EBV and HIV-Related Lymphoma
Michele Bibas and Andrea Antinori
Clinical Department, National Institute for Infectious Diseases “Lazzaro Spallanzani”, IRCCS, Rome, Italy
Published: December 29, 2009
Received: December 23, 2009
Accepted: December 27, 2009
Medit J Hemat Infect Dis 2009, 1(2): e2009032, DOI 10.4084/MJHID.2009.032
This article is available from: http://www.mjhid.org/article/view/5272
Abstract
HIV-associated lymphoproliferative disorders represent a heterogeneous group of diseases, arising in the presence of HIV-associated immunodeficiency. The overall prevalence of HIV-associated lymphoma is significantly higher compared to that of the general population and it continues to be relevant even after the wide availability of highly active antiretroviral therapy (HAART) (1). Moreover, they still represent one of the most frequent cause of death in HIV-infected patients. Epstein–Barr virus (EBV), a γ-Herpesviruses, is involved in human lymphomagenesis, particularly in HIV immunocompromised patients. It has been largely implicated in the development of B-cell lymphoproliferative disorders as Burkitt lymphoma (BL), Hodgkin disease (HD), systemic non Hodgkin lymphoma (NHL), primary central nervous system lymphoma (PCNSL), nasopharyngeal carcinoma (NC). Virus-associated lymphomas are becoming of significant concern for the mortality of long-lived HIV immunocompromised patients, and therefore, research of advanced strategies for AIDS-related lymphomas is an important field in cancer chemotherapy. Detailed understanding of the EBV lifecycle and related cancers at the molecular level is required for novel strategies of molecular-targeted cancer chemotherapy The linkage of HIV-related lymphoma with EBV infection of the tumor clone has several pathogenetic, prognostic and possibly therapeutic implications which are reviewed herein.
Registry linkage studies in the pre-highly active
antiretroviral
therapy (HAART) era found that the incidence of high grade B-cell
non-Hodgkin’s lymphoma (NHL) in HIV-infected individuals was 60-200
times higher than that in HIV-uninfected persons. The introduction of
HAART during the mid-1990s has been associated with a fall in incidence
of opportunistic infections and AIDS-associated malignancies, including
NHL [1,2]
Within the French Hospital Database on HIV Infection (FHDH), the
incidence of systemic NHL has decreased between 1993 and 1994 and
between 1997 and 1998, from 8.6 per 1,000 to 4.3 per 1,000
person-years, respectively [3]; the incidence in the same cohort was
2.8 per 1,000 person-years in 2006. This is consistent with reports of
decreased incidence of HIV-related NHL in the post HAART era from the
U.K., Australia, California [4]. Nevertheless, the incidence ratio of
NHL still remains relatively high in HIV-infected patients [5,6]. On
the contrary, the incidence of PCNSL has dramatically decreased since
the introduction of HAART [4]. Concerning HD, the relative risk is
increased, ranging from five- to 25-fold compared to that of the
general population [7,8,9].
Approximately 1–6% of HIV infected patients develop lymphoma each year.
In 2006 the World Health Organization estimated 39.5 million people
were living with HIV and that during that year there were 4.3 million
new infections with 65% of these occurring in sub-Saharan Africa. Major
increases were also seen in Eastern Europe and Central Asia, where it
appears that infection rates have risen by more than 50% since 2004.
Many of those with retroviral infection will either have limited access
to HAART or will be unaware of their HIV status. Therefore the
incidence of HIV-associated lymphomas will most likely increase
globally in the years to come [10,11].
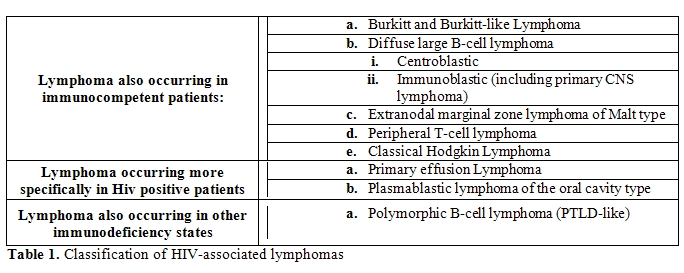
The WHO (12) classification of lymphoid neoplasms categorises (Table 1)
- Those also occurring in immunocompetent patients, as Burkitt and Burkitt –like lymphomas, Diffuse large B-cell lymphomas Centroblasti and Immnunoblastic (including primary CNS Lymphoma), Extranodal marginal zone lymphoma of Malt Type, Peripheral T-cell lymphoma, Classical Hodgkin lymphoma (80% of all HIV lymphomas)
- Those occurring more specifically in HIV-positive patients as Primary Effusion Lymphoma4 and Plasmablastic Lymphoma of oral cavity type and other variants (3%)
- Those also occurring in patients with other forms of immunosuppression as Polymorphic B-cell lymphoma (PTLD-like) (5% of all HIV lymphomas).
HIV is a lentivirus of the
retrovirus family, and thus integrates into host chromosomal DNA using
a DNA
intermediate. It has been generally believed that integration of HIV is
a
random process, and therefore this process is not in itself oncogenic [13].
Accordingly with this theory is the fact that Southern blot analysis of
HIV-associated
lymphomas has failed to detect HIV sequences [14],
with rare reports
of clonal integration restricted to T-cell neoplasms [15].
Although
the neoplastic cells are not themselves infected with HIV in most
cases, in
vitro evidence suggests that HIV does have transforming properties.
Laurence
and Astrin showed that HIV infection of B-cell lines derived from
EBV-seropositive individuals led to B-cell immortalisation,
dysregulation of
MYC, and activation of EBV [7]. Certain HIV gene
products,
particularly Tat, have been implicated as potentially oncogenic in
their role
as transactivators of cellular genes, such as IL6 and IL10 [7].
Tat
protein can more directly interfere with cell cycle control by
interaction with
the regulatory protein Rb2/p130 [8]. This role of the
Tat protein has
been proposed as a significant factor in the pathogenesis of
HIV-related
Burkitt lymphoma [8].
The predominant
contribution of HIV to lymphoma pathogenesis is believed to be through
indirect
mechanisms. The increased risk for lymphoma among HIV-infected
individuals
appears related to multiple factors, including duration and degree of
immunosuppression, induction of cytokines leading to B-cell
proliferation, and
opportunistic infections with oncogenic herpesviruses such as EBV and
HHV8 [14].
HIV-associated malignancies
are commonly considered to be the result of diminished immune
surveillance
against viruses and virus-infected tumor cells. The beneficial effects
of HAART
on these tumors have therefore been interpreted as the result of
drug-mediated
HIV suppression and immune reconstitution.
This is supported by
several findings. For example, EBV load is increased in patients before
development of B-cell lymphoma, whereas specific immune responses
against the
virus are decreased [16,17,18]. The relative risk of
AIDS-associated
malignancies increases progressively as a function of the progressive
decline
of CD4+ T-cell counts [19]. Nevertheless, the relation between immune
deficiency and tumor development is not straightforward.
In fact, only certain types
of AIDS-associated tumors arise in immunodeficient patients. In
particular, NHL
subtypes including Immunoblastic lymphomas and PCNSL, along with
Burkitt’s-like
lymphomas, typically develop in patients with very low CD4+ T-cell
counts. On
the other hand, the incidence of other NHL subtypes such as
Centroblastic
Diffuse Large-cell Lymphomas, along with classic Burkitt’s Lymphoma,
Hodgkin’s
disease, cervical cancer and, most notably, Kaposi’s sarcoma, increases
in
patients who have significantly higher CD4+ T-cell numbers [9,20,21].
The overall risk of tumour
development is very high in HIV-infected individuals, but the relative
increase
in tumor risk with stepwise decreases in CD4+ T-cell counts is only
marginal [22].
It has been observed that the risk of tumor development increases
steeply as
CD4+ T-cell counts decline below a certain threshold, nevertheless,
once below
this threshold, cancer risk becomes less dependent on further CD4+
T-cell loss [19].
However, evidence indicates
that this hypothetical CD4+ T-cell count threshold can be very high in
certain
individuals. In particular, in HIV-infected homosexual men, the
incidence rate
of Kaposi’s sarcoma increases by more than 1000-fold before a
consistent CD4+
T-cell decline [20]. So, CD4+ T-cell loss and
consequent immune
deficiency cannot fully explain the increased incidence of certain
malignancies
in HIV-infected individuals. Indeed, several recent studies show that
immune
activation causes and precedes the development of immune deficiency in
HIV
infection [23,24,25]. Sustained and uncontrolled HIV
replication leads
to continuous antigenic stimulation and to chronic T-cell activation
and
proliferation, which, in turn, generates a continuous drain of naive
and memory
T cells that become activated, proliferate, die by apoptosis or
re-enter the
pool of memory T cells. However, this exhausts the pool of naive T
cells,
impairing the capacity to mount antigen-specific immune responses [22-25].
Several other studies also
indicate that immune activation, rather than immune deficiency, is the
key
factor in the initiation of B-cell lymphomas. In particular,
AIDS-associated
B-cell lymphomas are described to be preceded by chronic antigen
dependent
B-cell stimulation leading to a persistent and generalized
lymphadenopathy that, in turn, promotes
the clonal expansion
of pre-neoplastic antigen-specific B-cell populations [26,27].
Furthermore, an increased
EBV load precedes the development of B-cell lymphoma [17],
whereas
extracellular Tat increases B-cell proliferation and induces B-cell
lymphomas
in mice [26,27].
Regarding
EBV, the percentage of cases within each histotypes with EBV viral
infection is
variable, ranging from 60% to 100%. In contrast to other lymphomas, a
high
frequency of EBV association has been shown in HL (80%-100%) tissues
from
HIV-infected people and the EBV-transforming protein, EBV-encoded
latent
membrane protein-1 (LMP-1), is expressed in virtually all HIV-HL cases [28,29].
On this basis, HL in HIV-infected persons appears to be an EBV-driven
lymphoma [30].
The spectrum of lymphomas
occurring in HIV-infected patients includes pathologic subtypes
displaying
specific association with distinct viruses. BL and DLBCL-IB with
plasmacytoid
differentiation are often HIV associated and closely linked to EBV
infection.
The HIV-associated DLBCL-IB
is distinct from other large cell lymphomas occurring in both
HIV-seropositive
and -seronegative patients because HIV-associated DLBCL-IB lymphomas
display a
plasma cell–related phenotype.
Most HIV-associated
lymphoproliferative disorders, including primary central nervous system
lymphoma, systemic DLBCL IB-plasmacytoid, PEL and its solid variant,
and PBLs
of the oral cavity type, display a phenotype related to plasma cells
and are
linked to EBV infection.
Burkitt
lymphoma:
Among
EBV-positive high-grade B cell Lymphomas, Burkitt Lymphoma (BL)
occupies a
particular position as being the tumor type in which EBV was
discovered. Burkitt
and Burkitt-like/atypical Burkitt lymphomas make up the largest
group of HIV-associated non-Hodgkin lymphomas, comprising up to 35–50%
of these
neoplasms in some studies [31].
Classification of these lymphomas in the HIV
setting follows the same diagnostic criteria as are used in the general
patient
population. That is, a diagnosis of Burkitt or Burkitt-like lymphoma
requires a
medium-sized CD10-positive B-cell population with a high proliferative
rate and
demonstration of a translocation involving the MYC gene [12].
Peripheral blood involvement is less common in HIV-infected patients
compared
to HIV-negative patients with Burkitt lymphoma, although it can occur [12,31,32].
Burkitt lymphoma occurring in the HIV setting is characterised by
multiple
genetic lesions, with the relative significance of each in the
pathogenesis of
this lymphoma unknown. In addition to the translocation involving MYC,
point
mutations in regulatory regions associated with MYC and within the TP53
tumour
suppressor gene are common [12].
In the context of HIV infection, EBV-encoded
RNA (EBER) can be detected by in situ hybridisation in tumor cells in
about 30%
of Burkitt lymphomas, 50–70% of Burkitt lymphomas with plasmacytoid
differentiation, and 30–50% of Burkitt-like lymphomas.
Similarly to sporadic or epidemic forms of
Burkitt lymphoma, in HIV-associated EBER-positive disease the viral
oncogenes
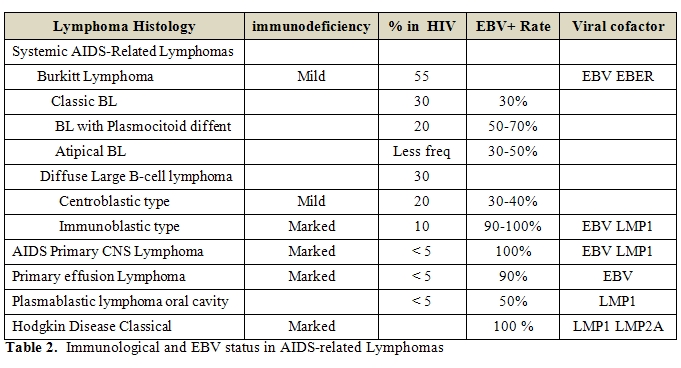
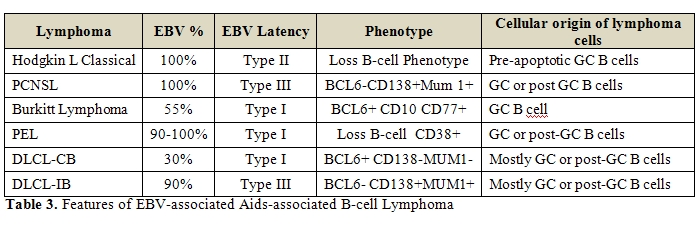
LMP-1 and EBNA-2 are not expressed (Table 2,
Table 3).
Although not essential in the pathogenesis of
BL, EBV supports tumor development. EBNA-1, a viral protein required
for the
replication and maintenance of the latent viral episomal DNA, is found
consistently
in BL cells [33]. The presence of latent EBV in BL
cells has been shown
to promote genetic instability [34], suggesting a
mechanism by which latent EBV
could contribute to genetic alterations required for the development of
BL.
This is in contrast to EBER-positive
immunoblastic DLBCL and PEL, which do show expression of these
EBV-associated
viral oncogenes. Thus EBV may not play the same role in oncogenesis in
these
different types of lymphoma. It is interesting to note that although
Burkitt
lymphoma is common in HIV-infected patients, it is not associated with
other
forms of immunosuppression.
This may indicate that the oncogenic properties
of HIV itself play a greater role in pathogenesis in this highly
proliferative
tumour compared with EBV or that there are other mechanisms.
Dysregulation of
cell cycle proteins has been implicated in the development of Burkitt
lymphoma.
Inactivating mutations of the tumour suppressor gene RBL2 (Rb2/p130)
are
frequently found in endemic Burkitt lymphoma, and are also found in
sporadic
cases [35].
By contrast, in HIV-associated cases,
abnormal overexpression of wild-type RBL2 is seen. This finding, in
conjunction
with studies indicating that the function of Rb2/p130 in the control of
the
G0/G1 transition can be negated by physical interaction with the Tat
protein of
HIV-1, may suggest a direct role for HIV proteins acting
synergistically with
MYC activation in the pathogenesis of Burkitt lymphoma [36].
Diffuse large
B-cell Lymphoma:
As in the HIV-negative
setting, the category of HIV associated DLBCL is a clinically and
pathologically heterogeneous group Lymphomas with a predominance of
centroblasts have been termed centroblastic DLBCL, whereas those with
greater
than 90% immunoblasts/plasmablasts have been termed immunoblastic DLBCL.
These two general morphological subtypes show
correlation with certain clinical features and molecular profiles. The
subtypes
occur with approximate equal frequency in HIVinfected patients, with
the
relative frequency of centroblastic DLBCL increasing and that of
immunoblastic
DLBCL decreasing in recent years due to advances in HIV therapy.
Centroblastic
DLBCL occurs in the setting of mild immunosuppression, has a low
frequency of
EBV positivity (30–40%) without expression of LMP-1, shows a germinal
centre B-cell
phenotype (expression of CD10 and BCL6, and lack of expression of CD138
and
MUM1), and frequently shows rearrangements of the BCL6 gene.
In contrast, immunoblastic DLBCL usually
occurs in the context of severe immunosuppression, has a high frequency
of EBV
positivity (80–90%) with frequent expression of LMP-1 and EBNA-2, shows
a
non-germinal centre B-cell/activated B-cell phenotype (lack of
expression of
CD10 and BCL6, expression of CD138 and MUM1), and lacks rearrangements
of BCL6 [35] (Table 2,
Table 3).
The transforming EBV protein LMP-1 is
frequently expressed [37,38]. LMP-1 plays a crucial
role in the
transformation of B-lymphocytes by EBV [40]. Thus,
LMP-1 transforms
rodent fibroblasts [40] transgenic mice that express
LMP-1 in B cells
show increased development of B-cell lymphomas [41]
and LMP-1 deletion
mutants of EBV are compromised in their ability to immortalize human
primary B
cells [42]. LMP-1 activates the NFkB as well as the
JNK and p38
pathways [39,40,41], by recruiting cellular TRAF 1-3
and TRADD molecules to 2
short sequence motifs, CTAR-1 and CTAR-2, respectively, in the
cytoplasmic
domain of the LMP-1 molecule [43,44,45].
In B cells, LMP-1 increases the expression of
the antiapoptotic proteins A20 and bcl-2, the adherence molecule
CD54/ICAM-1,
the cell-cycle regulator p27Kip,71 and many others [46].
In DLBCL, expression of LMP-1 correlates
inversely with the expression of BCL6, a marker for germinal center B
cells,
suggesting that, among DLBCLs, the impact of EBV LMP-1 is likely to be
strongest in tumors representing a post–germinal center plasmacytic
differentiation profile [47]. In addition, knockdown
of LMP-1 in cell
lines derived from AIDS-DLBCL results in apoptosis, indicating that
this viral
oncoprotein plays a role in lymphoma pathogenesis [48].
EBV-associated DLBCLs have t been considered
as EBV-driven lymphoproliferations occurring in the context of a
defective
T-cell immunity against EBV [49]. However, unlike
EBV-driven
lymphoproliferative disease in transplant recipients, which includes
monoclonal,
oligoclonal, as well as polyclonal B-cell proliferations, DLBCL is
always
monoclonal. This suggests that, in addition to the effects contributed
by EBV
LMP-1, additional factors such as genetic damage are likely to
contribute to
the pathogenesis of AIDS-DLBCL.
Primary CNS Lymphoma:
Accounting for 15% of
HIV-associated lymphomas, PCNSL has a reported incidence of over 1000
times
greater than in the non-HIV population [50]. This is
most likely a
reflection of the brain as a relatively immuno-privileged site. There has been a decline in its incidence
since HAART introduction [51], and it would confirm
the strong
association of this tumor with severe and prolonged immunosuppression. Clinical presentation results from
neurological deficits related to the site of the tumor, with mental
state
disturbance and seizures more common than in non-HIV PCNSL. Systemic B
symptoms
are also common [52,53].
These tumors have a
tendency to occur late in the course of HIV infection and show EBV
association in
virtually 100% of the cases [53]. A few studies have
reported that
detection of EBV in the cerebrospinal fluid of HIV-positive patients
with a CNS
lesion infers a diagnosis of lymphoma [54,55,56].
These lymphomas have
been reported to express all EBV latent encoded proteins (latency III) [57],
and there are observations consistent with their histogenetic
derivation from
germinal center-related B cells [58]. Nevertheless,
the exact role of EBV in
the pathogenesis of these disorders remains not completely defined (Table 2,
Table 3).
Most patients have CD4 counts <50/uL and
have multifocal lesions at time of diagnosis. Ocular involvement occurs
in up
to 20% of cases [59]. Full staging at time of
diagnosis is essential to
exclude system NHL involving the brain. MRI brain scan has a higher
diagnostic
yield than CT and is recommended for suspected intracranial masses [60].
Up to 30% of CNS lesions in HIV patients are found to be PCNSL with
toxoplasmosis and progressive multifocal leukoencephalopathy making up
the
remaining cases [60]. The most common histology is
immunoblastic
variant DLBCL.
Differentiation between PCNSL and
toxoplasmosis can be difficult, as both cause ring enhancing lesions
with mass
effect and oedema (although PCNSL lesions are more likely to be
periventricular) and up to 15% false negative rates for toxoplasmosis
serology [61,62].
Radionuclide scanning has also been
investigated. PCNSL lesions are avid by 201Thallium single
photon
emission CT and [18] fluorodeoxyglucose-positron
emission tomography
(FDG-PET), however improve specificity should be combined with PCR and
is
emerging as an alternative to brain biopsy [63,64].
This needs to be
further validated and brain biopsy is still the definitive diagnostic
procedure, but must be weighed against a mortality rate of 2–3% [64],
particularly in the post-HAART era during which it seems that EBV-DNA
detection
shows a reduced negative predictive value compared to that of the
pre-HAART
period.
In the new trials the use of EBV-DNA
measurement is used as a surrogate to brain biopsy. Response to therapy
may
also be monitored with EBV-DNA. There is
no standard therapy for PCNSL. Whole-brain radiation (WBRT) achieves CR
in up
to 50% but this is not translated to increased survival, with median
survival
no more than 3 months. Deaths are generally related to opportunistic
infections
due to overwhelming immunosuppression at time of diagnosis. Even though
many
patients are unable to tolerate the full dose of radiation, the
strongest
predictors of outcome are performance status and the ability to deliver
higher effective
radiation doses.
A promising alternative to WBRT was studied
in 15 patients using single-agent MTX intravenously at 3g/m2. The mean
CD4
count in these patients was 30/uL. Almost 50% had achieved CR with a
median
survival of 19 months and a relapse rate of only 14% [65].
There is a
survival benefit associated with the use of cART after diagnosis [66],
and there is evidence that cART may increase the radio-sensitivity of B
cells
within the lymphoma [67,68]. Given the very limited
benefit of current
modalities, patients should be referred to clinical trials.
Since there is universal association of EBV
in HIV-associated PCNSL, therapeutic options which target the virus
have been
explored. In this regard it should be noted that EBV-specific
allogeneic CTL
have been shown to cross the blood brain barrier and induce tumour
lysis. In
the absence of an available study, either first-line WBRT or
alternatively
high-dose MTX with the option of WBRT consolidation should be
considered.
Concomitant HAART therapy to enhance the immune system is critical to
successful outcomes
Classical Hodgkin
lymphoma:
HL
is the most common type of non-AIDS defining tumor. The risk of
developing HL
in HIV patients is up to 11-18 times above the general population [69].
It is associated with advanced disease and is more common in the
intravenous
drug group than in homosexual men. Its hallmark includes aggressive
clinical
presentation with systemic B symptoms, widespread non-contiguous
extranodal
lesions and frequent bone marrow involvement (in up to 50% of cases).
The
morphological patterns are similar to those seen in patients without
HIV
infection, although with a greater proportion of the subtypes (mixed
cellularity, lymphocyte depleted) with less favourable prognosis
compared to
the general population [70]. As noted above, the greater proportion of
mixed cellularity and lymphocyte depleted subtypes appears specifically
related
to severe immunocompromise in HIV, while HIV-infected patients with
modest
immunocompromise are more at risk for the development of the nodular
sclerosis
subtype.
The composition of the
reactive inflammatory infiltrate in HIV-associated HL is often
characterised by
a predominance of CD8-positive T lymphocytes over CD4-positive
lymphocytes, by
contrast with the background in HL without HIV infection [70].
This
finding may simply reflect the depleted peripheral CD4 counts in this
patient
population. The cytological and phenotypic features of the Hodgkin
Reed–Sternberg (HRS) cells in HIV-associated HL are similar to those in
non-HIV
associated HL. It has been determined that RS cells of all histologic
categories of HIV-HD consistently display the BCL-6(-)/syn-1(+)
phenotype and
thus reflect post-GC B cells [71].
The HRS cells typically
express CD15 and CD30, express CD20 in a minor subset, and lack
expression of
CD45 [70] In the vast majority of HIV associated HL
there is coincident
EBV infection. The latent EBV proteins EBNA-1, LMP-1, and LMP2A are
expressed
in the RS cells, the malignant cell population of this tumor [72]. RS
cells are derived from B cells that have passed through the germinal
center, as
shown by the presence of somatic mutations in the rearranged Ig
variable region
of their immunoglobulin genes [73] LMP2A interferes
with normal B-cell
development, allows BCR-negative B cells to leave the bone
marrow/colonize
peripheral lymphoid organs [74], and induces a
transcriptome pattern in
B cells, which resembles that of HL RS cells [75].
Following EBV
infection, LMP2A is essential for the survival and continued
proliferation of
germinal center B cells lacking a functional B-cell receptor [76,77].
LMP2A may therefore promote the survival of “crippled” germinal center
B cells
and could thus aid their development into RS cells (Table 2,
Table 3).
LMP-1 may also induce an
“HL-like” transcriptional program in germinal center B cells [78] Among
the cellular genes up-regulated by LMP-1 in HL cells is bmi-1, a
polycomb
family member known to cause lymphoma in transgenic mice and to
down-regulate
the ATM tumor suppressor [79]. EBNA-1 was shown to
induce CCL-20
secretion in RS cell lines and to thereby promote the migration of
regulatory T
cells, which could be envisaged to downmodulate EBV-specific T-cell
responses [80].
This association with EBV
is considerably stronger than that seen in HL in the non-HIV infected
population. HIV-associated HL most often presents at an advanced
clinical
stage, with B symptoms, frequent extranodal disease, as bone marrow
localization,
and an aggressive course [81]. Unusual extranodal
sites, such as the
skin, lung and gastrointestinal tract may be involved [82].
These sites
are essentially never involved by HL that is not associated with HIV.
HIV-HL patients have
reduced CR rates and survival compared with the HIV negative
population. In the
early years post-HAART therapy the incidence of HIV-HL appeared to be
in
decline however two studies showed that the incidence may actually have
increased [83,84].
The post-HAART era was also
associated with an improvement in survival which was attributed to
virological
response to antiretroviral therapy and a reduction in HIV-associated
mortality [85].
In another study of 47 patients in the post-HAART era, the median
survival was
not reached compared with 19 months in the pre-HAART era [86,87].
Optimal therapy for HIV-HL
has not been defined. Treatment regimes used are similar to those used
in HL in
the seronegative population [88,89].
Primary effusion
lymphoma (PEL):
[90]PEL
is a distinct clinicopathological entity occurring almost
exclusively
in HIV-infected patients. This lymphoma subtype comprises less than 5%
of all
HIV-associated NHL. Cases of this type were first described by Knowles
et al in
1989, but its distinctive features were not fully recognised until
after the identification of the Kaposi sarcoma-associated
herpesvirus/human herpesvirus
8 (KSHV/HHV8) in 1994 [91,92], PEL is a distinct
type of B-cell
non-Hodgkin lymphoma (NHL) that presents most frequently in body
cavities as
lymphomatous effusions without an associated tumor mass.
The tumor cells have large
round to irregular nuclei with prominent nucleoli, and abundant deeply
basophilic and occasionally vacuolated cytoplasm. These are described
as
immunoblastic/plasmablastic or anaplastic morphological features.
Recent
studies have broadened the scope of PEL to include those presenting as
a solid
tumour mass with or without an associated effusion [93,94,95].
The
so-called ‘‘extracavitary’’ or ‘‘solid variant’’ of PEL most commonly
involves
the gastrointestinal tract or soft tissue, but can also involve lymph
nodes.
Some studies have suggested that the extracavitary variant of PEL has a
slightly better prognosis when compared with cases presenting with
effusion [95,96].
A defining property of PEL
is its consistent association with KSHV infection. Most cases are also
co-infected by EBV. It is believed that KSHV, rather than EBV, is a
driving
force in these tumors, as in PEL, at least 5 KSHV viral genes are
expressed,
which provide proliferative and antiapoptotic signals. In contrast, EBV
has a
restricted latency pattern of gene expression in PEL, where only EBNA1
and
EBERs are expressed [97]. However, the viral
oncoprotein LMP-1 is
generally not expressed [94-97] (Table 2,
Table 3).
The immunophenotypic
features of PEL often make it difficult to confirm B-cell lineage, as
the
neoplasm usually lacks expression of most B-cell associated antigens
including
CD19, CD20, CD79a and immunoglobulins. The most frequently expressed
antigens
include those associated with activation or plasmacytic
differentiation, such
as CD30, CD45, EMA, CD71, MUM1, and CD138. Aberrant expression of Tcell
associated antigens CD3 and CD7 has been reported [98-102].
B-cell origin of PELs, can
be demonstrated by the presence of clonal immunoglobulin gene
rearrangements.
Evidence points toward a post–germinal center B-cell derivation, as
most PELs
contain somatic hypermutation of Ig genes as well as frequent somatic
hypermutation of the noncoding region of the BCL6 gene [103,104].
Consistent with this notion is the expression of plasma cell markers
such as
CD138/Syndecan-1. Recently, gene expression analysis of PEL showed
features
most similar to AIDS immunoblastic lymphoma and multiple myeloma, again
indicating a pre–plasma cell or “plasmablastic” profile [105].
Again, the exact role of
EBV has been debated; but the fact that both viruses are detected
together in
most of the cases suggests that EBV may act as a cofactor in the
initiating
events (because it can immortalize and transform B cells in vitro and
HHV-8
cannot), whereas HHV-8 may be the driving force for the tumor [106].
With or without therapy, PEL is invariably associated with an adverse
prognosis. There is limited data on the treatment of PEL. The
destruction of
local tissue despite aggressive therapy leads to shortened survival [107].
Chemotherapy and
radiotherapy may result in responses but these are seldom durable and
survival
is generally less than 12 months although a small series suggests the
addition
of high-dose MTX may improve outcome [107] .
Interestingly, a patient
treated with a combination of zidovudine and a-interferon
(a-IFN)
entered into durable remission after only 5 days [108].
Study of the primary tumour cells derived from this patient
demonstrated that
azidothymidine (AZT) blocked nuclear translocation of NFkB and
potentiated the
pro-apoptotic effect of a-IFN (which induces another death receptor
ligand,
TRAIL). Further clinical studies of this combinationare under way. In a
murine
system sirolimus showed promising activity which was in part mediated
by
inhibition of IL-10 signaling [108]. Given the
relative rarity of this
lymphoma, patients should be enrolled in clinical trials where
possible.
Concomitant administration of HAART is advised and there are several
reports of
remission of PEL with use of HAART alone.
Plasmablastic
lymphoma is a distinct type of diffuse large B-cell lymphoma that
occurs most
often in the oral cavity or jaw of HIV-infected individuals [110]. This
rare lymphoma subtype accounts for 2.6% of HIV-related NHL [111] The
first description designated this tumour as a lymphoma of the oral
cavity68;
however, subsequent reports have described less frequent involvement of
extraoral sites such as the anal cavity, gastrointestinal tract, lung,
paranasal sinus, skin, spermatic cord, testicle, bone and lymph nodes [112-118].
Regardless of the site of occurrence,
plasmablastic lymphoma shows similar morphological and phenotypic
features. The
neoplastic cells are intermediate to large in size, with round nuclear
contours
and occasional multinucleation . Plasmacytic differentiation is usually
apparent, with a cytological spectrum including a minor population of
small
plasmacytoid cells with condensed chromatin ranging to large cells with
dispersed chromatin, prominent central nucleoli and abundant basophilic
cytoplasm
with a paranuclear hof [112,113,114]. The
neoplastic population
generally expresses CD45 and plasmacytic markers such as CD138, EMA and
MUM1,
and usually lacks expression of pan-B-cell antigens such as CD20 and
PAX5.1 [110,116].
In early reports, slightly more than 50% of cases were EBER positive as
shown
by in situ hybridisation studies [110] in more
recent series all cases
of plasmablastic lymphoma have been shown to be EBER positive [115], 73
EBER-positive cases generally lack expression of EBNA2 and LMP-1 [115,116] (Table 2,
Table 3). HHV8
infection is not
implicated in the pathogenesis of plasmablastic lymphoma, with all
cases
negative for LNA1 when tested by immunohistochemistry. While there is
morphological and phenotypic overlap with anaplastic myeloma,
extramedullary
presentation and frequent EBV infection are distinctive features. A potential role for EBV
in the pathogenesis of the disease remains unknown, especially with the
highly
restricted latency expression pattern.
Despite the use of aggressive chemoteraphy and HAART the prognosis
remains poor [119].
Polymorphic B-cell
Lymphoma (PTLD-like):
HIV infection
results in a reduction of T-cell immunity similar to that
iatrogenically induced in transplant patients. Is not surprising that
polymorphic lymphoid proliferations resembling post-transplant
lymphoproliferative disorders (PTLD) have been reported in HIV-infected
adults
and children. According to the WHO
classification, they are divided into early lesions (reactive
plasmacytic hyperplasia
and mononucleosis-like syndrome), polymorphic lesions, monomorphic
lesions, and
Hodgkin-like lesions [120]. Similarly to PTLD,
these infiltrates are
often associated with EBV infection. By contrast with HIV-associated
lymphoma,
these polymorphic infiltrates often show more limited disease
distribution,
lack oncogene and tumour suppressor gene alterations, and may be
polyclonal or
show a minor B-cell clone in a polyclonal background. Regression of
polymorphic
B-cell lymphoma in an HIV-infected patient after anti-retroviral
therapy has
been reported [121].
EBV has been linked to most
PTLDs, with a near 100% association in the early-occurring cases
(within a
year) and in PTLD-associated Hodgkin lymphoma [122].
The EBV-negative
PTLDs constitute approximately 20% of all cases, have a tendency to
late
occurrence and have an unknown etiology. Type III latency is exhibited
by the
EBV-positive B cells in PTLD, although some studies have reported a
more
restricted latency pattern [123]. The wide
expression of the latent
EBV-encoded proteins strongly suggests an important role that EBV may
play in
the oncogenic process (Table 2,
Table 3).
The mechanism by which EBV is thought to contribute to the pathogenesis of PTLD is similar to its presumed role in Hodgkin lymphoma. Because approximately 50% of PTLD cases are derived from GC B cells lacking a functional BCR because of certain crippling mutations, and because these cells manage to escape apoptosis despite lacking antigen affinity, it is believed that EBV aids in rescuing these cells from an imminent programmed cell death [124,125]. As in Hodgkin cases, LMP1 and LMP2A may replace survival signals induced by activated BCR and CD40 receptors and also activate the NF-κB signaling pathway, inducing proliferation of neoplastic cells. The decreased cytotoxic T-cell surveillance because of immunosuppression in PTLD patients is also believed to greatly facilitate the actions of EBV. The similar role that EBV is thought to play in inducing the survival and neoplastic transformation of infected GC cells in both PTLD and Hodgkin lymphoma, in addition to the near 100% EBV positivity in PTLD-associated Hodgkin lymphoma, has led some investigators to speculate a connection between the 2 diseases and the possibility that EBV infection and its GC effects may be the initiating role in the pathogenesis of both entities [124].
Conclusions:
HIV-associated lymphomas represent a
particular setting characterizing specific pathogenetic models
prevalently
driven by EBV and by immunodeficiency. The impact of combined
antiretroviral
therapy has substantially changed the risk and prognosis of lymphoma in
HIV-infected population, as well as the relationship with the natural
history
of HIV disease. As a consequence of cART, many authors now strongly
recommend
that patients with lymphoma and HIV infection should be treated as
patients
with lymphoma of the general population.
In fact, due to the
improvement of morbidity and mortality related with cart exposure, more
aggressive treatment protocols can be taken into consideration, on the
bases of
the results in terms of efficacy and tolerability reported in the
general
population, such as the use of high-dose chemotherapy in combination
with PBSC
transplantation in HIV-NHL which showed response rates similar to those
obtained in HIV- negative patients.
The concurrent use of
antiblastic chemotherapy and cART should be considered a potential
advantage
for tumor prognosis and for reducing risk of toxicities associated to
antineoplastic drugs, even though concerns due to drug-drug
interactions could
be suggested. In perspectives, the molecular and epidemiological
linkage
between AIDS-related malignancies and EBV-infection suggests that viral
gene
products would be potential targets for molecular-targeted
chemotherapy. Detailed
understanding of the EBV lifecycle and
related cancers at the molecular level may lead to the development of
novel
strategies of molecular-targeted cancer chemotherapy to specific viral
oncogenes to which the lymphoma cells are addicted, and that will
provide
therapeutic benefits.
- Ledergerber B, Telenti A, Egger M.Risk of
HIV related Kaposi’s sarcoma and Non-Hodgkin’s lymphoma with potent
antiretroviral therapy: prospective cohort study. Br Med J
1999;319:23-24.

- Stebbing J, Gazzard B, Mandalia S, et al.
Antiretroviral treatment regimens and immune parameters in the
prevention of systemic AIDS-related non-Hodgkin’s lymphoma. J
Clin Oncol 2004;22:2177-2183.

- May T, Lewden C, Bonnet F, et al. Causes
and characteristics of death among HIV-1 infected patients with
immunovirologic response to antiretroviral treatment. Presse Med
2004;33:1487-1492.

- Besson C, Goubar A, Gabarre J, et
al:Changes in AIDS-related lymphoma since the era of highly active
antiretroviral therapy. Blood 2001;98:2339-2344.

- Clifford GM, Polesel J, Rickenbach M, et
al. Cancer risk in the Swiss HIV cohort study: associations with
immunodeficiency, smoking, and highly active antiretroviral therapy. J
Natl Cancer Inst 2005;97:425-432.

- Engels EA, Pfeiffer RM, Goedert JJ, et al.
Trends in cancer risk among people with AIDS in the United States
1980-2002. AIDS 2006;20:1645-1654.

- Herndier B, Shiramizu B, Jewett N, et al.
Acquired immunodeficiency syndrome associated T-cell lymphoma: evidence
for human immunodeficiency virus type 1- associated T-cell
transformation. Blood 1992;79:1768–74.

- Bellan C, Lazzi S, DeFalco G, et al.
Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin
Pathol 2003;56:188–93

- Tirelli U, Errante D, Dolcetti R, Gloghini
A, Serraino D, Vaccher E,Franceschi S, Boiocchi M, Carbone A. Hodgkin d
isease and human immunodeficiency virus infection: clinicopathologic
and virologic features of 114 patients from the Italian Cooperative
Group on AIDS and Tumors. J. Clin. Oncol. 13, 1758–1767 (1995).

- INSERM U720: Epidemiologie Clinique et Traitement de l’Infection a` VIH: Retour d’Informations Clinico-Epidemiologiques. Septembre 2007, http://www.code.fr
- Tran H, Nourse L, Hall S, Green M,
et al. Blood Reviews 2008;22,261–281.

- Raphael M, Borisch B, Jaffe E. Lymphomas associated with infection by the human immune deficiency virus (HIV). In: Jaffe E, Harris N, Stein H, Vardiman J,eds. World Health Organization classification of tumours, pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press, 2001:260–3.
- Jarrett R. Viruses and lymphoma/leukaemia.
J Pathol 2006;208:176–86.

- Knowles D. Etiology and pathogenesis of
AIDS-related non-Hodgkin’s lymphoma. Hematol Oncol Clin N Am
2003;17:785–820.

- Laurence J, Astrin S. Human
immunodeficiency virus induction of malignant transformation in human B
lymphocytes Proc Natl Acad Sci 1991;88:7635–9

- Kersten, M. J., Klein, M. R., Holwerda, A.
M., Miedema, F. & van Oers, M. H.
Epstein–Barr virus-specific cytotoxic T cell
responses in HIV-1 infection: different kinetics in patients
progressing to opportunistic infection or non-Hodgkin’s lymphoma. J.
Clin. Invest. 99, 1525–1533 (1997).

- Van Baarle, D. et al. Dysfunctional
Epstein–Barr virus (EBV)-specific CD8+ T lymphocytes
and increased EBV load in HIV-1 infected individuals progressing to
AIDSrelated non-Hodgkin lymphoma. Blood 98, 146–155 (2001).

- Van Baarle, D. et al. Lack of Epstein–Barr
virus- and HIVspecific CD27-CD8+ T cells is associated with progression
to viral disease in HIV-infection. AIDS 16, 2001–2011

- Mbulaiteye, S. M., Biggar, R. J., Goedert,
J. J. & Engels, E. A. Immune deficiency and risk for malignancy
among persons with AIDS. J. Acquir. Immune. Defic. Syndr. 32, 527–533
(2003).

- Mu˝oz A, Schrager LK, Bacellar H, Speizer
I, Vermund SH, Detels R, Saah AJ,Kingsley LA, Seminara D, Phair JP.
Trends in the incidence of outcomes defining acquired immunodeficiency
syndrome (AIDS) in the Multicenter AIDS Cohort Study: 1985–1991. Am.
J.Epidemiol. 137, 1985–1991 (1993).

- Kirk O, Pedersen C, Cozzi-Lepri A, Antunes
F, Miller V, Gatell JM, Katlama C, Lazzarin A, Skinh°j P, Barton SE;
EuroSIDA Study Group.Non-Hodgkin lymphoma in HIV-infected patients in
the era of highly active antiretroviral therapy. Blood 98, 3406–3412
(2001).

- Grossman, Z., Meier-Schellersheim, M.,
Sousa, A. E., Victorino, R. M. & Paul, W. E. CD4+ T-cell depletion
in HIV infection: are we closer to understanding the cause? Nature Med.
8, 319–323 (2002).

- Hellerstein, M. K. et al. Subpopulations
of long-lived and short-lived T cells in advanced HIV-1 infection. J.
Clin.Invest. 112, 956–966 (2003).

- Kovacs JA, Lempicki RA, Sidorov IA,
Adelsberger JW, Herpin B, Metcalf JA,Sereti I, Polis MA, Davey RT,
Tavel J, Falloon J, Stevens R, Lambert L, Dewar R, Schwartzentruber DJ,
Anver MR, Baseler MW, Masur H, Dimitrov DS, Lane HC.Identification of
dynamically distinct subpopulations of T lymphocytes that are
differentially affected by HIV. C.

- Ribeiro, R. M., Mohri, H., Ho, D. D. &
Perelson, A. S. In vivo dynamics of T cell activation, proliferation,
and death in HIV-1 infection: why are CD4+ but not CD8+ T cells
depleted? Proc. Natl Acad. Sci. USA 99, 15572–15577 (2002)

- Pelicci PG, Knowles DM 2nd, Arlin ZA,
Wieczorek R, Luciw P, Dina D, Basilico C, Dalla-Favera R. Multiple
monoclonal B cell expansions and c-myc oncogene rearrangements in
acquired immune deficiency syndrome-related lymphoproliferative
disorders. Implications for lymphomagenesis. J. Exp. Med. 164,2049–2060
(2003).

- Carbone, A. Emerging pathways in the
development of AIDS-related lymphomas. Lancet Oncol. 4, 22–29 (2003).

- Rezk SA, Weiss LM. Epstein-Barr
virus-associated lymphoproliferative disorders. Hum Pathol
2007;38:1293-1304.

- Said JW. Immunodeficiency-related Hodgkin
lymphoma and its mimics. Adv Anat Pathol. 2007;14:189-194.

- Carbone A, Gloghini A, Larocca LM, et al.
Human immunodeficiency virus-associated Hodgkin’s disease derives from
post-germinal center B cells. Blood. 1999;93:2319-2326.

- Spina M, Tirelli U, Zagonel V, et al.
Burkitt’s lymphoma in adults with and without human immunodeficiency
virus infection. Cancer 1998;82:766–74.

- Gold J, Castella A, Zalusky R. B-cell
acute lymphocytic leukemia in HIV-antibodypositive patients. Am J
Hematol 1989;32:200–4.

- Young LS, Rickinson AB. Epstein-Barr
virus: 40 years on. Nat Rev Cancer. 2004;4:757-768.

- Kamranvar SA, Gruhne B, Szeles A, Masucci
MG. Epstein-Barr virus promotes genomic instability in Burkitt’s
lymphoma. Oncogene. 2007;26: 5115-5123.

- Grogg KL, Miller RF, and Dogan A. HIV
infection and lymphoma: J. Clin. Pathol. 2007;60;1365-1372.

- Bellan C, Lazzi S, DeFalco G, et al.
Burkitt’s lymphoma: new insights into molecular pathogenesis . J
Clin Pathol 2003;56:188–93

- Hamilton-Dutoit SJ, Rea D, Raphael M, et
al. Epstein- Barr virus-latent gene expression and tumor cell phenotype
in acquired immunodeficiency syndrome-related non-Hodgkin’s lymphoma:
correlation of lymphoma phenotype with three distinct patterns of viral
latency. Am J Pathol. 1993;143:1072-1085.

- Carbone A, Tirelli U, Gloghini A, Volpe
R,Boiocchi M. Human immunodeficiency virus-associated systemic
lymphomas may be subdivided into two main groups according to
Epstein-Barr viral latent gene expression. J Clin Oncol.
1993;11:1674-1681.

- Young LS, Rickinson AB. Epstein-Barr
virus: 40 years on. Nat Rev Cancer. 2004;4:757-768.

- Wang D, Liebowitz D, Kieff E. An EBV
membrane protein expressed in immortalized lymphocytestransforms
established rodent cells. Cell. 1985;43:831-840.

- Kulwichit W, Edwards RH, Davenport EM,
Baskar JF, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr
virus latent membrane protein 1 induces B cell lymphoma in transgenic
mice. Proc Natl Acad Sci U S A. 1998;95:11963-11968.

- Dirmeier U, Neuhierl B, Kilger E, Reisbach
G, Sandberg ML, Hammerschmidt W. Latent membrane protein 1 is critical
for efficient growth transformation of human B cells by Epstein-Barr
virus. Cancer Res. 2003;63:2982-2989.

- Huen DS, Henderson SA, Croom-Carter D,
Rowe M. The Epstein-Barr virus latent membrane protein- 1 (LMP1)
mediates activation of NF-kappa B and cell surface phenotype via two
effector regions in its carboxy-terminal cytoplasmic domain. Oncogene.
1995;10:549-560.

- Devergne O, Hummel M, Koeppen H, et al.
Anovel interleukin-12 p40-related protein induced by latent
Epstein-Barr virus infection in B lymphocytes. J Virol.
1996;70:1143-1153.

- Izumi KM, Kaye KM, Kieff ED. The
Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis
factor receptor associated factors is critical for primary B lymphocyte
growth transformation. Proc Natl Acad Sci U S A. 1997;94: 1447-1452.

- Brinkmann MM, Schulz TF. Regulation of
intracellular signalling by the terminal membrane proteins of members
of the Gammaherpesvirinae. J Gen Virol. 2006;87:1047-1074.

- Gaidano G, Carbone A, Dalla-Favera R.
Pathogenesis of AIDS-related lymphomas: molecular and histogenetic
heterogeneity. Am J Pathol. 1998;152:623-630.

- Guasparri I, Bubman D, Cesarman E. EBV
LMP2A affects LMP1-mediated NF-kappaB signaling and survival of
lymphoma cells by regulating regulating TRAF2 expression. Blood.
2008;111:3813-3820

- Rowe M, Young LS, Crocker J, Stokes H,
Henderson S, Rickinson AB. Epstein-Barr virus(EBV)-associated
lymphoproliferative disease in the SCID mouse model: implications for
the pathogenesis of EBV-positive lymphomas in man. J Exp Med.
1991;173:147-158.

- Flinn IW, Ambinder RF. AIDS primary
central nervous system lymphoma. Curr Opin Oncol 1996;8:373–6.

- Besson C, Goubar A, Gabarre J et al.
Changes in AIDSrelated lymphoma since the era of highly active
antiretroviral therapy. Blood 2001;98:2339–4

- Raez LE, Patel P, Feun L, Restrepo A, Raub
Jr WA, Cassileth PA. Natural history and prognostic factors for
survival in patients with acquired immune deficiency syndrome
(AIDS)-related primary central nervous system lymphoma (PCNSL). Crit
Rev Oncog 1998;9:199–208

- Cohen JI. Clinical aspects of Epstein-Barr virus infection. In: Robertson ES, editor. Epstein-Barr virus. Norfolk: Caister Academic press; 2005. p. 35-55.
- Cinque P, Vago L, Dahl H, et al.
Polymerase chain reaction on cerebrospinal fluid for diagnosis of
virus-associated opportunistic diseases of the central nervous system
in HIV-infected patients. AIDS 1996;10:951-8.

- Cingolani A, De Luca A, Larocca LM, et al.
Minimally invasive diagnosis of acquired immunodeficiency
syndrome-related primary central nervous system lymphoma. J Natl Cancer
Inst 1998;90:364-369.

- Ivers LC, Kim AY, Sax PE. Predictive value
of polymerase chain reaction of cerebrospinal fluid for detection of
Epstein-Barr virus to establish the diagnosis of HIV-related primary
central nervous system lymphoma. Clin Infect Dis 2004;38:1629-32 .

- MacMahon EM, Glass JD, Hayward SD, et al.
Epstein-Barr virus in AIDS-related primary central nervous system
lymphoma. Lancet 1991;338:969-973.

- Larocca LM, Capello D, Rinelli A, et al.
The molecular and phenotypic profile of primary central nervous system
lymphoma identifies distinct categories of the disease and is
consistent with histogenetic derivation from germinal center-related B
cells. Blood 1998;92:1011-1019.

- Maher EA, Fine HA. Primary CNS lymphoma.
Semin Oncol 1999;26:346–56.

- Johnson BA, Fram EK, Johnson PC,
Jacobowitz R. The variable MR appearance of primary lymphoma of the
central nervous system: comparison with histopathologic features. AJNR
Am J Neuroradiol 1997;18:563–72.

- Antinori A, Ammassari A, De Luca A, et al.
Diagnosis of AIDS-related focal brain lesions: a decision-making
analysis based on clinical and neuroradiologi characteristics combined
with polymerase chain reaction assays in CSF. Neurology 1997;48:687-694.

- Antinori A, De Rossi G, Ammassari A et al.
Value of combined approach with thallium-201 single-photon emission
computed tomography and Epstein-Barr virus DNA polymerase chain
reaction in CSF for the diagnosis of AIDSrelated primary CNS lymphoma.
J Clin Oncol 1999;17: 554–60.

- Castagna A, Cinque P, d’Amico A, Messa C,
Fazio F, Lazzarin A. Evaluation of contrast-enhancing brain lesions in
AIDS patients by means of Epstein-Barr virus detection in cerebrospinal
fluid and 201thallium single photon emission tomography. AIDS
1997;11:1522–3.

- Antinori A, Ammassari A, Luzzati R, et al.
Role of brain biopsy in the management of focal brain lesions in
HIV-infected patients. Gruppo Italiano Cooperativo AIDS & Tumori.
Neurology 2000;54:993-997.

- Jacomet C, Girard PM, Lebrette MG, Farese
VL, Monfort L, Rozenbaum W. Intravenous methotrexate for primary
central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS.
1997 Nov 15;11(14):1725-30.

- Newell ME, Hoy JF, Cooper SG et al. Human
immunodeficiency virus-related primary central nervous system lymphoma:
factors influencing survival in 111 patients. Cancer 2004;100:2627–36.

- Hoffmann C, Tabrizian S, Wolf E et al.
Survival of AIDS patients with primary central nervous system lymphoma
is dramatically improved by HAART-induced immune recovery. AIDS
2001;15:2119–27.

- Pajonk F, McBride WH. Survival of AIDS
patients with primary central nervous system lymphoma may be improved
by the radiosensitizing effects of highly active antiretroviral
therapy. AIDS 2002;16:1195–6.

- Goedert JJ, Cote TR, Virgo P et al.
Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:
1833–9.

- Thompson L, Fisher M, Chu W, et al.
HIV-associated Hodgkin lymphoma. Am J Clin Pathol 2004;121:727–38.

- Carbone A, Gloghini A, Larocca LM, et al.
Human immunodeficiency virus-associated Hodgkin's disease derives from
post-germinal center B cells. Blood 1999;93:2319-2326.

- Young LS, Murray PG. Epstein-Barr virus
and oncogenesis: from latent genes to tumours. Oncogene.
2003;22:5108-5121.

- Kuppers R, Rajewsky K, Zhao M, et al.
Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from
histological sections show clonal immunoglobulin gene rearrangements
and appear to be derived from B cells at various stages of development.
Proc Natl Acad Sci U S A. 1994;91:10962-10966.

- Caldwell RG, Wilson JB, Anderson SJ,
Longnecker R. Epstein-Barr virus LMP2A drives B cell development and
survival in the absence of normal B cell receptor signals.
Immunity.1998;9:405-411.

- Portis T, Dyck P, Longnecker R.
Epstein-Barr Virus (EBV) LMP2A induces alterations in gene
transcription similar to those observed in Reed-Sternberg cells of
Hodgkin lymphoma. Blood 2003;102:4166-4178

- Mancao C, Altmann M, Jungnickel
B,Hammerschmidt W. Rescue of “crippled” germinal center B cells from
apoptosis by Epstein-Barr virus. Blood. 2005;106:4339-4344.

- Mancao C, Hammerschmidt W. Epstein-Barr
virus latent membrane protein 2A is a B-cell receptor mimic and
essential for B-cell survival. Blood. 2007;110:3715-3721.

- Vockerodt M, Morgan S, Kuo M, et al. The
Epstein- Barr virus oncoprotein, latent membrane protein-1, reprograms
germinal centre B cells towards a Hodgkin’s Reed-Sternberg-like
phenotype. J Pathol. 2008;216:83-92.

- Dutton A, Woodman CB, Chukwuma MB, et al.
Bmi-1 is induced by the Epstein-Barr virus oncogene LMP1 and regulates
the expression of viral target genes in Hodgkin lymphoma cells. Blood
2007;109:2597-2603.

- Baumforth KR, Birgersdotter A, Reynolds
GM, et al. Expression of the Epstein-Barr virus-encoded Epstein-Barr
virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates
up-regulation of CCL20 and the migration of regulatory T cells. Am J
Pathol. 2008;173:195-204.

- Thompson L, Fisher M, Chu W, et al.
HIV-associated Hodgkin lymphoma. Am J Clin Pathol 2004;121:727–38.

- Doweiko J, Dezube B, Pantanowitz L.
Unusual sites of Hodgkin’s lymphoma.J Clin Oncol 2004;22:4227–31

- Biggar RJ, Jaffe ES, Goedert JJ,
Chaturvedi A, Pfeiffer R,Engels EA. Hodgkin lymphoma and
immunodeficiency in persons with HIV/AIDS. Blood 2006;108:3786–91

- Herida M, Mary-Krause M, Kaphan R et al.
Incidence of non-AIDS-defining cancers before and during the
highlyactive antiretroviral therapy era in a cohort of human
immunodeficiency virus-infected patients. J Clin Oncol 2003;21:3447–53.

- Palella Jr FJ, Delaney KM, Moorman AC et
al. Declining morbidity and mortality among patients with advanced
human immunodeficiency virus infection. HIV Outpatient Study
Investigators. N Engl J Med. 1998;338:853–60.

- Gerard L, Galicier L, Boulanger E et al.
Improved survival in HIV-related Hodgkin’s lymphoma since the
introduction of highly active antiretroviral therapy. Aids 2003;17:81–7.

- Glaser SL, Clarke CA, Gulley ML et al.
Population-based patterns of human immunodeficiency virus-related
Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988-1998.
Cancer 2003;98:300–9.

- Hartmann P, Rehwald U, Salzberger B et al.
BEACOPP therapeutic regimen for patients with Hodgkin’s disease and HIV
infection. Ann Oncol 2003;14:1562–9.

- Spina M, Gabarre J, Rossi G et al.
Stanford V regimen and concomitant HAART in 59 patients with Hodgkin
disease and HIV infection. Blood 2002;100:1984–8.

- Knowles D, Inghirami G, Ubriaco A, et al.
Molecular genetic analysis of three AIDS-associated neoplasms of
uncertain lineage demonstrates their B-cell derivation and the possible
pathogenetic role of the Epstein-Barr virus. Blood 1989;73:792–9.

- Nador R, Cesarman E, Chadburn A, et al.
Primary effusion lymphoma: a distinct clinicopathologic entity
associated with the Kaposi’s sarcoma-associated herpesvirus. Blood
1996;88:645–56.

- Cesarman E, Chang Y, Moore P, et al.
Kaposi’s sarcoma-associated herpesviruslike DNA sequences in
AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186–91.

- Chang Y, Cesarman E, MS P, et al.
Identification of herpesvirus-like DNAsequences in AIDS-associated
Kaposi’s sarcoma. Science 1994;266:1865–9.

- Carbone A, Gloghini A, Vaccher E, et al.
Kaposi’s sarcoma-associated herpesvirus/human herpesvirus type
8-positive solid lymphomas. J Mol Diagn 2005;7:17–27.

- Chadburn A, Hyjek E, Mathew S, et al.
KSHV-positive solid lymphomas represent an extra-cavitary variant of
primary effusion lymphoma. Am J Surg Pathol 2004;28:1401–16.

- Deloose S, Smit L, Pals F, et al. High
incidence of Kaposi sarcoma-associated herpesvirus infection in
HIV-related solid immunoblastic/plasmablastic diffuse large B-cell
lymphoma. Leukemia 005;19:851–5.

- Carbone A, Cesarman E, Spina M, Gloghini A
and Thomas F. Schulz: HIV-associated lymphomas and gamma-herpesviruses:
Blood ; 2009 113: 1213-1224

- Boulanger E, Hermine O, Fermand J-P, et
al. Human herpesvirus 8 (HHV8)-associated peritoneal primary effusion
lymphoma (PEL) in two HIV-negative elderly patients. Am J Hematol
2004;76:88–91.

- Oksenhendler E, Boulanger E, Galicier L,
et al. High incidence of Kaposi sarcoma-associated herpesvirus-related
non-Hodgkin lymphoma in patients with HIV infection and multicentric
Castleman disease. Blood 2002;99:2331–6.

- Said J, Shintaku I, Asou H, et al.
Herpesvirus 8 inclusions in primary effusion lymphoma: report of a
unique case with T-cell phenotype. Arch Pathol Lab Med 2001;123:257–60.

- Beaty M, Kumar S, Sorbara L, et al. A
biphenotypic human herpesvirus 8- associated primary bowel lymphoma. Am
J Surg Pathol 1997;21:719–24

- Gaidano G, Capello D, Cilia AM, et al.
Genetic characterization of HHV-8/KSHV-positive primary effusion
lymphoma reveals frequent mutations of BCL6: implications for disease
pathogenesis and histogenesis. Genes Chromosomes Cancer. 1999;24:16-23.

- Matolcsy A, Nador RG, Cesarman E, Knowles
DM. Immunoglobulin VH gene mutational analysis suggests that primary
effusion lymphomas derive from different stages of B cell maturation.
Am J Pathol. 1998;153:1609-1614.

- Jenner RG, Maillard K, Cattini N.
Kaposi’s sarcoma- associated herpesvirus-infected primary effusion
lymphoma has a plasma cell gene expression profile. Proc Natl Acad Sci
U S A. 2003;100:10399-10404.

- Fan W, Bubman D, Chadburn A, Harrington
Jr WJ, Cesarman E,Knowles DM. Distinct subsets of primary effusion
lymphoma can be identified based on their cellular gene expression
profile and viral association. J Virol 2005;79:1244-51.

- Simonelli C, Spina M, Cinelli R et al.
Clinical features and outcome of primary effusion lymphoma in
HIV-infected patients: a single-institution study. J Clin Oncol
2003;21: 3948–54.

- Boulanger E, Daniel MT, Agbalika F,
Oksenhendler E. Combined chemotherapy including high-dose methotrexate
in KSHV/HHV8-associated primary effusion lymphoma. Am J Hematol
2003;73:143–8.

- Ghosh SK, Wood C, Boise LH et al.
Potentiation of TRAILinduced apoptosis in primary effusion lymphoma
through azidothymidine-mediated inhibition of NF-kappa B. Blood
2003;101:2321–7.

- Sin SH, Roy D, Wang L et al. Rapamycin is
efficaciousagainst primary effusion lymphoma (PEL) cell lines in vivo
by inhibiting autocrine signaling. Blood 2007;109: 2165–73.

- Delecluse H, Anagnostopoulos I,
Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new
entity associated with the human immunodeficiency virus infection.
Blood 1997;89:1413–20.

- Folk G, Abbondanzo S, Childers E, et al.
Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn
Pathol 2006;10:8–12.

- Tavora F, Gonzalez-Cuyar L, Chen-Chih J,
et al. Extra-oral plasmablastic lymphoma: report of a case and review
of literature. Human Pathol 2006;37:1233–6.

- Schichman S, McClure R, Schaefer R, et
al. HIV and plasmablastic lymphoma manifesting in sinus, testicles, and
bones: a further expansion of the disease spectrum. Am J Hematol
2004;77:291–5.

- Chetty R, Hlatswayo N, Muc R, et al.
Plasmablastic lymphoma in HIV+ patients: an expanding spectrum.
Histopathology 2003;42:605–9.

- Dong H, Scadden D, de Leval L, et al.
Plasmablastic lymphoma in HIV-positive patients: an aggressive
Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. Am J
Surg Pathol 2005;29:1633–41.

- Lin Y, Rodrigues G, Turner J, et al.
Plasmablastic lymphoma of the lung: report of a unique case and review
of the literature. Arch Pathol Lab Med 2001;125:282–5.

- Pruneri G, Graziadei G, Ermellino L, et
al. Plasmablastic lymphoma of the stomach. Haematologica 1998;83:87–9.

- Hausermann P, Khanna N, Buess M, et al.
Cutaneous plasmablastic lymphoma in an HIV-positive male: an
unrecognized cutaneous manifestation. Dermatology 2004;208:287–90.

- Castillo L, Pantanowitz L, Dezube
BJ.HIV-associated plasmablastic lymphoma: lessons learned from 112
published cases: Am J Hematol. 2008 Oct;83(10):763-4.

- Harris NL, Swerdlow SH, Frizzera G, Knowles DM. Post transplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization classification of tumors. Pathology and genetics of tumors of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. p. 264-70.
- Martin S, Zukerberg L, Robbins G.
Reactive Epstein-Barr virus-related polyclonal lymphoproliferative
disorder in a patient with AIDS. Clin Infect Dis 2005;41:e76–9.

- Thompson MP, Kurzrock R. Epstein-Barr
virus and cancer. Clin Cancer Res 2004;10:803-21 .

- Brink AA, Dukers DF, van den Brule AJ, et
al. Presence of Epstein- Barr virus latency type III at the single cell
level in posttransplantation lymphoproliferative disorders and AIDS
related lymphomas. J Clin Pathol 1997;50:911-8.

- Timms JM, Bell A, Flavell JR, et al.
Target cells of Epstein-Barr-virus (EBV)–positive post-transplant
lymphoproliferative disease: similarities to EBV-positive Hodgkin's
lymphoma. Lancet 2003;361:217-23.

- Capello D, Rossi D, Gaidano G.
Post-transplant lymphoproliferativedisorders: molecular basis of
disease histogenesis and pathogenesis. Hematol Oncol 2005;23:61-7