Viral hepatitis A to E in South Mediterranean Countries
Sanaa M. Kamal, Sara Mahmoud, Tamer Hafez and Runia EL-Fouly
Department of Tropical Medicine, Gastroenterology and Liver Disease, Ain Shams University, Cairo, Egypt
Correspondence to: Prof. Sanaa M. Kamal Department of Tropical Medicine, Gastroenterology and Liver Egypt.
E-mail: sanaakamal@link.net
Published: February 10, 2010
Received: January 19, 2010
Accepted: February 4, 2010
Medit J Hemat Infect Dis 2010, 2(1): e2010001, DOI 10.4084/MJHID.2010.001
This article is available from: http://www.mjhid.org/article/view/5424
Abstract
Viral hepatitis represents an important health problem in the South Mediterranean Countries (SMC), Egypt, Libya, Tunisia, Algeria and Morocco. Emerging natural history and epidemiological information reveal differences in the overall epidemiology, risk factors and modes of transmission of viral hepatitis A, B, C, D, E infections in the SMC. The differences in the in incidence and prevalence of viral hepatitis across North African countries is attributed to variations in health care and sanitation standards, risk factors and immunization strategies. The active continuous population movement through travel, tourism and migration from and to the SMC contribute to the spread of infections due to hepatitis viruses across borders leading to outbreaks and emergence of new patterns of infection or introduction of uncommon genotypes in other countries, particularly in Europe.
There are some similarities but also there substantial differences between SMC. The epidemiology of viral hepatitis in SMC is dynamic and affected by many factors including hygiene, socioeconomic status and vaccination coverage. For example, hepatitis B (HBV) [1,2] and hepatitis C (HCV) viruses [3] have a high tendency to persist and establish chronic hepatitis with long-term sequels. HBV- and HCV-related chronic hepatitis is considered the main cause of cirrhosis, hepatocellular carcinoma (HCC) and liver transplantation in the region[2,3]. While HCV related liver disease represents a huge health and economic problem in Egypt, HCV is not considered a public health problem in Morocco. In this review, we will explore the epidemiology, patterns and trends of viral hepatitis in the SMC namely, Egypt, Libya, Tunisia, Algeria and Morocco.
Hepatits A Virus
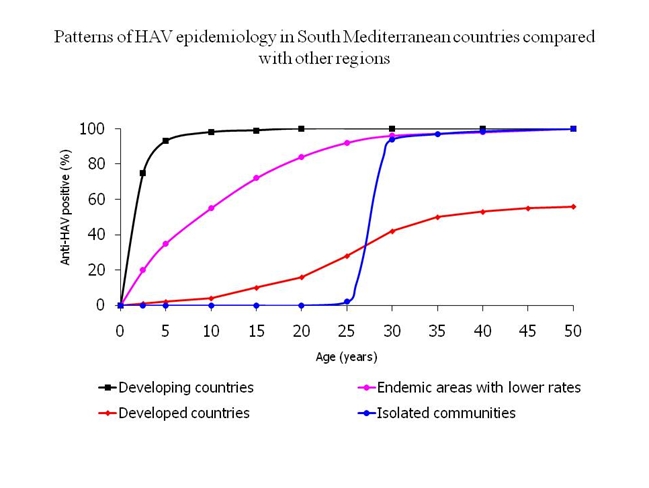
HAV still represents a public health problem in the SMC which is considered an area with high HAV prevalence.[4] HAV is a frequent travel acquired infection that has caused several outbreaks among European tourists.[5,6,7,8,9] HAV infection is largely acquired through fecal-oral transmission.5 Consumption of contaminated food or raw sea food or drinking contaminated water represents the main routes of transmission in the SMC. HAV is a resilient virus that survives for prolonged periods in food and drink.[7,8] HAV infection in SMC has been characterized with a lifetime risk of infection greater than 90%. Most infections occur in early childhood and those infected may not experience any noticeable symptoms. Epidemics are uncommon because older children and adults are generally immune. Disease rates in these areas are low and outbreaks are rare. Recently, a shift from high to intermediate HAV endemicity patterns is observed in countries with transitional economies such as Egypt, Tunisia and Morocco. In these countries, the improvement of economic and sanitary conditions has been associated with decline in early childhood HAV infections that started to occur in older age groups. Immunization against HAV is not incorporated in the routine childhood immunization programs in any of the countries of the region.
HAV in Egypt
HAV is endemic in Egypt and represents a major cause of acute viral hepatitis being responsible for more than half of acute hepatitis cases. HAV genotype IB is the prevalent genotype in Egypt. Person-to-person transmission, consumption of food contaminated in fields or through infected food handlers is very common as well as consumption of contaminated mollusks (clams, oysters) and unsafe drinking water represent the major routes of HAV transmission in Egypt. In children, day care centers contribute to HAV spread among young children, siblings and parents of daycare center attendees.[10,11,12]
Generally, HAV is acquired early in life with most infections occurring between 5-15 years with no sexual predilection. However, it is unknown what proportions of infected persons are aware of their infection given acute HAV tends to be asymptomatic particularly in children <5 years of age.[10,13] Thus, many individuals are neither tested nor reported. Individuals living in rural areas are at higher risk of hepatitis A infection compared to the urban population due to overcrowding, poor sanitation, certain social practices, and lack of a reliable clean water resource.[12] A study showed that in Egypt, HAV could be detected in 72%, 50% and 43% of raw sewage, Nile and treated effluent sewage samples respectively.[14] posing a potential health risk for people.
Within the Egyptian socioeconomic framework and social class structure, differing frequencies of hepatitis A virus infection with different age peaks are observed. In rural or semi-urban regions and lower socioeconomic classes, most of the population is exposed to HAV at a very young age, acquires the infection with minimal or no symptoms and develops immunity.[12,13] Cross-sectional studies conducted in rural areas revealed very high‐prevalence of anti-HAV IgG reaching 100% of adults.[13] Consumption of common village water, use of indoor dry pits, and contamination of drinking water with sewage represent the major risk factors for HAV acquisition in rural Egypt.[16,17] In the past 2 decades, there has been significant emphasis on improving sanitation measures and hygiene in Egypt coupled with extension of municipal potable water, sewage, and solid‐waste management systems projects. Therefore, in large Egyptian cities and among high social classes, children are infrequently exposed to hepatitis A virus at a young age with subsequent decline in herd immunity and a change to the epidemiology of the illness with steady increases in the mean age of occurrence of illness attributed to acute hepatitis A virus infection. Thus, in urban Egypt despite the overall decrease in the incidence of HAV infection, more HAV infections are encountered at older age groups.
HAV infection in children and adolescents is mostly asymptomatic or might present as flu-like illness or anicteric hepatitis. Symptomatic cases with jaundice that terminates in complete resolution are less common in young age groups. However, in spite of this jaundice, the patient feels well. A proportion of patients who acquire infection at older may develop symptomatic HAV infection with prolonged clinical course and jaundice or may have recurrence within a short period of improvement.[5,11,12] Fulminant hepatitis due to HAV is very rare in Egypt although recently few fulminant HAV cases that required liver transplantation have been reported in adult patients who acquired the infection at older age.
Currently, immunization for HAV is not included within the compulsory children immunizations programs in Egypt due to the limited clinical seriousness of the infection among the general Egyptian population.[11] Immunization is reserved to special groups, namely household/close contacts of patients with HAV, patients with chronic liver disease or HCV infection. Newborns and children may be immunized depending on epidemiology.
Recently several outbreaks of HAV have been reported among European travelers and European tourists returning from Egypt. A survey conducted in 13 European countries traced the source of the outbreaks to food or drinks in hotels or Nile cruises. The reported hepatitis A attack rates varied between tourists from different European countries. Thus, the health authorities in EU countries recommended active vaccination with hepatitis A vaccine for people travelling to Egypt. Adoption of the public health policies and immunization prior to travel are likely to reduce the potential of acquiring HAV infection by non-immune travelers.[6,7,8,9,18]
HAV in Libya
Similar to Egypt, most HAV infections are acquired during childhood. Surveys showed that HAV antibodies could be detected in 60-70% of three-year old children. By the age of 7 years almost 100% of children are HAV immune.[19]
HAV in Tunisia
In Tunisia, there is no national program for virological surveillance of hepatitis cases or assessment of epidemiology of these infections. However, some studies showed that HAV prevalence rates range between 84.0 and 92%. [20,21,22] Primary infection with HAV in Tunisia is progressively shifting to older ages, probably due to the improvement of sanitary conditions. A survey conducted to assess the prevalence of HAV among Tunisian children and adolescents revealed an overall seroprevalence among this population was 60% (44%, in children < 10 years old, 58% in those 10-15 years of age, and 83% in those > 15 years of age). Prevalence rates varied according to areas of residence. More than 90% of those living in rural areas had antibodies to HAV compared to 30-50% among those living in the urban areas. The lowest anti-HAV prevalence rates are found in coastal regions probably due to the higher socioeconomic level of the population living in such regions. [20] To date, mass vaccination programs for HAV are not indicated in Tunisia.
Several outbreaks of HAV infection were erupted among European tourists following visits to Tunisia. By age-group, the highest incidence was found in unprotected children travelers 0 to 14 years. Thus, travelers, and especially children, are strongly encouraged to receive HAV vaccination before travelling to SMC including Tunisia.[23,24,25]
HAV in Algeria
Hepatitis A is common in Algeria where 96% of individuals have anti-HAV antibodies. Patients come into contact with the virus before the age of 10. No symptoms are found in 95% of cases while icteric cases are mostly encountered among infants and young children.[26,27,28]
HAV in Morocco
Morocco is an intermediate endemic area for HAV infection. The prevalence of anti-HAV antibodies among Moroccan children varies with age. Anti-HAV antibodies could be detected in 45% and 70% in children less than 6 years and children between 7-14 years respectively. Socioeconomic factors, city dwelling and parents education correlated with the prevalence of anti-HAV-IgG. Thus, Morocco is entering a transitional phase with less children exposed to HAV at early childhood. Introduction of hepatitis A vaccination in early childhood might reduce the prevalence of this infection and prevent outbreaks.[29]
Hepatits B Virus
Hepatitis B virus (HBV) is an ubiquitous virus with a global
distribution. Of the approximately 2 billion people who have been
infected worldwide, more than 350 million are chronic carriers of HBV.[30] Approximately 15-40% of infected patients will
develop cirrhosis, liver failure, or hepatocellular carcinoma (HCC).31
HBV infection accounts for 500.000 to 1.2 million deaths each year and
is the 10th leading cause of death worldwide.[30]
SMC have been previously been classified as a high endemicity countries
for HBV.[30,31] Horizontal transmission used to
occurs among children and adults. This results in chronic carriage, but
less often than when infection occurs vertically or perinatally,
leading to a gradual dwindling of the reservoir of chronic carriers
with fewer long-term complications and deaths. There is a correlation
between the endemicity level of HBV infection and the frequency of HBV
related chronic hepatitis and cirrhosis. In fact, where the endemicity
level is intermediate or high, HBV infection is common in newborn
babies and children and in these groups, progression towards chronicity
is frequent. [31,33] On the other hand, late onset
of infection such as a result of sexual activity would result in
chronic carrier rates of 3-5% become, but 30% may show signs and
symptoms of acute infection.
Recent surveys suggest a shift in the HBV endemicity patterns in
most SMC from high endemicity towards intermediate or low
endemicity patterns [33] with some limited
hyperendemic pockets.[30] Since the early nineties
of the last century, Tunisia, Morocco, Libya and Egypt adopted highly
effective infants’ vaccination programs which were sometimes
extended to the several paediatric and adult groups,
producing a dramatic decrease in the incidence of HBV in birth cohorts
born after implementation of the vaccination programs. Furthermore, the
increases in the public awareness and modes of transmission of HBV in
addition to the improvements in healthcare infrastructure and the
socioeconomic conditions contributed to reduce the infection rate in
the region.
HBV is divided into eight genotypes (A-H) according to overall
nucleotide sequence variation of the genome. The genotypes have a
distinct geographical distribution and are used in tracing the
evolution and transmission of the virus. Genotype D in is prevalent in
North African countries. Differences between genotypes affect the
disease severity, course and likelihood of complications, and response
to treatment and possibly vaccination.[34,35]
The incidence and prevalence of HBV showed recent decline following the effective HBV immunization program. In 1985, a study [36] conducted on 1,866 apparently healthy Egyptians from Upper and Lower Egypt showed that the overall prevalence rate of HBsAg was 10.1%. with higher rates in Upper Egypt (11.7% ) compared to the Nile Delta (8.0%). HBsAg was more frequent in young males than females and the prevalence gradually increased with age. A recent study [37] conducted 20 years later screened [55],922 potentially healthy asymptomatic blood donors for HBsAg. The cumulative seroprevalence of HBV infection was 1.3% with decline in the annual seroprevalence throughout the study period from 2.3% to 0.9%. Another study 13that compared the frequencies of hepatitis viruses as etiological agents for acute viral hepatitis over the past 2 decades found that the frequency of acute hepatitis B virus infection decreased from 43.4% in 1983 to 28.5% in 2002 (P<.01).
The significant decline in HBV rates indicates the effectiveness of the universal hepatitis B virus immunization of infants that was initiated in 1991. Cross-sectional studies conducted over a decade among schoolchildren in an endemic area in Egypt for screening of HBV virus markers also showed a significant decrease of HBV prevalence among school children confirming the effectiveness of the immunization campaigns.[38] To evaluate the protective efficacy of hepatitis B vaccine, 720 children aged 10 years who were vaccinated in infancy were tested for hepatitis B serologic markers. Among the vaccinated children, 37.9% had protective anti-HBs, 0.6% had HBsAg while HBV infection occurred in 6.8% of the vaccinated children and it induced a boosting effect on anti-HBs level. only of the vaccinated children.[39] Despite the decline in the incidence and prevalence of HBV in Egypt, higher HBV prevalence rates are encountered in special patient groups such as patients undergoing hemodialysis [40], patients with hematological malignancies [41], hemophiliacs and injecting drug users (IDUs).[42]
Adults particularly from Upper Egypt had higher prevalence of HBV. In Egypt, various risk factors for HBV transmission were identified including shaving at barbers shops, injecting drug use, recent (<1 year) marriage, home deliveries, occupational or nosocomial exposure in health care facilities.[13,43]
In Egypt, HBV genotype D is the predominant HBV genotype (37.1%) followed by genotype B that constituted 25.7%. and mixed D & B infections in 15.7% of patients. HBV genotypes A and C infections were the less observed constituting 10% and 8.6% respectively of the total infections. In children with pediatric malignancies, a relatively high prevalence of mixed A/D genotype infections.[41]
The association of HBV and liver cancer is well documented. The burden of hepatocellular carcinoma (HCC) has increased in Egypt with a doubling in the incidence rate in the past 10 years. The prevalence rates for HBV and HCV were 25.9% and 78.5% among HCC cases. Among HCC cases, HBV significantly decreased over time (p=0.001) while HCV did not, suggesting a shift in the relative influences of these viruses in HCC etiology in Egypt.[44]
HBV in Libya
Libya is considered a country
with intermediate endemicity for HBV. The seroprevalence of HBsAg among
general population in Libya ranges between 5.8% and 1.3% depending on
the method of diagnosis and the geographic region. [45,46]
However, recent surveys reported an over all prevalence
of 2.2% with 120.000-150.000 chronic HBsAg carriers in
Libya associated with viral transmission.[47]HBV
infection is related to male gender, use of skin scarifications,
high-risk behaviors, unsafe injections, history of blood
transfusion, family history of hepatitis B or contact with hepatitis B
patients. Vertical transmission is responsible for some cases of
neonatal HBV infections. In Libya, universal newborns hepatitis B
vaccine was introduced since 1993. The vaccine was also recommended to
be used to household contacts of HBV-infected individuals and
healthcare workers.[47]
HBV genotype D is the
prevalent HBV genotype as in other countries in North Africa. HBV
infection is responsible for the majority of chronic liver disease
cases in the country. A high prevalence rate of
HBeAg-negative/anti-HBe-positive chronic hepatitis B has been reported,
which accounted for 80% of chronic HBV patients. HBV is a leading cause
of hepatocellular carcinoma in Libya.[46,47]
HBV in Tunisia
Tunisia is considered a
country with intermediate endemicity of HBV infection. Many
infections are clustered among children and teenagers. Geographically,
HBsAg seropositivity varied throughout the country, ranging from 3% to
13% with higher prevalence in the south and central-west regions.
Studies conducted in the eighties and nineties showed high
overall seroprevalence of HBV in Tunisia reaching 37.5%.[48]
However, a more recent survey estimated the overall hepatitis B carrier
rates in the general Tunisian population at 3% to7% suggesting a
decline in HBV in Tunisia. This could be attributed to the hepatitis B
vaccination which was incorporated in the national schedule of
new-born vaccination in 1995.[52]
The magnitude of vertical and perinatal transmission of HBV is not
clear but has been addressed in a study conducted on a cohort of 2303
Tunisian pregnant women of whom 4% were HBsAg positive and 1.4% were
vaccinated previously against hepatitis B. Analysis of the risk factors
revealed association between the HBsAg status and presence of
intrafamilial hepatitis cases (p<0.05). The maternal DNA levels
ranged from 34 to 108copies/ml to 10 4 copies/ml. Vertical and
perinatal transmission of HBV in the first 3 months of life occurred in
0.4% of 177 mother and child pairs. HBV seroprevalence was 10.7% in
infants under 5 years old and increased with age rapidly till 25 years
of age and then more slowly in adulthood, reaching 54% for people aged
over 40 years.[51]
HBV genotypes, (D, A, and
E) have been detected in Tunisia with prevalence rates 80%, 8%,
and 9%, respectively indicating that genotype D is the most prevalent
HBV genotype.[50] A novel subgenotype, D7, was
the most common subgenotype found in asymptomatic Tunisian HBsAg
carriers.[53] A high frequency of HBV precore mutants
has been reported from Tunisia.[49] Precore mutants
have been more frequently found in chronically and in acutely infected
individuals, in patients with severe and asymptomatic infections, in
HBeAg positive as well as HBeAg negative individuals. [50]
HBV in Algeria
Algeria is considered a
country with intermediate endemicity for HBV. A survey was
conducted to assess the serologic markers of hepatitis B (HBsAg ,
anti-HBc in 1,112 apparently healthy blood donors and 715 pregnant
women in different regions in Algeria. HBsAg was detected in 3.6 % of
blood donors and 1.6 % of pregnant women while anti-HBc antibodies in
13 % of blood donors and 11.1 % of pregnant women.[54]
Another Algerian study evaluated the characteristics of hepatitis B
viral strains in chronic carrier patients from North-East Algeria. The
median age of patients was 35 years and 80% of them had normal
transaminase level. Liver histology revealed different
necroinflammatory changes in 63% of the patients and established
cirrhosis in 21%. "Precore" mutant serological profile without HBe Ag
was detected in 87% of patients. Genotype D was predominant (93%)
followed by genotype A (5%) and E for one patient. Algerian strains
clustered independently from other genotype D reference sequences,
suggesting a possible new D subtype.[55]
HBV in Morocco
Morocco is among the countries
with intermediate/low endemicity of HBV infection. To date, there is no
concrete figures for overall prevalence rates of HBV in Morocco.
However, some studies showed that 2% of chronic haemodialysis patients
treated in a haemodialysis unit in Casablanca show evidence of HBV
infection.[58] Genotype D is the most prevalent HBV
genotype (97.5%) followed by genotype A, a pattern which is consistent
with the predominance of genotype D in the Mediterranean basin. [56] Phylogenetic analysis based on pre-S/S sequences
revealed that genotype D in Morocco differed from others D strains
subgenotypes (D1, D2, D3 and D4). Pre-core mutant are highly prevalent
in Morocco as a study showed that HBeAg-negative/anti-HBe-positive and
HBV DNA positive was detected in 86% of 91 patients with chronic
hepatitis B. [57]
Hepatits C Virus
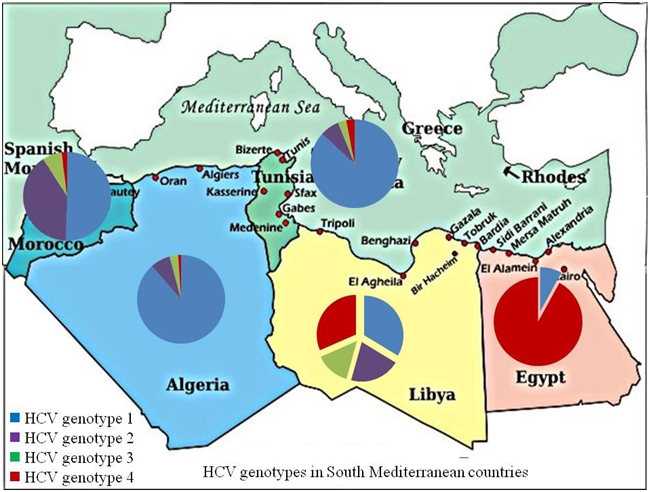
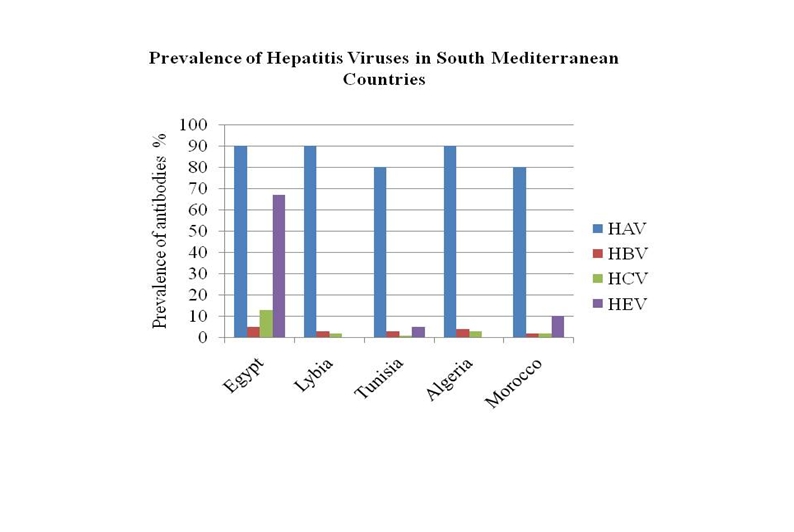
Hepatitis C virus (HCV) is a major public health problem and the primary causative agent of chronic liver disease in SMC. Compared to other hepatitis viruses, HCV shows vast variations across SMC. Geographically, HCV prevalence rates decrease along the Mediterranean coast from the East towards the West. Egypt has the highest HCV prevalence worldwide with 13% of Egyptians are infected while the prevalence of HCV in Morocco and Algeria is 1-2%. The incidence of HCV in the individual countries is hard to estimate accurately given that many patients with acute HCV infection are asymptomatic. The risk factors and modes of transmission differ among the 5 SMC. HCV genotypes distribution also differ from prevalence of HCV G4 in Egypt and the predominance of genotype 1a or 1b in western areas such as Tunisia and Morocco.
HCV in Egypt
Egypt has the highest prevalence of HCV in the world (13%).[59,60] The current sequence diversity and phylogenetic tree structure of HCV G4a in Egypt is compatible with the introduction of HCV into that population through parenteral treatment for schistosomiasis with non disposable and poorly sterilized needles in the 1950s and 1960s.[62,63] Although subtype 4a is the dominant Egyptian HCV strain, recent studies revealed that other subtypes are also present, indicating that HCV G4 in Egypt is extremely variable.[64,65,66] HCV accounts for 31% of acute viral hepatitis cases in Egypt.[67] In Egypt, high rates of infection are observed in all age groups including young individuals, indicating an ongoing high risk for acquiring HCV infection. More than 60% of acute HCV infections are in persons below the age of 25 yr.[68,69] High incidence rates (14.1 per 1,000 person-years) have been detected in Egyptian children younger than 10 yr of age living in households with an anti-HCV-positive.[68] This high incidence in young persons could lead to future increases in chronic disease in these individuals and persistence of the high magnitude of the burden of HCV-related chronic disease in Egypt. The prevalence of HCV in both genders is not well documented although some studies showed higher HCV incidence among men and high spontaneous resolution n rates in women.[69]
In Egypt, relatively higher rates of sexual transmission have been reported and reflect the higher background prevalence in this country. In rural Egypt, sexual transmission between monogamous spouses ranged between 3% and 34% (95% CI 0–49).[70,71] Acute HCV is associated with a high temporal risk of transmission of HCV to sexual partners. Sexual transmission, confirmed by phylogenetic analysis, was detected in 15% of sexual partners of individuals with acute HCV.[71] Although some cases of acute HCV have been related to sexual transmission, the degree to which sexual transmission of HCV occurs is controversial because sexual transmission is difficult to confirm given that partners might have other risk factors for HCV transmission such as IDU. Phylogenetic analysis to identify genetic relatedness of HCV viral isolates in partners is required to confirm sexual transmission. All studies on the sexual transmission of hepatitis C are limited by the potential of the confounding variable of IDU or shared items such as razors and other items among sexual partners.[72]
HCV infection has been associated in Egypt with health-care-related procedures performed by traditional healers and folk medicine providers, acupuncture, tattooing, body piercing, and commercial barbering. Informal health-care providers and traditional healers perform services that may be associated with HCV transmission such as injections, dentistry, wound treatment, circumcision, excision, and scarification.[73,74] In rural Egypt, about 50% of deliveries are attended by traditional birth assistants.[74] Lack of appropriate cleaning and disinfection of equipment used in these procedures contribute to HCV transmission and the emergence of new HCV cases.[71]
Infection with HCV is an important occupational hazard for Egyptian health-care workers. The risk of occupational HCV transmission increases with deep injuries and after procedures involving hollow-bore needles. In Egypt, occupational transmission among health-care workers through needlesticks and sharps injuries contributes to new HCV cases given that needlestick-prevention devices are not yet adopted most hospitals and health-care units in addition to inadequate compliance with universal, standard precautions in some health facilities.[75,77,78]
The role of intrafamilial HCV transmission is still controversial. However, several studies reported HCV spread in families with HCV-infected index cases. In rural Egypt, intrafamilial HCV transmission is considered an important route of transmission where living in a house with an infected family member is a risk factor for HCV transmission. Analysis of data collected during surveys of Egyptian rural communities show that children whose parents had antibodies to HCV were at higher risk for contracting anti-HCV than children whose parents did not.[75]
HCV in Libya
In 1994, a study that tested 266 healthy Libyan subjects for anti-HCV antibodies found that 21 (7.9%) subjects had evidence of HCV infection suggesting a high frequency of 'community-acquired' HCV in the normal Libyan population.[79] However, the national sero-epidemiological survey in 2006 showed that the prevalence of HCV antibodies in the general population in Libya was 1.2%.[80] In a Tripoli, screening of sera collected over a 2 year period showed that the prevalence HCV of 1.6%, 1.2%, 2% among the general population, blood donors, hospital health care workers respectively. HCV prevalence was 20.5% among renal dialysis patients and 10.8% in the multiple blood transfusion group.[81] History of hospitalization and/or surgical procedures risk (33%), history of blood transfusion. (22.7%), past history of intravenous drug abuse (IVDA) (15%) and history of dental procedures. (15.9%) [81] or medical waste handling [82] were important risk factors for HCV infection. It is not clear whether the discrepancy in prevalence rates between the 1994 report and the 2006 survey report is due to actual reduction or changes in the sensitivity and specificity of the diagnostic tests used.
The largest documented outbreak of nosocomial HIV transmission to approximately 400 Libyan children in 1998 was coupled by HCV and/or HBV transmission.[83] Among the HIV infected children, 47% and 33% had HCV and HBV coinfection respectively. Vertical transmission was ruled out by analysis of parents' serology. Prospective follow up of 160 HIV/HCV coinfected showed that half of the children progressed to moderate or severe immunosuppression and/or moderate or severe clinical symptoms three years after infection. In those who progressed during follow-up, 85% had done so within three years of infection. Children progressing to moderate or severe immunosuppression and/or clinical symptoms were significantly more likely to be receiving ART. [83]
The major HCV genotypes circulating in Libya include genotype 1 (G1) in 30.9% of cases followed by G4 (29.2%). Genotype 2 in 19.3% and G3 in 13.6%. The frequency of HCV genotype 4 in the Libya might suggest introduction from neighboring countries mostly from Egypt and Central African countries, where genotype 4 is predominant. Few of the Libyan isolates were closely related to the European ones where the majority of patients are infected with genotype 1, 2 or 3.[83]
HCV C in Tunisia
The seroprevalence of HCV in the Tunisian general population is low (0.4%).[48] However, another study showed high prevalence of HCV infection among Tunisian dialysis patients with rates of 51% suggesting that the spread may be nosocomial rather than transfusion-related.[85] HCV isolates from 93 Tunisians, including 16 haemophiliacs, were genotyped. In non-haemophiliacs, subtype 1b was largely predominant (79%), types 1a, 2a, 2b, 3a and 4a occurred much less frequently at 5, 7, 3, 3 and 1% of cases, respectively. In the group of haemophiliacs, co-dominance between subtypes 1a and 1b was noticed (38%).[85]
HCV in Algeria
The prevalence of HCV in Algeria is not high. In a 1995 survey of 1,112 apparently healthy blood donors and 715 pregnant women in Algeria54, anti-HCV antibodies were detected in 0.18% of blood donors and 0.19 % of pregnant women. Recently, the Algerian Ministry of health estimates the prevalence of HCV in Algeria to be around 2.5%.[86]
Occupational exposure and health related transmission seem important risk factors for HCV transmission in Algeria. A survey on the frequency of occupational exposure to HCV among health care personnel in an Algeria hospital revealed 108 exposures in 2 years. Needle stick injuries represented 81% of cases. Source patient serology was unknown in most of the cases, negative in 9% of cases and positive in 10% of cases. 62% of exposed health workers received immediate serology, follow up and screening as of the first day of exposure, 12% after 3 months and 36% after 6 months. No seroconversion case was noted. Cleaning staff and hygiene workers are at high risk of blood contamination as well as nurses, and more than one-third of injuries occurred because of mismanagement of healthcare waste produced in the hospital environment, where needles were not disposed of appropriately in a hard container. Thus, 41.66% of injuries could be avoided if objects were thrown away correctly in specific containers. It is urgent to raise awareness of health care personnel and strengthen adherence to standard precautions as well as to provide suitable containers for the collection and disposal of needles and sharp objects.[87]
HCVin Morocco
HCV prevalence rate in Morocco
is estimated to be 1.93 %.[88] However, higher HCV
prevalence rates reaching 35.1 and 42.4% are detected among
special populations such as patients undergoing haemodialysis and
haemophiliacs respectively.[89,90,91]
The prevalent HCV genotypes in Morroco are 1b (47.6%), 2a/2c (37.1%)
and 1a (2.8%) . Interestingly, the HCV genotypes distribution varies
with age as genotype 1b is more prevalent among older patients, whereas
subtype 2a/2c is mainly found among younger ones. [88]
Although Morocco belongs to the African continent, the circulating HCV
strains are similar to those observed in Europe.
Hepatitis D Virus
Chronic hepatitis D virus (HDV) infection is still an
important health problem in the Mediterranean basin region such as in
Italy where coinfection with HDV was reported in 9.7%.[92]
Hepatitis D virus (HDV) is an RNA virus that is structurally
unrelated to hepatitis A, B, or C virus. It was. HDV causes a unique
infection that requires the assistance of viral particles from
HBV to replicate and infect other hepatocytes. Its clinical
course is varied and ranges from acute self-limited infection to acute
fulminant liver failure. Chronic liver infection can lead to end-stage
liver disease and associated complications.[92]
Approximately 15 million people are infected worldwide. North Africa
and the Middle East are considered regions that high prevalence
of HDV infections. HDV infection is not associated with a sex
predilection. HDV infection is more common among adults than children.
However, children from underdeveloped, HDV-endemic countries are more
likely to contract HDV infection through breaks in the skin due to the
presence of skin lesions.[93]
HDV in Egypt
Hepatitis D virus (HDV) infection among Egyptian HBV carriers and
patients has not been adequately evaluated. Earlier studies
conducted in 1990, demonstrated HDV antibodies in 16.94% of patients
with acute HBV and 23.53% in patients with chronic HBV.[94,95]
A more recent study detected anti-HDV in 20% of HBsAg positive
subjects and HDV RNA in 60%. The proportion of the patients with liver
disease was higher in HBV carriers of anti-HDV positive with HDV RNA
than in HBV carriers of anti-HDV positive without HDV RNA (P <
0.05). Phylogenetic analysis based on the sequences in nucleotide
position 853-1267 of HDV revealed HDV genotype 92.1% of the samples
confirming the prevalence of HBV genotype D and HDV genotype I are most
prevalent in Egypt. [96] In a prospective study of
45 consecutively Egyptian children (2-15 years) with chronic hepatitis
B, anti-delta antibody (IgG anti-HD) was detected in only four children
with an overall prevalence of 8.9% (4/45). A substantial association
between delta infection and the state of hepatic illness was detected.[97]
HDV in Tunisia
Screening of serum samples from [33],363 healthy
people in Tunisia for serological markers of hepatitis B and delta
showed an overall seroprevalence for HDV of 0.4%. HDV
superinfection occurred later than HBV and increased with age in
parallel with HBV. HDV superinfection seems common in Tunisia and
occurs in almost 44% of individuals infected with HBV.
In contrast with the high endemicity of delta agent reported in other countries of North Africa, the incidence of delta infections seems to be very low in Morocco. Considering the possibility the high frequency of severe hepatitis B (HB) observed in this country could be the contemporary presence of HDV, delta antigen (D Ag) and antibodies to delta agent (D Ab) were tested by radio-immuno-assay in the sera from 85 HBs Ag positive patients, hospitalized in Casablanca: among them 57 suffered from acute or fulminant HB (12 deceased), 10 from chronic hepatitis and 18 from cirrhosis. Neither D Ag nor D Ab were found, excepted once in a patient with cirrhosis having shown the presence of D Ab.[98]
Hepatitis E Virus
Hepatitis E virus (HEV),
previously known as epidemic non-A, non-B hepatitis, is a
positive-sense single-stranded RNA icosahedral virus with a 7.5
kilobase genome. HEV as the prototypic member of the genus Hepevirus.[99] HEV has been classified further into four major genotypes
(I to IV). Genotype I includes Asian strains from India, Burma, Nepal,
China, and and African strains from Chad, Algeria, Tunisia, Morocco,
Egypt, and Namibia.[100]
This enterically transmitted virus is prevalent throughout much
of the developing world. It is constantly present (endemic) in
countries where human waste is allowed to get into drinking water
without first being purified. Large outbreaks (epidemics) linked to
contaminated waterborne sources have occurred in the Indian
subcontinent and South American countries where there is poor
sanitation. HEV is also a zoonosis. Anti-HEV antibodies have been
detected in rodent, swine, sheep, bovine and poultry.[99,
100]
In general, hepatitis E is a short-lived, self-limiting viral infection
followed by recovery. Prolonged viraemia or faecal shedding are unusual
and chronic infection does not occur. The incidence of hepatitis E is
highest in juveniles and adults between the ages of 15 and 40. Though
children often contract this infection as well, they less frequently
become symptomatic. Mortality rates are generally low. Occasionally, a
fulminant form of hepatitis develops, with overall patient population
mortality rates ranging between 0.5%-4.0%. Fulminate hepatitis occurs
more frequently in pregnancy and induces a mortality rate of 20% among
pregnant women in the 3rd trimester and can also cause premature births.[99,100]
The prevalence and endemicity of HEV vary across SMC. The
features of HEV infection in North Africa is different from that
in the Indian subcontinent. Despite the high prevalence rates in SMC,
HEV infection is rarely symptomatic in the region and fulminant
hepatitis cases among pregnant women are uncommon. However, given that
there is no immunization against HEV, travelers from non-endemic areas
to these countries must be aware of the risk of HEV infection and
should adhere to strict hygienic measures, such as drinking bottled
water and eating cooked food.
Hepatitis E virus is probably endemic in Egypt and seems to be a frequent infection. Genotypic characterization of HEV circulating in Egypt showed the predominance of genotype 1 HEV related to other North African isolates is circulating in acute symptomatic patients in Egypt.[101]
HEV accounts for 20—40% of adult and pediatric hospitalized cases of acute viral hepatitis.[67,95,101] Community surveillance revealed a high prevalence of anti-HEV antibodies within several rural populations.[102,103,104] A cross sectional survey in several villages in Upper Egypt and the Nile Delta revealed anti-HEV antibodies in 67.7% of healthy adults [102] while screening of Egyptian blood donors in Cairo demonstrated anti-HEV antibodies in 24% of studied subjects.
The seroprevalence of anti-HEV is high among Egyptian children and the rate of positive antibodies increases with age. In the first decade of life, the prevalence of antibodies has been shown to exceed 60% then it peaked to 76% in the second decade and remained around 70% thereafter. HEV serological markers are frequently encountered among children with acute viral hepatitis in conjunction with hepatitis B viruses. Dual infection with HEV and other hepatotropic viruses has been reported in children with great elevation of aspartate and alanine aminotransferases. Among children (1-11 years old), with acute symptomatic hepatitis, HEV was recognized as etiological factor in 22% of cases.[102]
The prevalence of HEV antibodies in pregnant females in rural areas in Egypt is very high. A cross-sectional survey of a cohort of pregnant women living in three rural villages in the Nile Delta demonstrates the high prevalence of anti-HEV that reaches 85%, suggesting universal community exposure to this enterically transmitted virus. Despite reports of fulminant HEV hepatitis during pregnancy in Asia, there is little evidence that HEV-caused overt hepatitis in the pregnant women and there have been no reports of the effects of HEV on pregnant women and their offspring in Egypt. [105]
Thus, the prevalence of HEV in Egypt is among the highest reported in the world. Despite the very high prevalence of anti-HEV, HEV epidemics are uncommon in Egypt and almost no one of the screened subjects gave a prior history of jaundice, hepatitis or liver disease, and none of these symptoms were significantly associated with anti-HEV. This lack of HEV-associated morbidity could be due to the presence of a less virulent circulating strain of HEV in the population (and possibly in all of Egypt); the presence of cross-reacting antibodies to an HEV-like virus which is not associated with clinical disease; or a high prevalence of exposures during the early years, which may be more likely to cause asymptomatic, anicteric illness, as is the case for HAV in endemic regions. Exposures to HEV in early childhood may modify responses to subsequent exposures, such that clinical illness due to HEV is rare, as it is in endemic HAV.[101]
HEV in Libya
Epidemic and sporadic water-related diseases that might be attributes to HEV have been reported in Libya.[19,106] However, no accurate estimates of the prevalence of HEV are available. Transmission of the HEV in Libya might be related to eating raw or uncooked shellfish. Farm animals, may serve as a viral reservoirs as HEV is one of the few viruses which has been shown to be transmitted directly from animals through food. HEV in Tunisia
The prevalence of HEV in Tunisia is about 4.3%.20 No epidemics attributed to HEV have been reported in Tunisia suggesting that the virus might be circulating among the Tunisian population as sporadic cases. Higher anti-HEV IgG prevalence rates reaching 12.1 % have been reported among pregnant women. In multivariate analysis, age (>30 years) and the number of persons per room (>2) in the house were independent factors predicting HEV infection. History of agricultural work, kind of water, sewage treatment, use detergent to wash vegetables, contact with animals and parenteral risk factors were not correlated with the presence of anti-HEV IgG.[107]
HEV in Algeria
Hepatitis E is endemic in Algeria but the accurate ratesare not available. Sporadic cases frequently emerge or may go unrecognized or under-reported. However, several epidemics of hepatitis E have been reported in Algeria such as the HEV Algerian epidemic between 1978-1980 and the epidemic in Tanefdour, Algeria, in 1986-1987.[108] Before the identification of hepatitis E virus in 1990, such hepatitis outbreaks were denominated enterically-transmitted non-A, non-B hepatitis. In January and February 1987, an outbreak of acute hepatitis occurred in the eastern territories of Algeria. Among the affected individuals, two pregnant women died by fulminant hepatitis. The epidemiological investigation traced the epidemic to a common source water contamination. Genotyping of the isolates showed that all the cases were due to HEV genotype I. Most isolates shared 99.7-100 % sequence identity and the remainder showed 1-1.3 % divergence. Interestingly, intra-patient heterogeneity revealed sequence diversity ranging from 0.11 to 3.4 suggesting a probable quasispecies organization of HEV during epidemics and could explain the adaptable behavior of the virus in the host-pathogen interrelations. Comparing the partial nucleotide sequences and derived peptide sequences of hepatitis E virus (HEV) from two outbreaks of hepatitis E in Algeria and Chad revealed 92 and 95%, homology at the nucleic acid level and 98% the peptide level respectively.[108,109] Thus, the African strains of HEV appear to be a distinct phylogenetic group, separate from the Mexican and Asian strains.
HEV in Morocco
HEV is also endemic in Morocco. Among healthy adult Moroccans, anti-HEV IgG has been detected in 6.1% - 10.4% of subjects particularly in the west and the south of Morocco. Subclinical HEV infection is frequent in children and young adults. A longitudinal study showed that anti-HEV IgG in healthy contacts decreased significantly after 30 years of age. The incidence of clinical acute HEV infections increased significantly with age.[110] In 1994, an of acute HEV outbreak erupted in the south of Morocco. HEV was confirmed using recombinant antigen-based enzyme immunoassays and reverse transcription polymerase chain reaction in 77.3% of patients. Furthermore, HEV-specific IgM was positive in 84.0% of cases and was associated with subclinical HEV infection in contacts of index cases. Faecal contamination of drinking water samples collected from the epidemic city was observed. It also appeared that primary infection with HEV accounted for more than 86% of the cases. A longitudinal study showed waning of anti-HEV antibodies in patients and healthy contacts six months after the initial testing.[111]
Conclusions
Taken together, the epidemiology, frequency and patterns of hepatitis viruses show variations across the SMC depending on several demographic and socioeconomic factors. The prevalence of viral hepatitis infection is still high and represents a serious public health challenge. Hepatitis B and C viruses remain to be the major causes of chronic hepatitis in the region. Hepatitis A and E are endemic in the region and despite the mainly subclinical features of the infections among residents, HAV and HEV represent a risk to tourists and expatriates visiting the SMC. Better control of viral hepatitis is required to decrease the burden of the disease. Immunization against hepatitis A virus and hepatitis B virus universal use of disposable syringes and implementation of better hygienic conditions are crucial measures to reduce the impact of viral hepatitis in SMC.
References
- World Health Organization. Hepatitis. Available at: http://www.who.int/topics/hepatitis/en/. Accessed 12/09/2009.
- Cooke GS, Main J, Thursz MR Treatment for
hepatitis B BMJ. 2010 5;340:b5429.

- Thomas DL, Seeff LB. Natural history of
hepatitis C. Clin Liver Dis 2005;9:383–98.

- World Health Organization. Hepatitis C.
Available at:
http://www.who.int/water_sanitation_health/diseases/hepatitis/en/index.html Accessed 12/09/2009. - Jeong SH, Lee HS. Hepatitis A: clinical
manifestations and management. Intervirology. 2010;53:15-9.

- Marano C, Freedman DO. Global health
surveillance
and travelers' health. Curr Opin Infect Dis. 2009;22:423-9.

- Couturier E, Roque-Afonso AM, Letort MJ,
Dussaix
E, Vaillant V, de Valk H. Cluster of cases of hepatitis A with a travel
history to Egypt, September-November 2008, France. Euro Surveill. 2009.

- Bernard H, Frank C. Cluster of hepatitis A
cases
among travellers returning from Egypt, Germany, September through
November 2008. Euro Surveill. 2009 22;14.

- Robesyn E, Micalessi MI, Quoilin S, Naranjo
M,
Thomas I. Cluster of hepatitis A cases among travellers returning from
Egypt, Belgium, September through November 2008. Euro Surveill. 2009
22;14.

- Salama II, Samy SM, Shaaban FA, Hassanin
AI, Abou
Ismail LA. Seroprevalence of hepatitis A among children of different
socioeconomic status in Cairo. East Mediterr Health J. 2007
Nov-;13:1256-64.

- El-Karaksy H, El-Sayed R, El-Raziky M,
El-Koofy
N, Mansour S. Cost-effectiveness of prescreening versus empirical
vaccination for hepatitis A in Egyptian children with chronic liver
disease. East Mediterr Health J. 2008;14:804-9.

- Omar AA, Hashish MH.. Screening for
hepatitis A
virus antibodies among a disadvantaged group of preschool children in
Alexandria. J Egypt Public Health Assoc. 2000;75:529-39.

- Meky FA, Stoszek SK, Abdel-Hamid M, Selim
S,
Abdel-Wahab A, Mikhail N, El-Kafrawy S, El-Daly M, Abdel-Aziz F, Sharaf
S, Mohamed MK, Engle RE, Emerson SU, Purcell RH, Fix AD, Strickland GT.
Active surveillance for acute viral hepatitis in rural villages in the
Nile Delta. Clin Infect Dis. 2006;42:628-33.

- Pintó RM, Alegre D, Domínguez A,
El-Senousy WM,
Sánchez G, Villena C, Costafreda MI, Aragončs L, Bosch A.Hepatitis A
virus in urban sewage from two Mediterranean countries. Epidemiol
Infect. 2007;135:270-3.

- El Gaafary MM, Rekacewicz C, Abdel-Rahman
AG,
Allam MF, El Hosseiny M, Hamid MA, Colombani F, Sultan Y, El-Aidy S,
Fontanet A, Mohamed MK. Surveillance of acute hepatitis C in Cairo,
Egypt. J Med Virol. 2005;76:520-5.

- Divizia M, Gabrieli R, Stefanoni ML,
Renganathan
E, El Ghazzawi E, Kader OA, Gamil F, El Sawaf G, El Sherbini E, Saleh
E, Degener AM, Noce A, Zaratti L, Modesti A, Panŕ A. HAV and HEV
infection in hospitalised hepatitis patients in Alexandria, Egypt. Eur
J Epidemiol. 1999;15:603-9.

- Darwish MA, Faris R, Clemens JD, Rao MR,
Edelman
R. High seroprevalence of hepatitis A, B, C, and E viruses in residents
in an Egyptian village in The Nile Delta: a pilot study. Am J Trop Med
Hyg. 1996;54:554-8.

- Ward M, Borgen K, Mazick A, Muehlen M.
Hepatitis
A vaccination policy for travellers to Egypt in eight European
countries, 2004. Euro Surveill. 2006;11:37-9.

- Gebreel AO, Christie AB. Viral hepatitis
in children: a study in Libya. Ann Trop Paediatr. 1983;3:9-11.

- Rezig D, Ouneissa R, Mhiri L, Mejri S,
Haddad-Boubaker S, Ben Alaya N, Triki H. Seroprevalences of hepatitis A
and E infections in Tunisia. Pathol Biol (Paris). 2008;56:148-53.

- Gharbi-Khelifi H, Sdiri K, Ferre V,
Harrath R,
Berthome M, Billaudel S, Aouni M. A 1-year study of the epidemiology of
hepatitis A virus in Tunisia. Clin Microbiol Infect. 2007
Jan;13(1):25-32.

- Letaief A, Kaabia N, Gaha R, Bousaadia A,
Lazrag
F, Trabelsi H, Ghannem H, Jemni L. Age-specific seroprevalence of
hepatitis a among school children in central Tunisia. Am J Trop Med
Hyg. 2005;73:40-3.

- Pröll S, Nothdurft HD. The risk of
contracting
hepatitis A or hepatitis B run by visitors to the Mediterranean and
Eastern Europe. MMW
Fortschr Med. 2004 May 13;146(20):51-4.

- Arya SC, Agarwal N. Hepatitis A and E:
Update on prevention and epidemiology. Vaccine. 2009 Dec 29.

- Fitz Simons D, Hendrickx G, Vorsters A,
Van Damme
P. Hepatitis A and E: Update on prevention and epidemiology. Vaccine.
2010
8;28:583-8.

- Smahi MC, Rahmoun L, Ghomari SM,
Benmansour S,
Sendani H, Bendeddouche AS, Gendrel D. Seroprevalence and risk factors
of hepatitis A among children in Tlemcen (north-west Algeria). Arch
Pediatr. 2009;16:844-6.

- Hamdi-Cherif M, Touabti A, Belabbes EH.
Prevalence of hepatitis virus A in the city of Sétif. Arch Inst Pasteur
Alger. 1986;55:285-7.

- Khalfa S, Ardjoun H. Epidemiology of viral
hepatitis in Algeria. Med Trop (Mars). 1984;44:247-52.

- Bouskraoui M, Bourrous M, Amine M.
Prevalence of
anti-hepatitis A virus antibodies in chidren in Marrakech. Arch
Pediatr. 2009 Oct;16 Suppl 2:S132-6.

- World Health Organization. Hepatitis.
Available
at:
http://www.who.int/diagnostics_laboratory/evaluations/hepb/en/index.html Accessed: 12/14/2009 - Margolis HS, Alter MJ, Hadler S. Viral hepatitis. In: Evans AS, Kaslow 1. RA, eds. Viral infections of humans: Epidemiology and control. 4th ed. Springer, 1997:363-418.
- André F. Hepatitis B epidemiology in Asia,
the Middle East and Africa. Vaccine. 2000 18;18 Suppl 1:S20-2.

- Crockett SD, Keeffe EB. Natural history
and
treatment of hepatitis B. virus and hepatitis C virus coinfection. Ann
Clin Microbiol Antimicrob 2005;4:13.

- Sanchez Tapias JM, Costa J, Mas A, et al.
Influence of hepatitis B 4. virus genotype on the long-term outcome of
chronic hepatitis B in western patients. Gastroenterology
2002;123:1848-56.

- Kramvis A, Kew MC. Epidemiology of
hepatitis B
virus in Africa, its genotypes and clinical associations of genotypes.
Hepatol Res. 2007;37:S9-S19.

- Sherif MM, Abou-Aita BA, Abou-Elew MH,
el-Kafrawi
AO. Hepatitis B virus infection in upper and lower Egypt. J Med Virol.
1985;15:129-35.

- Ismail AM, Ziada HN, Sheashaa HA, Shehab
El-Din
AB. Decline of viral hepatitis prevalence among asymptomatic Egyptian
blood donors: a glimmer of hope. Eur J Intern Med. 2009;20:490-3.

- El Sherbini A, Mohsen SA, Seleem Z, Ghany
AA,
Moneib A, Abaza AH. Hepatitis B virus among schoolchildren in an
endemic area in Egypt over a decade: impact of hepatitis B vaccine. Am
J Infect Control. 2006;34:600-2.

- Shaaban FA, Hassanin AI, Samy SM, Salama
SI, Said
ZN. Long-term immunity to hepatitis B among a sample of fully
vaccinated children in Cairo, Egypt. East Mediterr Health J.
2007;13:750-7.

- Ibrahim S, el-Din S, Bazzal I. Antibody
level
after hepatitis-B vaccination in hemodialysis patients: impact of
dialysis adequacy, chronic inflammation, local endemicity and
nutritional status. J Natl Med Assoc. 2006.

- Zekri AR, Hafez MM, Mohamed NI, Hassan ZK,
El-Sayed MH, Khaled MM, Mansour T. Hepatitis B virus (HBV) genotypes in
Egyptian pediatric cancer patients with acute and chronic active HBV
infection. Virol J. 2007;15;4:74.

- Badr II, Farghaly AG, Koura MR, Mohamed
HF,
Hassan EM, Kotkat AM. Health status assessment of drug addicts in
Alexandria. J Egypt Public Health Assoc. 1998;73:275-96.

- Paez Jimenez A, El-Din NS, El-Hoseiny M,
El-Daly
M, Abdel-Hamid M, El Aidi S, Sultan Y, El-Sayed N, Mohamed MK, Fontanet
A. Community transmission of hepatitis B virus in Egypt: results from a
case-control study in Greater Cairo. Int J Epidemiol. 2009;38:757-65.

- Lehman EM, Wilson ML. Epidemiology of
hepatitis
viruses among hepatocellular carcinoma cases and healthy people
in Egypt: a systematic review and meta-analysis. Int J Cancer. 2009
1;124:690-7.

- Elzouki A N. Hepatitis B infection in Libya: The magnitude of the problem. The Libyan Journal of Infectious Diseases, 2008, 2: 20-25.
- Elzouki A, Esmeo M, Samod M, et al. Prevalence of hepatitis B, C and HIV infection in Libya: a population- based nationwide sero- epidemiological study. Liver International 2006; 26: Supp 1, 20.
- Elzouki A, Esmeo M, Samod M, et al. Prevalence of hepatitis B, C and HIV infection in Libya: a population- based nationwide sero- epidemiological study. Liver International 2006; 26: Supp 1, 20
- Triki H, Said N, Ben Salah A, Arrouji A,
Ben
Ahmed F, Bouguerra A, Hmida S, Dhahri R, Dellagi K. Seroepidemiology of
hepatitis B, C and delta viruses in Tunisia. Trans R Soc Trop Med Hyg.
1997;91:11-4.

- Triki H, Ben Slimane S, Ben Mami N, Sakka
T, Ben
Ammar A, Dellagi K. High circulation of hepatitis B virus (HBV) precore
mutants in Tunisia, North Africa. Epidemiol Infect. 2000;125:169-74.

- Bahri O, Cheikh I, Hajji N, Djebbi A,
Maamouri N,
Sadraoui A, Mami NB, Triki H. Hepatitis B genotypes, precore and core
promoter mutants circulating in Tunisia. J Med Virol. 2006;78:353-7.

- Hannachi N, Bahri O, Mhalla S, Marzouk M,
Sadraoui A, Belguith A, Triki H, Boukadida J. Hepatitis B virus
infection in Tunisian pregnant women: risk factors and viral DNA levels
in HBe antigen negative women. Pathol Biol (Paris). 2009;57:e43-7.

- Langar H, Triki H, Gouider E, Bahri O,
Djebbi A,
Sadraoui A, Hafsia A, Hafsia R. Blood-transmitted viral infections
among haemophiliacs in Tunisia. Transfus Clin Biol. 2005;12:301-5.

- Meldal BH, Moula NM, Barnes IH, Boukef K,
Allain
JP. A novel hepatitis B virus subgenotype, D7, in Tunisian blood
donors. J Gen Virol. 2009;90:1622-8.

- Ayed, Z., Houinato, D., Hocine, M., Ranger-Rogez, S., Denis, F. Prevalence of hepatitis B and C among Algerian blood donors and pregnant women. Bulletin de la Société de Pathologie Exotique, 2006. CABI.
- Khelifa F, Thibault V. Characteristics of
hepatitis B viral strains in chronic carrier patients from North-East
Algeria. Pathol Biol 2009;57:107-13.

- Sbai A, Bennani A, Benjouad A, Hassar M.
HBV genotypes in Morocco. J Clin Virol. 2007;38:184-5.

- Ezzikouri S, Chemin I, Chafik A, Wakrim L,
Nourlil J, Malki AE, Marchio A, Dejean A, Hassar M, Trepo C, Pineau P,
Benjelloun S. Genotype determination in Moroccan hepatitis B chronic
carriers. Infect Genet Evol. 2008;8:306-12.

- Boulaajaj K, Elomari Y, Elmaliki B,
Madkouri B,
Zaid D, Benchemsi N. Prevalence of hepatitis C, hepatitis B and HIV
infection among haemodialysis patients in Ibn-Rochd university
hospital, Casablanca] Nephrol Ther. 2005;1:274-84.

- World Health Organization. Hepatitis C. Available at: http://www.who.int/vaccine research/diseases/viral cancers/ en/index2.html. Accessed 11/29/2009.
- The Global Burden of Hepatitis C Working
Group. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol
2004;44:20–9.

- Shepard CW, Finelli L, Alter MJ. Global
epidemiology of hepatitis C virus infection. Lancet Infect Dis
2005;5:558–67.

- Strickland GT. Liver disease in Egypt:
hepatitis
C superseded schistosomiasis as a result of iatrogenic and biological
factors. Hepatology. 2006;43:915-22.

- Kamal S, Madwar M, Bianchi L, Tawil AE,
Fawzy R,
Peters T, et al. Clinical, virological and histopathological features:
long-term follow-up in patients with chronic hepatitis C co-infected
with S. mansoni. Liver 2000;20:281-289.

- Simmonds P, Holmes EC, Cha T-A, Chan SW,
McOmish
F, Irvine B, et al. Classification of hepatitis C virus into six major
genotypes and a seriesof subtypes by phylogenetic analysis of the NS-5
region. J Gen Virol 1993;74:2391-2399.

- Bhattacherjee V, Prescott LE, Pike I,
Rodgers B,
Bell H, El-Zayadi AR, et al. Use of NS-4 peptides to identify type
specific antibody to hepatitis C virus genotypes 1, 2, 3, 4, 5 and 6. J
Gen Virol 1995;76:1737-1748.

- Chamberlain R, Adams N, Saeed AA, Simmonds
P,
Elliott RM. Complete nucleotide sequence of a type 4 hepatitis C virus
variant, the predominant in the Middle East. J Gen Virol
1997;78:1341-1347.

- Zakaria S, Fouad R, Shaker O, Zaki S,
Hashem A,
El-Kamary SS, Esmat G, Zakaria S. Changing patterns of acute viral
hepatitis at a major urban referral center in Egypt. Clin Infect Dis.
2007 15;44:e30-6.

- Meky FA, Stoszek SK, Abdel-Hamid M, et al.
Active
surveillance for acute viral hepatitis in rural villages in the Nile
Delta. Clin Infect Dis 2006;42:628–33.

- Bakr I, Rekacewicz C, El Hosseiny M, et
al.
Higher clearance of hepatitis C virus infection in females compared
with males. Gut 2006;55:1183–7.

- Magder LS, Fix AD, Mikhail NN, et al.
Estimation
of the risk of transmission of hepatitis C between spouses in Egypt
based on seroprevalence data. Int J Epidemiol 2005;34:160-5.

- Kamal SM, Amin A, Madwar M, et al.
Cellular
immune responses in seronegative sexual contacts of acute hepatitis C
patients. J Virol 2004;78:12252–8.

- Kamal SM, Nasser I. Hepatitis C genotype
4: What
we now and what we do not yet know. Hepatology. 2008;47:1371-83.

- El Katsha S, Labeeb S, Watts S, et al.
Informal
health providers and the transmission of hepatitis C virus: Pilot study
in two Egyptian villages. East Mediterr Health J 2006;12:758–67.

- Stoszek SK, Abdel-Hamid M, Narooz S, et
al.
Prevalence of and risk factors for hepatitis C in rural pregnant
Egyptian women. Trans R Soc Trop Med Hyg 2006;100:102–7.

- Talaat M, Kandeel A, El-Shoubary W, et al.
Occupational exposure to needlestick injuries and hepatitis B
vaccination coverage among health care workers in Egypt. Am J Infect
Control 2003;31:469–74.

- Mohamed MK, Abdel-Hamid M, Mikhail NN, et
al.
Intrafamilial transmission of hepatitis C in Egypt. Hepatology
2005;42:683–7.

- Kamal SM, Ismail A, Graham CS, et al.
Pegylated
interferon a therapy in acute hepatitis C: Relation to hepatitis C
virusspecific T cell response kinetics. Hepatology 2004;39:1721–31.

- Kamal SM, Moustafa KN, Chen J, et al.
Duration of
peginterferon therapy in acute hepatitis C: A randomized trial.
Hepatology 2006;43:923–31.

- Saleh, M. G., Pereira, L. M. M. B., Tibbs,
C. J.,
Ziu, M et al. High prevalence of hepatitis C virus in the normal
Libyan population. Tropical Medicine and Hygiene, 1994; 88: 292-294.

- Elzouki A, Esmeo M, Samod M, et al. Prevalence of hepatitis B, C and HIV infection in Libya: a population- based nationwide sero- epidemiological study. Liver International 2006; 26: Supp 1, 20.
- Daw MA, Elkaber MA, Drah AM,
Werfalli MM,
Mihat AA, Siala IM. Prevalence of hepatitis C virus antibodies among
different populations of relative and attributable risk. Saudi Med J.
2002;23:1356-60.

- Franka E, El-Zoka AH, Hussein AH, Elbakosh
MM,
Arafa AK, Ghenghesh KS. Hepatitis B virus and hepatitis C virus in
medical waste handlers in Tripoli, Libya. J Hosp Infect. 2009;72:258-61.

- Yerly S, Quadri R, Negro F, Barbe KP,
Cheseaux
JJ, Burgisser P, Siegrist CA, Perrin L. Nosocomial outbreak of multiple
blood borne viral infections. J Infect Dis. 2001 1;184:369-72.

- Sassi F, Gorgi Y, Ayed K, Abdallah TB,
Lamouchi
A, Maiz HB. Hepatitis C virus antibodies in dialysis patients in
Tunisia: a single center study. Saudi J Kidney Dis Transpl.
2000;11:218-22.

- Djebbi, A, Triki, H, Bahri, O. et al.
Genotypes
of hepatitis C virus circulating in Tunisia. Epidemiol. Infect. 2003,
130: 501-505.

- National Travel Health Network and Centre (NaTHNaC): Country Information, Algeria, Tunisia and Morocco, 2009.
- Beghdadli B, Ghomari O, Taleb M, Belhaj Z,
Belabed A, Kandouci del AK, Fanello S. Personnel at risk for
occupational blood exposure in a university hospital in West Algeria.
Sante Publique. 2009;21:253-6.

- Benouda A, Boujdiya Z, Ahid S, Abouqal R,
Adnaoui
M.: Prevalence of hepatitis C virus infection in Morocco and
serological tests assessment of detection for the viremia prediction.
Pathol Biol 2009, 57:368-72.

- Sekkat S, Kamal N, Benali B, Fellah H,
Amazian K,
Bourquia A, El Kholti A, Benslimane A. Prevalence of anti-HCV
antibodies and seroconversion incidence in five haemodialysis units in
Morocco. Nephrol Ther. 2008;4:105-10.

- Boulaajaj K, Elomari Y, Elmaliki B,
Madkouri B,
Zaid D, Benchemsi N. Prevalence of hepatitis C, hepatitis B and HIV
infection among haemodialysis patients in Ibn-Rochd university
hospital, Casablanca. Nephrol Ther. 2005;1:274-84.

- Zahraoui-Mehadji M, Baakrim MZ, Laraqui S,
Laraqui O, El Kabouss Y, Verger C, Caubet A, Laraqui CH. Infectious
risks associated with blood exposure for traditional barbers and their
customers in Morocco. Sante. 2004;14:211-6.

- Wedemeyer H, Manns MP. Epidemiology,
pathogenesis
and management of hepatitis D: update and challenges ahead. Nat Rev
Gastroenterol Hepatol. 2010;7:31-40.

- Rizzetto M. Hepatitis D: thirty years
after. J Hepatol. 2009;50:1043-50.

- Darwish MA, Shaker M, Raslan OS,
Abdel-Raouf T.
Delta virus infection in Egypt. J Egypt Public Health
Assoc.1992;67:147-61.

- El-Zimaity DM, Hyams KC, Imam IZ, Watts
DM,
Bassily S, Naffea EK, Sultan Y, Emara K, Burans J, Purdy MA, et al.
Acute sporadic hepatitis E in an Egyptian pediatric population. Am J
Trop Med Hyg. 1993;48:372-6.

- Saudy N, Sugauchi F, Tanaka Y, Suzuki S,
Aal AA,
Zaid MA, Agha S, Mizokami M. Genotypes and phylogenetic
characterization of hepatitis B and delta viruses in Egypt. J Med
Virol. 2003;70:529-36.

- Morcos MM, Mikhail TH, Hanna WM,
Abdel-Fattah S,
el-Rasad MM, Wassef EL. The prevalence of delta virus infection in
chronic liver disease in Egyptian children in comparison with some
other countries. Panminerva Med. 2000;42:97-100.

- Rioche M, Himmich H, Hansson BG,
Nordenfelt E.
Low occurrence of delta agent infections in Morocco. Bull Soc Pathol
Exot Filiales. 1987;80:741-4.

- Meng XJ. Recent advances in Hepatitis E
Virus. J Viral Hepat. 2009;21.

- Fitz Simons D, Hendrickx G, Vorsters A,
Van Damme P. Hepatitis A and E: update on prevention and epidemiology.
Vaccine.
2010 8;28:583-8.

- Blackard JT, Rouster SD, Nady S, Galal G,
Marzuuk N, Rafaat MM, Daef E, El Din SS, Purcell RH, Emerson SU,
Sherman KE, Shata MT. Genotypic characterization of symptomatic
hepatitis E virus (HEV) infections in Egypt. J Clin Virol.
2009;46:140-4.

- Stoszek SK, Engle RE, Abdel-Hamid M,
Mikhail N,
Abdel-Aziz F, Medhat A, Fix AD, Emerson SU, Purcell RH, Strickland GT.
Hepatitis E antibody seroconversion without disease in highly endemic
rural Egyptian communities. Trans R Soc Trop Med Hyg. 2006;100:89-94.

- Darwish MA, Faris R, Darwish N, Shouman
A,
Gadallah M, El-Sharkawy MS, Edelman R, Grumbach K, Rao MR, Clemens JD.
Hepatitis c and cirrhotic liver disease in the Nile delta of Egypt: a
community-based study. Am J Trop Med Hyg. 2001;64:147-53.

- Fix AD, Abdel-Hamid M, Purcell RH,
Shehata MH,
Abdel-Aziz F, Mikhail N, el Sebai H, Nafeh M, Habib M, Arthur RR,
Emerson SU, Strickland GT. Prevalence of antibodies to hepatitis E in
two rural Egyptian communities. Am J Trop Med Hyg. 2000;62:519-23.

- Stoszek SK, Abdel-Hamid M, Saleh DA, El
Kafrawy
S, Narooz S, Hawash Y, Shebl FM, El Daly M, Said A, Kassem E, Mikhail
N, Engle RE, Sayed M, Sharaf S, Fix AD, Emerson SU, Purcell RH,
Strickland GT. High prevalence of hepatitis E antibodies in pregnant
Egyptian women. Trans R Soc Trop Med Hyg. 2006;100:95-101.

- Ghannoum MA, Moore KE, Al-Dulaimi M, Nasr
M. The
incidence of water-related diseases in the Brak area, Libya from 1977
to 1979, before and after the installation of water treatment plants.
Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173:501-8.

- Hannachi N, Hidar S, Harrabi I, Mhalla S,
Marzouk M, Ghzel H, Ghannem H, Khairi H, Boukadida J Seroprevalence and
risk factors of hepatitis E among pregnant women in central Tunisia.
Biol (Paris). 2009 Nov 4.

- Van Cuyck-Gandré H, Zhang HY, Tsarev SA,
Clements NJ, Cohen SJ, Caudill JD, Buisson Y, Coursaget P, Warren RL,
Longer CF. Characterization of hepatitis E virus (HEV) from Algeria and
Chad by partial genome sequence. J Med Virol. 1997;53:340-7.

- Grandadam M, Tebbal S, Caron M,
Siriwardana M,
Larouze B, Koeck JL, Buisson Y, Enouf V, Nicand E. Evidence for
hepatitis E virus quasispecies. J Gen Virol. 2004;85:3189-94.

- Bernal MC, Leyva A, Garcia F, Galan I,
Piedrola
G, Heyermann H, Maroto MC. Seroepidemiological study of hepatitis E
virus in different population groups. Eur J Clin Microbiol Infect Dis.
1995;14:954-8.

- Benjelloun S, Bahbouhi B, Bouchrit N,
Cherkaoui
L, Hda N, Mahjour J, Benslimane A. Seroepidemiological study of an
acute hepatitis E outbreak in Morocco. Res Virol. 1997;148:279-87.
