.

- Sohan K, Billington M, Pamphilon D,
Goulden N, Kyle P.Normal growth and development following in
utero diagnosis and treatment of homozygous alpha-thalassaemia.
Bjog 2002;109: 1308-1310.

- Steinberg MH, Forget BG, Higgs DR, Nagel RL . Disorders of hemoglobin: Genetics, Pathophysiology and Clinical Management, 2001a; Cambridge, UK: Cambridge University Press.
- Steinberg MH, Forget BG, Higgs DR, Nagel RL (2001b) Molecular Mechanism of ▀ Thalassemia; Bernard G. Forget, Cambridge, UK: Cambridge University Press.
- Suter D, Tomasini R, Reber U, Gorman L,
Kole R, Schumperli D. Double-target antisense U7 snRNAs promote
efficient skipping of an aberrant exon in three human beta-thalassemic
mutations. Hum Mol Genet 1999;8: 2415-2423.

- Suwanmanee T, Sierakowska H, Fucharoen S,
Kole R . Repair of a splicing defect in erythroid cells from
patients with beta-thalassemia/HbE disorder. Mol Ther 2002a; 6: 718-726.

- Suwanmanee T, Sierakowska H, Lacerra G,
Svasti S, Kirby S, Walsh CE, Fucharoen S, Kole R. Restoration of
human beta-globin gene expression in murine and human IVS2-654
thalassemic erythroid cells by free uptake of antisense
oligonucleotides. Mol Pharmacol 2002b; 62: 545-553.

- Svasti S, Suwanmanee T, Fucharoen S,
Moulton HM, Nelson MH, Maeda N, Smithies O, Kole R RNA repair
restores hemoglobin expression in IVS2-654 thalassemic mice. Proc Natl
Acad Sci U S A 2009; 106(: 1205-1210.

- Takahashi K, Tanabe K, Ohnuki M, Narita
M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent
stem cells from adult human fibroblasts by defined factors. Cell
2007;13: 861-872.

- Takahashi K, Yamanaka S. Induction
of pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell 2006; 126: 663-676.

- Thomas ED, Buckner CD, Sanders JE,
Papayannopoulou T, Borgna-Pignatti C, De Stefano P, Sullivan KM, Clift
RA, Storb R. Marrow transplantation for thalassaemia. 1982;
Lancet
2: 227-229.

- Vacek MM, Ma H, Gemignani F, Lacerra G,
Kafri T, Kole R . High-level expression of hemoglobin A in human
thalassemic erythroid progenitor cells following lentiviral vector
delivery of an antisense snRNA. Blood 2003;101: 104-111.

- Vichinsky EP . Changing patterns of
thalassemia worldwide. Ann N Y Acad Sci 2005;1054: 18-24.

- Weatherall DJ, Clegg JB. Inherited
haemoglobin disorders: an increasing global health problem. Bull World
Health Organ 2001; 79: 704-712.

- Wernig M, Meissner A, Foreman R,
Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R . In
vitro reprogramming of fibroblasts into a pluripotent ES-cell-like
state. Nature 2007; 448: 318-324.

- Yang B, Kirby S, Lewis J, Detloff PJ,
Maeda N, Smithies O . A mouse model for beta 0-thalassemia. Proc
Natl Acad Sci U S A 1995; 92: 11608-11612.

- Ye L, Chang JC, Lin C, Sun X, Yu J, Kan
YW . Induced pluripotent stem cells offer new approach to therapy
in thalassemia and sickle cell anemia and option in prenatal diagnosis
in genetic diseases. Proceedings of the National Academy of Sciences of
the United States of America 2009;106: 9826-9830.

- Zhao H, Pestina TI, Nasimuzzaman M, Mehta
P, Hargrove PW, Persons DA. Amelioration of murine
beta-thalassemia through drug selection of hematopoietic stem cells
transduced with a lentiviral vector encoding both gamma-globin and the
MGMT drug-resistance gene. Blood 2009;113: 5747-5756.
