Hepatitis C Virus Infection and Lymphoma
Emmanuel Bachy1, Caroline Besson2, Felipe Suarez1 and Olivier Hermine1
1Service d’hématologie adulte, Hôpital Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris, Université René Descartes Paris V, CNRS UMR 8147 Paris, France.
2Service d’hématologie Biologique, Hôpital du Kremlin Bicêtre, Université Paris Sud, France.
Correspondence
to: Pr. Olivier Hermine. Service d’hématologie Hôpital Necker,
149-161 rue de Sèvres, Paris 15, France. ohermine@gmail.com
Published: March 31.2010
Received: March 22.2010
Accepted: March 28.2010
Medit J Hemat Infect Dis 2010, 2(1): e2010004, DOI 10.4084/MJHID.2010.004
This article is available from: http://www.mjhid.org/article/view/5642
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Apart
from its well known role as an etiological agent for non-A and non-B
viral hepatitis, there is growing evidence that hepatitis C virus is
associated to B-cell non-Hodgkin lymphoma. The association between HCV
and lymphoproliferative disorders has been recently postulated based on
epidemiological data, biological studies and clinical observations.
Although various subtypes of lymphomas appear to be associated to HCV,
diffuse large B-cell lymphoma, small lymphocytic lymphoma/chronic
lymphocytic leukemia and marginal zone lymphoma appeared to be
particularly represented among HCV-positive patients. The
causative role of HCV in those disorders has been further supported by
the response to anti-viral therapy. Despite a better understanding of
pathophysiological processes at stake leading from HCV infection to
overt lymphoma, many issues still need to be further elucidated.
Although HCV has been demonstrated to directly infect peripheral blood
mononuclear cells both in vitro and, in some cases, in vivo, a strong
body of evidence rather supports the hypothesis of an indirect
transformation mechanism by which sustained antigenic stimulation leads
from oligoclonal to monoclonal expansion and sometimes to lymphoma,
probably through secondary oncogenic events. Here, we review
epidemiological and biological studies, as well as clinical data on
antiviral therapy, linking HCV-infection to B-cell non-Hodgkin lymphoma.
Introduction
Hepatitis C virus (HCV) is a small (9600 nucleotide) encapsulated positive strand RNA member of the Flaviviridae family. The virus lacks a reverse-transcriptase and its genome encodes a single open reading frame for a large polyprotein, which is subsequently cleaved to structural and non-structural (enzymatic) component viral proteins. For more than a decade, evidence from either epidemiological studies, therapeutic approaches or biological data have emerged giving strong support to an etiological role of HCV in non-Hogkin lymphoma (NHL) development [1,2].
Thus, many large case-control studies have reported a clear association between HCV infection and NHL development. Furthermore, HCV-associated NHL response to interferon (IFN) and ribavirin therapy in case of viral load decrease, previously described in several studies, also gave strong support for an etiopathological role of HCV in this kind of lymphoproliferative disorder.
Although HCV is the major etiologic agent of non-A and non-B chronic hepatitis, it can present with a broad spectrum of extrahepatic manifestations. Among them, immune-related disorders have been described such as type II mixed-cryoglobulinemia (MC), characterized by a monoclonal IgM with rheumatoid factor (RF) activity (i.e. an IgM with anti-IgG activity) with or without an overt cryoglobulinemia-associated vasculitis. About 8 to 10% of patients with MC ultimately develop a frank lymphoproliferative disorder and recent data demonstrated a 35 times increased risk of lymphoma for cryoglobulinemia carriers. Among pathogenetic hypotheses, HCV lymphotropism has been studied but no direct transformation leading to an overt lymphoproliferative disorder has been clearly demonstrated. Conversely, strong evidence for an indirect role for HCV in inducing lymphoproliferative disorders has been given by recent findings. In this regard, association between HCV-infection, mixed cryoglobulinemia (MC) and NHL lent support to a multistep model in which HCV would induce a protracted stimulation of antigen-specific B-cell clones, leading to MC and in a subset of patients to overt lymphomas.
Thus, cumulative evidence for a pathological link between HCV infection and lymphoma has emerged and will be reviewed in this paper.
HCV-associated lymphoma: evidence from epidemiological studies.
Several epidemiological studies have been performed to establish a link between HCV infection and NHL. Early studies based on relatively small number of patients provided conflicting results and suggested a significant increased risk of B-NHL in HCV-infected patients only in high prevalence areas.[3,4] Discrepancies concerning HCV prevalence in lymphoma patients demonstrated in case-control studies from North America and North European countries have thus been pointed out. In those studies, the prevalence of HCV infection among patients with NHL did not significantly differ from controls. This might be explained, at least in part, by a large difference in HCV prevalence itself (which is lower in those countries), or by yet unknown environmental and genetic factors.[5,6] Two large US studies involving the NCI-SEER registry and the US Veterans Affairs health system respectively, as well as an analysis by the European multicentre EPILYMPH consortium, have documented a positive, modestly increased risk of NHL in patients with HCV as compared to HCV-negative controls (relative risk (RR)=2–3).[7,8] These latter studies were additionally supported by a concurrent meta-analysis review of 15 case-control and 3 prospective studies in this field, showing a ‘‘pooled’’ RR of 2–2.5 depending on study design.9 Eventually, another recently published meta-analysis included 7 member studies from the International Lymphoma Epidemiology Consortium (InterLymph) based in Europe, North America, and Australia.[10] HCV infection was detected in 3.60% of NHL cases and in 2.70% of controls (odds ratio [OR], 1.78; 95% confidence interval [CI], 1.40-2.25). It thus appears that there is a greater propensity to develop NHL in the setting of HCV infection, and that the risk is most dramatically evident in populations with high HCV prevalence. Besides, geographic variability worldwide may indicate additional important environmental factors influencing the strength of this relationship. In addition, providing further evidence, a report from Japan demonstrated that 2,5% of patients infected by the virus were at risk of developing NHL within a 15-year period of follow-up. Interestingly, during the same period following antiviral therapy, none of the patients who cleared the virus developed NHL whereas those who remained PCR positive had the same risk as not treated patients.[11]
Not all lymphoma subtypes are equally represented among those lymphoproliferative disorders. Hence, splenic (with or without villous lymphocytes) or non splenic marginal zone lymphomas (MZL), diffuse large B-cell lymphomas (DLBCL), but not follicular lymphoma (FL) or T-cell lymphomas have been associated with HCV infection. Hence, the recent meta-analysis from the InterLymph Consortium demonstrated that, in subtype-specific analyses, HCV prevalence was associated with MZL (OR, 2.47; 95% CI, 1.44-4.23), DLBCL (OR, 2.24; 95% CI, 1.68-2.99), and lymphoplasmacytic lymphoma (OR, 2.57; 95% CI, 1.14-5.79) whereas risk estimates were not increased for FL (OR, 1.02; 95% CI, 0.65-1.60).[10] Thus, the most common lymphomas found in patients with HCV are low grade MZL particularly those of splenic origin and high-grade DLBCL with extranodal localizations.[11-13] These lymphoproliferations exhibit peculiar features: i. they usually occur following a long period of infection (more than 15 years), ii. are not associated with a specific virus genotype, iii. often involve extranodal sites particularly liver, spleen and salivary glands.
However, despite these epidemiological and clinical evidences, the role of HCV in lymphomagenesis has remained elusive, and only recently have pathophysiological models started to emerge.
HCV-associated lymphoma: evidence from antiviral therapy efficacy on lymphoma.
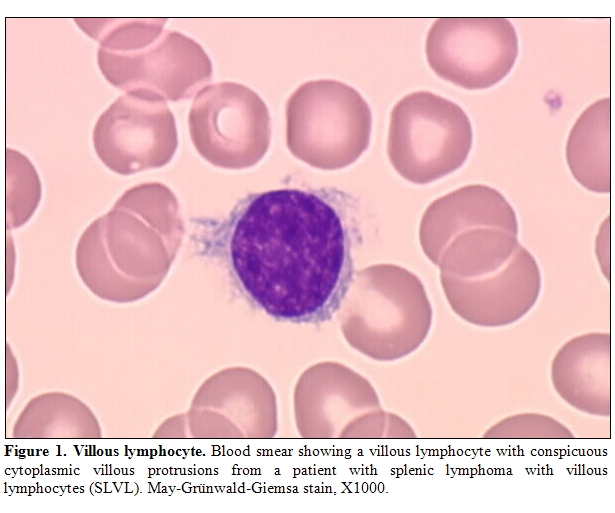
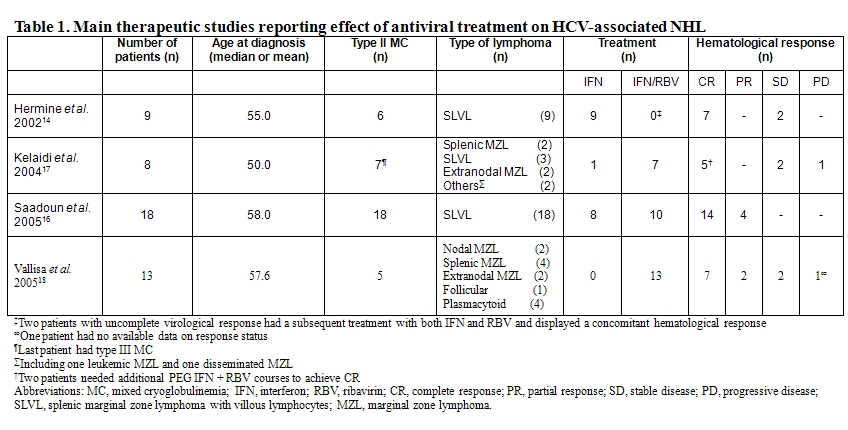
If HCV infection has been inferred to be a factor in the development of NHL on the basis of case-control epidemiological studies as previously discussed, strong line of evidence also arose from response of so called HCV-associated lymphoma to antiviral therapy. Hence, in 2002, we reported the outcome of 9 patients who had splenic MZL with villous lymphocytes and HCV infection treated with interferon alfa-2b (IFN) alone or in combination with ribavirin.[14] Splenic lymphoma with villous lymphocytes (SLVL) is a clonal chronic B-cell lymphoproliferative disorder characterized by splenomegaly and peripheral blood malignant circulating B lymphocytes with villous projections (Figure 1). Histologically, the lymphoid marginal zone surrounding the follicular areas is expanded by neoplastic cells that have cytological and phenotypical features of marginal zone B cells.[15] As a consequence, clonal expansion of villous lymphocytes is supposed to originate from the marginal zone of the spleen although definitive conclusion has not been clearly demonstrated to date. From a clinical point of view, the disease displays an indolent evolution with splenomegaly increasing over years and with a gradual progression of circulative malignant B cells. Of the 9 IFN-treated patients, 7 achieved a complete hematological remission, defined by the absence of abnormal lymphocytosis and the resolution of the splenomegaly, after HCV RNA load became undetectable. The remaining 2 patients experienced a partial or a complete response after addition of ribavirin and the loss of detectable HCV RNA.
Conversely, none of 6 similarly treated HCV-negative SLVL patients responded to therapy thereby suggesting that the observed response rate was not due to the effect of interferon itself. Those results gave support to the hypothesis that HCV might trigger, to some extent, clonal expansion and oncogenic events at least in a subgroup of indolent lymphomas patients. We also reported in 2005 on a series of 18 patients with HCV-associated SLVL.[16] All patients had MC, the majority of whom having symptomatic MC (72%), a much higher proportion than HCV-infected patients without SLVL. Apart from this finding, HCV-positive SLVL did not differ from HCV-negative SLVL patients. All patients were treated with alpha-IFN with (10 patients) or without (8 patients) ribavirin. Four patients had received prior therapy for SLVL including splenectomy or chemotherapy. Six patients received associated therapy for symptomatic MC (steroids, cyclophosphamide or plasmapheresis). Complete hematological remission was observed in 78% of the patients, most of them having concomitant complete virological responses (i.e. disappearance of HCV RNA). Two patients with major virological responses (more than 2 log reduction in HCV RNA) also achieved complete hematological remission, whereas the 2 patients with minor virological responses (less than 2 log reduction in HCV RNA) only achieved partial hematological responses (i.e. reduced albeit persistent circulating villous lymphocytes and splenomegaly). Moreover, in one patient with a virological relapse, villous lymphocytosis reappeared, but re-initiation of antiviral therapy was associated with a second complete hematological remission following HCV RNA reduction. The mean time to treatment responses was approximately 4 months for both virological and hematological responses. Mean duration of antiviral treatment was 17 months. Responses were sustained, as the mean duration of hematological response was 62 months. Clinical manifestations of MC subsided in all patients after antiviral treatment. Interestingly, viral genotype did not seem to correlate with the response as 4 out of 7 patients presenting with HCV genotype 1, usually associated with poor responses, achieved a complete hematologic response. Of note, even for patients who exhibited a complete hematological remission, B-cell clone could still be detected in peripheral blood but clinical relapses did not occur if viremia remained negative.
Overall, these observations strongly supported a causal relationship between HCV replication and lymphomagenesis in SLVL. Another report confirmed those results showing that among 8 patients with MZL of MALT or splenic (with or without villous lymphocytes) subtypes, alpha-IFN and ribavirin yielded a 60% response rate, which was correlated to virologic response in most cases.[17] A more recently published study extended these results by showing efficacy of antiviral therapy in other histological subtypes of indolent NHL associated with HCV infection, including follicular lymphoma and splenic MZL.[18]
Altogether, response to antiviral therapy in HCV-related NHL in case of viral load decrease gave further insights into the pathogenesis of those disorders and strengthened the association presumed from epidemiological studies.
HCV-associated lymphoma: evidence from biological studies.
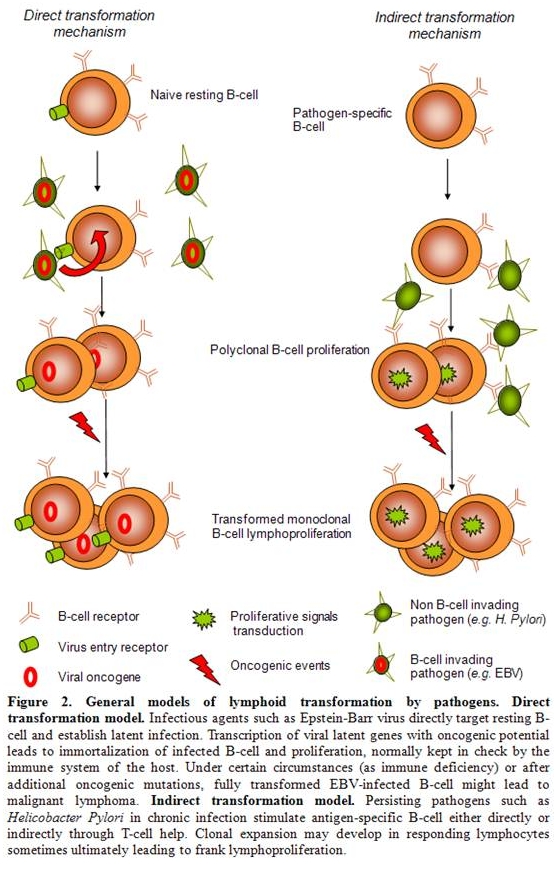
Although epidemiological studies and clinical data link NHL with HCV, underlying biological processes ultimately leading from infection to lymphoma are still poorly understood. Basically, relationships between infectious agents and lymphoproliferative disorders such as Helicobacter pylori and gastric MALT-lymphoma, Epstein-Barr virus (EBV) and lymphoproliferative disorders (LPD), human herpesvirus 8 (HHV8) and primary effusion lymphoma (PEL) or human T-lymphotropic virus 1 (HTLV-1) and adult T-cell leukemia/lymphoma have been extensively studied for the two last decades but no clear pathological model has emerged so far. Nevertheless, antigen-driven lymphoproliferation might be though of as being split into 2 distinct mechanisms. On one hand, direct lymphocyte transformation by a given agent such as lymphotropic transforming viruses (EBV, HHV8, or HTLV1) expressing viral oncogenes has been clearly demonstrated.[19-21] On the other hand, a more recently described model for an indirect transformation mechanism of lymphocytes ultimately leading to clonal expansion has emerged, among which Helicobacter Pylori-associated gastric MALT lymphoma might be the best characterized (Figure 2).[22]
As a matter of fact, growing evidence show that, when exposed to chronic antigenic stimulation, B cells accumulate genetic lesions through inherent genomic instability during activation-induced deaminase (AID)-mediated variable-determining-joining V(D)J class switch recombination (CSR) and/or somatic hypermutation (SHM).[23] Both of these reactions have been shown to produce double-strand DNA break intermediates that might be aberrantly resolved as chromosomal translocations. [24,25]
Several oncogenes have been described as targets for somatic hypermutation or translocations with immunoglobulin heavy-chains regions (IgH). In most cases, these anomalies alarm the DNA damage response system that either allows for DNA repair or eliminates the aberrant B-cell clones.[19] Occasionally, those repair systems fail and B-cell malignancies may arise.
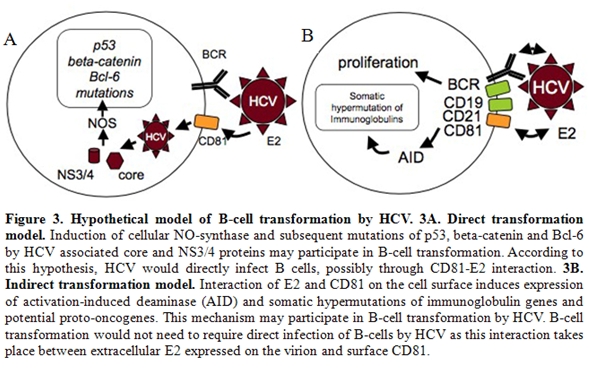
As regards to hepatitis C virus, few experimental data support the hypothesis of a direct transformation mechanism accounting for HCV-associated lymphomagenesis. In in vitro studies, CD81 has been shown to be an entry receptor for HCV and could be involved in infection of B cells by the virus. [26] In vitro, infection of B-cell lines with HCV leads to somatic mutations of several oncogenes and tumor suppressor genes such as p53, beta-catenin and Bcl6. [27,28] The expression of the HCV core protein (C) and non-structural protein 3 (NS3) has been associated with the induction of nitric oxide synthase (NOS) which might be responsible for these mutations (Figure 3A). Similarly, frequent chromosomal polyploidy in peripheral blood mononuclear cells (PBMC) from HCV-positive patients was recently demonstrated, as well as in splenocytes from HCV core protein-expressing transgenic mice, suggesting that HCV infection may inhibit the mitotic checkpoint. [29] In vivo, as it lacks a reverse transcriptase, HCV requires the cellular machinery to efficiently produce negative strand from positive strand viral DNA. Therefore, identification of negative strand RNA sequences in cells is indicative of active virus replication. Using a specific and sensitive method for HCV minus strand RNA detection, Sansonno et al. demonstrated active HCV replication in PBMC in nearly half of HCV-positive MC patients whereas no sign of infection was found in HCV-infected individuals without MC. [30]
Notably, in 7 patients with B-NHL without MC, active HCV replication was not found. So far, direct infection of the malignant clone has been described in a single case of B-cell lymphoma associated with HCV. [31] Therefore, although it can infect B cells in vivo and despite an association between PBMC infection and MC, an etiological role of the virus in HCV-associated lymphoproliferative disorders by a direct transformation mechanism is poorly supported by those data as half of MC patients and virtually all NHL patients do not display active HCV replication in PBMC or in the malignant clone respectively.
On the contrary, many studies support the role of HCV as an indirect transformation agent by chronically stimulating B-cell immunologic response and ultimately leading to overt lymphoma in some cases. In this regard, data on the association between MC and NHL have been of great interest. Frequent polyclonal or monoclonal B-cell proliferation can be detected in the blood, bone marrow or liver biopsies of HCV patients and association with type II MC (characterized by a monoclonal IgM with anti-IgG – i.e. a rheumatoid factor - activity) has been clearly demonstrated. Hence, 50 to 90% of patients with MC harbor a positive HCV serology and conversely, nearly 40-50% of HCV patients exhibit circulating cryoprecipitating complexes. Along with data on the response of lymphoma to therapy, antiviral treatment has been associated with disappearance of MC and in many cases of B-cell clones. [32-35] About 8 to 10% of patients with cryoglubulinemia ultimately develop a frank lymphoproliferative disorder and recent data demonstrated a 35 times increased risk of lymphoma for cryoglobulinemia carriers compared with general population. Nevertheless, NHL does not always evolve out of MC in what can be thought of as a stepwise evolution and many issues still need to be further elucidated.
Data from V(D)J region analyses among patients with MC or frank NHL also brought new insights into the pathological process from HCV-infection to lymphoma. Restricted usage of VH1-69 and V3-20/15 regions have been demonstrated, for instance, among patients with MC and HCV-associated NHL thereby giving strong support to an antigenic selection driven process underlying lymphoma development in HCV-positive patients.[36,37] Sequencing of IgV regions also revealed that they constitute a target for SHM, suggestive of a maturation process under antigenic stimulation. [37] Furthermore, De Re et al. showed BCR from some HCV-related patients recognized both IgG-Fc and HCV-NS3 protein rising the possibility that rheumatoid factor might emerge from cross-reaction between a virus-associated epitope and IgG autoantigen. [38] Cross-reactivity between E2 directed antibodies and anti-IgG IgM RF in type II MC has also been demonstrated. [39] One may imagine that Ig-HCV immunocomplexes in chronically infected patients would amplify the phenomenon by stimulating cross-reactive RF producing clones. Other recent data support a major role for HCV envelope glycoprotein E2 in indirect transformation. Not only can it promote HCV cellular entry by binding to CD81 on B-cell surface but when E2 binds to B cells via CD81, this latter then associates with CD19 and CD21, forming a complex that lowers the activation threshold. [40] The synergy between CD81-CD19-CD21 complex signaling and BCR cross-activation by envelope glycoprotein E2 and/or NS3 is thought to promote B-cell proliferation, emergence of autoreactive clones, genomic instability and NHL development. To further support this hypothesis, direct infection of lymphocytes by HCV would not be necessary in vivo to induce somatic mutations of several oncogenes and tumor suppressor genes such as p53, beta-catenin and Bcl6 as recombinant HCV E2 binding to surface CD81 has also been shown to induce somatic hypermutation of the immunoglobulin gene locus (Figure 3B).[41] As the E2 glycoprotein is expressed on the virion surface, this mechanism of mutagenesis would not require direct infection of B cells by HCV.
Altogether, those biological data have led to the concept of a multi-step lymphomagenesis process in HCV-related B-cell clonal disorders by an indirect transformation mechanism.
Perspective: towards a multi-step lymphomagenesis model.
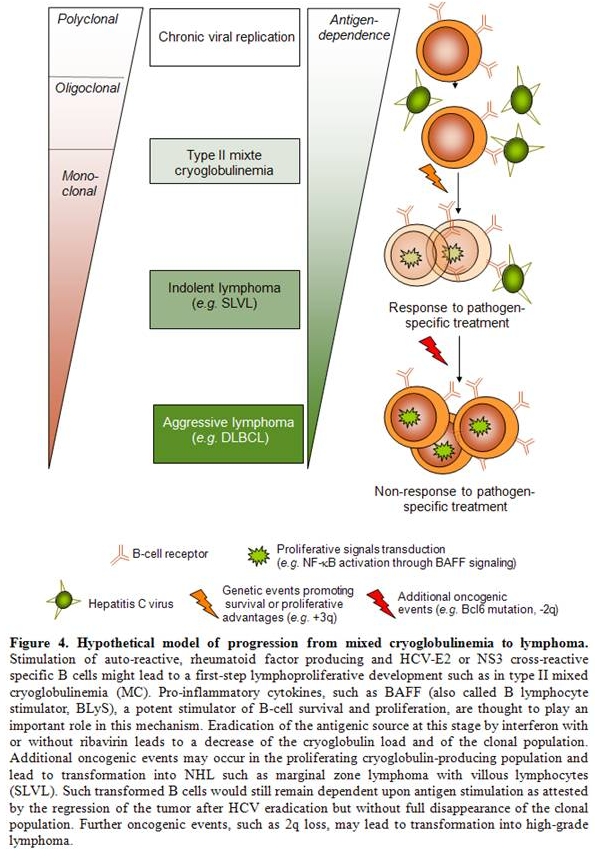
The finding that MC represents an independent risk factor for the development of NHL in HCV-infected patients,[42] ]as well as the finding that, among HCV-infected patients with NHL, antiviral treatment was more effective in those with associated MC16 lend support to a model in which HCV would induce a protracted stimulation of antigen-specific B-cell clones, leading to MC and in a subset of patients to overt lymphomas.
Besides chronic antigenic stimulation, cytokines and growth factors produced within the inflammatory context of chronic infection are now suspected to play a key role in B-cell transformation. As a matter of fact, BAFF (for B-cell activating factor of the TNF family) has been described as a critical survival factor for B cells, promoting their activation and maturation, mainly through the nonclassical NF-B pathway. [43-47] BAFF has also been shown to promote the survival of autoreactive B-cell clones in case of abnormal production thus triggering autoimmune diseases. [48] In HCV infection, BAFF deregulation has been demonstrated, consistent with a role of the virus as a trigger for BAFF upregulation possibly predisposing to B-cell proliferation and clonal expansion. [49,50] A role for BAFF in promoting B-cell survival in an autocrine regulation loop has also been suggested in B-cell lymphoproliferative disorders. Hence, MC HCV-positive patients with NHL displayed higher BAFF levels and more frequently upregulated BAFF levels than MC HCV-positive counterparts without NHL. [51] Studying other pro-inflammatory cytokines such as IL-17 might also be of interest as this cytokine has recently been described to act in synergy with BAFF in human B-cell survival, proliferation and differentiation into immunoglobulin-secreting cells. [46] Similarly to BAFF, higher IL-17 serum levels were also demonstrated in patients suffering from systemic lupus erythematosus compared to healthy donors46 and a role of IL-17-producing T helper cells in other autoimmune disorders such as rheumatoid arthritis, psoriasis or multiple sclerosis has been suggested. [52] A recent study demonstrated that PBMC from HCV Ag-positive patients secreted IL-17 in response to stimulation with the HCV nonstructural protein 4 (NS4).53 However, NS4 also induced TGF- and IL-10 expression at high levels by monocytes from HCV-infected patients and those cytokines were shown to significantly suppressed NS4 specific human Th17 cells. The balance between IL17 expected role in protective immunity to HCV and its demonstrated role in inducing autoimmunity has to be further studied in MC and NHL HCV-positive patients to bring new insights into the pathogenesis of these disorders.
Concerning oncogenic events leading to NHL development, cytogenetic information on HCV-associated lymphomas is scarce. Matteucci et al. recently studied genomic imbalances in low- and high-grade HCV-related lymphomas and found trisomy 3 or +3q in 4 out of 6 splenic MZL as typically reported in low-grade splenic non HCV-related MZL.54 As those 3q+ splenic MZL exhibited an indolent clinical course and regressed after HCV eradication, and since trisomy 3 was also found in circulating B cells of one patient with MC without lymphoma, 3q gain is not supposed to be oncogenic ‘per se’. Conversely, 2q loss was associated with more aggressive B-cell lymphomas (4 out of 5 DLBCL) with no response to antiviral treatment. Of note, 2 of these DLBCL were derived from low-grade underlying lymphoma. Regarding IgH/BCL2 translocations, unexpected results in HCV-related NHL have been published so far. Hence, whereas the presence of IgH/BCL2 clones is frequent in HCV-infected patients, especially when associated to MC, the translocation was not shown to be any more frequent among HCV-infected NHL patients than among HCV-negative NHL patients. [55-57] This finding suggests that IgH/BCL2, which is present at a low frequency in healthy subjects, [58] may be amplified by a bystander effect due to a non specific stimulation of the immune system rather than being an additional oncogenic hit that leads to malignant transformation.
Response to treatment from our cohort of patients illustrated the hypothesis of additional oncogenic events driving oligoclonal proliferation in MC to monoclonal expansion in lymphoma. Accordingly, despite the efficacy of antiviral treatment in a majority of patients, leading to complete virological and hematological responses including the disappearance of detectable MC, the B-cell clones were still detectable in all patients.[16] Those results contrasts with previous findings in HCV-associated patients with MC [32,59] where B-cell clones were not detectable anymore in case of complete virological response. This may reflect differences in oncogenic potential between HCV-driven MC-producing clones and SLVL malignant B cells. In the latter, B-cell clones are therefore likely to have accumulated unknown additional oncogenic events, inducing a survival benefit for this expanded clonal population that remains however still dependent upon antigenic stimulation as demonstrated by response to antiviral treatment.
Aside from SLVL and other low-grade B-cell NHL, HCV is epidemiologically associated with DLBCL. [3,4] We have recently shown that DLBCL in HCV-positive patients were more frequently transformed from low-grade B-cell lymphomas than DLBCL in HCV-negative patients. [60] The finding that splenic involvement was more frequent in HCV-positive cases also supports a possible transformation from an underlying low-grade B-cell NHL. Furthermore, some differences exist in the clinical outcome of these transformed DLBCL. Surprisingly, patients in the HCV-positive group exhibited a longer event-free survival although the overall survival was significantly shorter in HCV positive patients. [60] These findings suggest a difference in the pathophysiological mechanisms underlying lymphomagenesis in HCV-associated DLBCL, and, by extension, a causal role for HCV in this lymphoma subtype. Therefore, DLBCL might be seen as the ultimate stage of lymphomagenesis in HCV-positive patients, ranging from indolent lymphoma responsive to therapy like SLVL to aggressive disease independent of antigenic stimulation like DLBCL.
Hepatitis C virus (HCV) is a small (9600 nucleotide) encapsulated positive strand RNA member of the Flaviviridae family. The virus lacks a reverse-transcriptase and its genome encodes a single open reading frame for a large polyprotein, which is subsequently cleaved to structural and non-structural (enzymatic) component viral proteins. For more than a decade, evidence from either epidemiological studies, therapeutic approaches or biological data have emerged giving strong support to an etiological role of HCV in non-Hogkin lymphoma (NHL) development [1,2].
Thus, many large case-control studies have reported a clear association between HCV infection and NHL development. Furthermore, HCV-associated NHL response to interferon (IFN) and ribavirin therapy in case of viral load decrease, previously described in several studies, also gave strong support for an etiopathological role of HCV in this kind of lymphoproliferative disorder.
Although HCV is the major etiologic agent of non-A and non-B chronic hepatitis, it can present with a broad spectrum of extrahepatic manifestations. Among them, immune-related disorders have been described such as type II mixed-cryoglobulinemia (MC), characterized by a monoclonal IgM with rheumatoid factor (RF) activity (i.e. an IgM with anti-IgG activity) with or without an overt cryoglobulinemia-associated vasculitis. About 8 to 10% of patients with MC ultimately develop a frank lymphoproliferative disorder and recent data demonstrated a 35 times increased risk of lymphoma for cryoglobulinemia carriers. Among pathogenetic hypotheses, HCV lymphotropism has been studied but no direct transformation leading to an overt lymphoproliferative disorder has been clearly demonstrated. Conversely, strong evidence for an indirect role for HCV in inducing lymphoproliferative disorders has been given by recent findings. In this regard, association between HCV-infection, mixed cryoglobulinemia (MC) and NHL lent support to a multistep model in which HCV would induce a protracted stimulation of antigen-specific B-cell clones, leading to MC and in a subset of patients to overt lymphomas.
Thus, cumulative evidence for a pathological link between HCV infection and lymphoma has emerged and will be reviewed in this paper.
HCV-associated lymphoma: evidence from epidemiological studies.
Several epidemiological studies have been performed to establish a link between HCV infection and NHL. Early studies based on relatively small number of patients provided conflicting results and suggested a significant increased risk of B-NHL in HCV-infected patients only in high prevalence areas.[3,4] Discrepancies concerning HCV prevalence in lymphoma patients demonstrated in case-control studies from North America and North European countries have thus been pointed out. In those studies, the prevalence of HCV infection among patients with NHL did not significantly differ from controls. This might be explained, at least in part, by a large difference in HCV prevalence itself (which is lower in those countries), or by yet unknown environmental and genetic factors.[5,6] Two large US studies involving the NCI-SEER registry and the US Veterans Affairs health system respectively, as well as an analysis by the European multicentre EPILYMPH consortium, have documented a positive, modestly increased risk of NHL in patients with HCV as compared to HCV-negative controls (relative risk (RR)=2–3).[7,8] These latter studies were additionally supported by a concurrent meta-analysis review of 15 case-control and 3 prospective studies in this field, showing a ‘‘pooled’’ RR of 2–2.5 depending on study design.9 Eventually, another recently published meta-analysis included 7 member studies from the International Lymphoma Epidemiology Consortium (InterLymph) based in Europe, North America, and Australia.[10] HCV infection was detected in 3.60% of NHL cases and in 2.70% of controls (odds ratio [OR], 1.78; 95% confidence interval [CI], 1.40-2.25). It thus appears that there is a greater propensity to develop NHL in the setting of HCV infection, and that the risk is most dramatically evident in populations with high HCV prevalence. Besides, geographic variability worldwide may indicate additional important environmental factors influencing the strength of this relationship. In addition, providing further evidence, a report from Japan demonstrated that 2,5% of patients infected by the virus were at risk of developing NHL within a 15-year period of follow-up. Interestingly, during the same period following antiviral therapy, none of the patients who cleared the virus developed NHL whereas those who remained PCR positive had the same risk as not treated patients.[11]
Not all lymphoma subtypes are equally represented among those lymphoproliferative disorders. Hence, splenic (with or without villous lymphocytes) or non splenic marginal zone lymphomas (MZL), diffuse large B-cell lymphomas (DLBCL), but not follicular lymphoma (FL) or T-cell lymphomas have been associated with HCV infection. Hence, the recent meta-analysis from the InterLymph Consortium demonstrated that, in subtype-specific analyses, HCV prevalence was associated with MZL (OR, 2.47; 95% CI, 1.44-4.23), DLBCL (OR, 2.24; 95% CI, 1.68-2.99), and lymphoplasmacytic lymphoma (OR, 2.57; 95% CI, 1.14-5.79) whereas risk estimates were not increased for FL (OR, 1.02; 95% CI, 0.65-1.60).[10] Thus, the most common lymphomas found in patients with HCV are low grade MZL particularly those of splenic origin and high-grade DLBCL with extranodal localizations.[11-13] These lymphoproliferations exhibit peculiar features: i. they usually occur following a long period of infection (more than 15 years), ii. are not associated with a specific virus genotype, iii. often involve extranodal sites particularly liver, spleen and salivary glands.
However, despite these epidemiological and clinical evidences, the role of HCV in lymphomagenesis has remained elusive, and only recently have pathophysiological models started to emerge.
HCV-associated lymphoma: evidence from antiviral therapy efficacy on lymphoma.
If HCV infection has been inferred to be a factor in the development of NHL on the basis of case-control epidemiological studies as previously discussed, strong line of evidence also arose from response of so called HCV-associated lymphoma to antiviral therapy. Hence, in 2002, we reported the outcome of 9 patients who had splenic MZL with villous lymphocytes and HCV infection treated with interferon alfa-2b (IFN) alone or in combination with ribavirin.[14] Splenic lymphoma with villous lymphocytes (SLVL) is a clonal chronic B-cell lymphoproliferative disorder characterized by splenomegaly and peripheral blood malignant circulating B lymphocytes with villous projections (Figure 1). Histologically, the lymphoid marginal zone surrounding the follicular areas is expanded by neoplastic cells that have cytological and phenotypical features of marginal zone B cells.[15] As a consequence, clonal expansion of villous lymphocytes is supposed to originate from the marginal zone of the spleen although definitive conclusion has not been clearly demonstrated to date. From a clinical point of view, the disease displays an indolent evolution with splenomegaly increasing over years and with a gradual progression of circulative malignant B cells. Of the 9 IFN-treated patients, 7 achieved a complete hematological remission, defined by the absence of abnormal lymphocytosis and the resolution of the splenomegaly, after HCV RNA load became undetectable. The remaining 2 patients experienced a partial or a complete response after addition of ribavirin and the loss of detectable HCV RNA.

Conversely, none of 6 similarly treated HCV-negative SLVL patients responded to therapy thereby suggesting that the observed response rate was not due to the effect of interferon itself. Those results gave support to the hypothesis that HCV might trigger, to some extent, clonal expansion and oncogenic events at least in a subgroup of indolent lymphomas patients. We also reported in 2005 on a series of 18 patients with HCV-associated SLVL.[16] All patients had MC, the majority of whom having symptomatic MC (72%), a much higher proportion than HCV-infected patients without SLVL. Apart from this finding, HCV-positive SLVL did not differ from HCV-negative SLVL patients. All patients were treated with alpha-IFN with (10 patients) or without (8 patients) ribavirin. Four patients had received prior therapy for SLVL including splenectomy or chemotherapy. Six patients received associated therapy for symptomatic MC (steroids, cyclophosphamide or plasmapheresis). Complete hematological remission was observed in 78% of the patients, most of them having concomitant complete virological responses (i.e. disappearance of HCV RNA). Two patients with major virological responses (more than 2 log reduction in HCV RNA) also achieved complete hematological remission, whereas the 2 patients with minor virological responses (less than 2 log reduction in HCV RNA) only achieved partial hematological responses (i.e. reduced albeit persistent circulating villous lymphocytes and splenomegaly). Moreover, in one patient with a virological relapse, villous lymphocytosis reappeared, but re-initiation of antiviral therapy was associated with a second complete hematological remission following HCV RNA reduction. The mean time to treatment responses was approximately 4 months for both virological and hematological responses. Mean duration of antiviral treatment was 17 months. Responses were sustained, as the mean duration of hematological response was 62 months. Clinical manifestations of MC subsided in all patients after antiviral treatment. Interestingly, viral genotype did not seem to correlate with the response as 4 out of 7 patients presenting with HCV genotype 1, usually associated with poor responses, achieved a complete hematologic response. Of note, even for patients who exhibited a complete hematological remission, B-cell clone could still be detected in peripheral blood but clinical relapses did not occur if viremia remained negative.
Overall, these observations strongly supported a causal relationship between HCV replication and lymphomagenesis in SLVL. Another report confirmed those results showing that among 8 patients with MZL of MALT or splenic (with or without villous lymphocytes) subtypes, alpha-IFN and ribavirin yielded a 60% response rate, which was correlated to virologic response in most cases.[17] A more recently published study extended these results by showing efficacy of antiviral therapy in other histological subtypes of indolent NHL associated with HCV infection, including follicular lymphoma and splenic MZL.[18]
Altogether, response to antiviral therapy in HCV-related NHL in case of viral load decrease gave further insights into the pathogenesis of those disorders and strengthened the association presumed from epidemiological studies.
HCV-associated lymphoma: evidence from biological studies.
Although epidemiological studies and clinical data link NHL with HCV, underlying biological processes ultimately leading from infection to lymphoma are still poorly understood. Basically, relationships between infectious agents and lymphoproliferative disorders such as Helicobacter pylori and gastric MALT-lymphoma, Epstein-Barr virus (EBV) and lymphoproliferative disorders (LPD), human herpesvirus 8 (HHV8) and primary effusion lymphoma (PEL) or human T-lymphotropic virus 1 (HTLV-1) and adult T-cell leukemia/lymphoma have been extensively studied for the two last decades but no clear pathological model has emerged so far. Nevertheless, antigen-driven lymphoproliferation might be though of as being split into 2 distinct mechanisms. On one hand, direct lymphocyte transformation by a given agent such as lymphotropic transforming viruses (EBV, HHV8, or HTLV1) expressing viral oncogenes has been clearly demonstrated.[19-21] On the other hand, a more recently described model for an indirect transformation mechanism of lymphocytes ultimately leading to clonal expansion has emerged, among which Helicobacter Pylori-associated gastric MALT lymphoma might be the best characterized (Figure 2).[22]

As a matter of fact, growing evidence show that, when exposed to chronic antigenic stimulation, B cells accumulate genetic lesions through inherent genomic instability during activation-induced deaminase (AID)-mediated variable-determining-joining V(D)J class switch recombination (CSR) and/or somatic hypermutation (SHM).[23] Both of these reactions have been shown to produce double-strand DNA break intermediates that might be aberrantly resolved as chromosomal translocations. [24,25]
Several oncogenes have been described as targets for somatic hypermutation or translocations with immunoglobulin heavy-chains regions (IgH). In most cases, these anomalies alarm the DNA damage response system that either allows for DNA repair or eliminates the aberrant B-cell clones.[19] Occasionally, those repair systems fail and B-cell malignancies may arise.
As regards to hepatitis C virus, few experimental data support the hypothesis of a direct transformation mechanism accounting for HCV-associated lymphomagenesis. In in vitro studies, CD81 has been shown to be an entry receptor for HCV and could be involved in infection of B cells by the virus. [26] In vitro, infection of B-cell lines with HCV leads to somatic mutations of several oncogenes and tumor suppressor genes such as p53, beta-catenin and Bcl6. [27,28] The expression of the HCV core protein (C) and non-structural protein 3 (NS3) has been associated with the induction of nitric oxide synthase (NOS) which might be responsible for these mutations (Figure 3A). Similarly, frequent chromosomal polyploidy in peripheral blood mononuclear cells (PBMC) from HCV-positive patients was recently demonstrated, as well as in splenocytes from HCV core protein-expressing transgenic mice, suggesting that HCV infection may inhibit the mitotic checkpoint. [29] In vivo, as it lacks a reverse transcriptase, HCV requires the cellular machinery to efficiently produce negative strand from positive strand viral DNA. Therefore, identification of negative strand RNA sequences in cells is indicative of active virus replication. Using a specific and sensitive method for HCV minus strand RNA detection, Sansonno et al. demonstrated active HCV replication in PBMC in nearly half of HCV-positive MC patients whereas no sign of infection was found in HCV-infected individuals without MC. [30]
Notably, in 7 patients with B-NHL without MC, active HCV replication was not found. So far, direct infection of the malignant clone has been described in a single case of B-cell lymphoma associated with HCV. [31] Therefore, although it can infect B cells in vivo and despite an association between PBMC infection and MC, an etiological role of the virus in HCV-associated lymphoproliferative disorders by a direct transformation mechanism is poorly supported by those data as half of MC patients and virtually all NHL patients do not display active HCV replication in PBMC or in the malignant clone respectively.

On the contrary, many studies support the role of HCV as an indirect transformation agent by chronically stimulating B-cell immunologic response and ultimately leading to overt lymphoma in some cases. In this regard, data on the association between MC and NHL have been of great interest. Frequent polyclonal or monoclonal B-cell proliferation can be detected in the blood, bone marrow or liver biopsies of HCV patients and association with type II MC (characterized by a monoclonal IgM with anti-IgG – i.e. a rheumatoid factor - activity) has been clearly demonstrated. Hence, 50 to 90% of patients with MC harbor a positive HCV serology and conversely, nearly 40-50% of HCV patients exhibit circulating cryoprecipitating complexes. Along with data on the response of lymphoma to therapy, antiviral treatment has been associated with disappearance of MC and in many cases of B-cell clones. [32-35] About 8 to 10% of patients with cryoglubulinemia ultimately develop a frank lymphoproliferative disorder and recent data demonstrated a 35 times increased risk of lymphoma for cryoglobulinemia carriers compared with general population. Nevertheless, NHL does not always evolve out of MC in what can be thought of as a stepwise evolution and many issues still need to be further elucidated.
Data from V(D)J region analyses among patients with MC or frank NHL also brought new insights into the pathological process from HCV-infection to lymphoma. Restricted usage of VH1-69 and V3-20/15 regions have been demonstrated, for instance, among patients with MC and HCV-associated NHL thereby giving strong support to an antigenic selection driven process underlying lymphoma development in HCV-positive patients.[36,37] Sequencing of IgV regions also revealed that they constitute a target for SHM, suggestive of a maturation process under antigenic stimulation. [37] Furthermore, De Re et al. showed BCR from some HCV-related patients recognized both IgG-Fc and HCV-NS3 protein rising the possibility that rheumatoid factor might emerge from cross-reaction between a virus-associated epitope and IgG autoantigen. [38] Cross-reactivity between E2 directed antibodies and anti-IgG IgM RF in type II MC has also been demonstrated. [39] One may imagine that Ig-HCV immunocomplexes in chronically infected patients would amplify the phenomenon by stimulating cross-reactive RF producing clones. Other recent data support a major role for HCV envelope glycoprotein E2 in indirect transformation. Not only can it promote HCV cellular entry by binding to CD81 on B-cell surface but when E2 binds to B cells via CD81, this latter then associates with CD19 and CD21, forming a complex that lowers the activation threshold. [40] The synergy between CD81-CD19-CD21 complex signaling and BCR cross-activation by envelope glycoprotein E2 and/or NS3 is thought to promote B-cell proliferation, emergence of autoreactive clones, genomic instability and NHL development. To further support this hypothesis, direct infection of lymphocytes by HCV would not be necessary in vivo to induce somatic mutations of several oncogenes and tumor suppressor genes such as p53, beta-catenin and Bcl6 as recombinant HCV E2 binding to surface CD81 has also been shown to induce somatic hypermutation of the immunoglobulin gene locus (Figure 3B).[41] As the E2 glycoprotein is expressed on the virion surface, this mechanism of mutagenesis would not require direct infection of B cells by HCV.
Altogether, those biological data have led to the concept of a multi-step lymphomagenesis process in HCV-related B-cell clonal disorders by an indirect transformation mechanism.

Perspective: towards a multi-step lymphomagenesis model.
The finding that MC represents an independent risk factor for the development of NHL in HCV-infected patients,[42] ]as well as the finding that, among HCV-infected patients with NHL, antiviral treatment was more effective in those with associated MC16 lend support to a model in which HCV would induce a protracted stimulation of antigen-specific B-cell clones, leading to MC and in a subset of patients to overt lymphomas.
Besides chronic antigenic stimulation, cytokines and growth factors produced within the inflammatory context of chronic infection are now suspected to play a key role in B-cell transformation. As a matter of fact, BAFF (for B-cell activating factor of the TNF family) has been described as a critical survival factor for B cells, promoting their activation and maturation, mainly through the nonclassical NF-B pathway. [43-47] BAFF has also been shown to promote the survival of autoreactive B-cell clones in case of abnormal production thus triggering autoimmune diseases. [48] In HCV infection, BAFF deregulation has been demonstrated, consistent with a role of the virus as a trigger for BAFF upregulation possibly predisposing to B-cell proliferation and clonal expansion. [49,50] A role for BAFF in promoting B-cell survival in an autocrine regulation loop has also been suggested in B-cell lymphoproliferative disorders. Hence, MC HCV-positive patients with NHL displayed higher BAFF levels and more frequently upregulated BAFF levels than MC HCV-positive counterparts without NHL. [51] Studying other pro-inflammatory cytokines such as IL-17 might also be of interest as this cytokine has recently been described to act in synergy with BAFF in human B-cell survival, proliferation and differentiation into immunoglobulin-secreting cells. [46] Similarly to BAFF, higher IL-17 serum levels were also demonstrated in patients suffering from systemic lupus erythematosus compared to healthy donors46 and a role of IL-17-producing T helper cells in other autoimmune disorders such as rheumatoid arthritis, psoriasis or multiple sclerosis has been suggested. [52] A recent study demonstrated that PBMC from HCV Ag-positive patients secreted IL-17 in response to stimulation with the HCV nonstructural protein 4 (NS4).53 However, NS4 also induced TGF- and IL-10 expression at high levels by monocytes from HCV-infected patients and those cytokines were shown to significantly suppressed NS4 specific human Th17 cells. The balance between IL17 expected role in protective immunity to HCV and its demonstrated role in inducing autoimmunity has to be further studied in MC and NHL HCV-positive patients to bring new insights into the pathogenesis of these disorders.
Concerning oncogenic events leading to NHL development, cytogenetic information on HCV-associated lymphomas is scarce. Matteucci et al. recently studied genomic imbalances in low- and high-grade HCV-related lymphomas and found trisomy 3 or +3q in 4 out of 6 splenic MZL as typically reported in low-grade splenic non HCV-related MZL.54 As those 3q+ splenic MZL exhibited an indolent clinical course and regressed after HCV eradication, and since trisomy 3 was also found in circulating B cells of one patient with MC without lymphoma, 3q gain is not supposed to be oncogenic ‘per se’. Conversely, 2q loss was associated with more aggressive B-cell lymphomas (4 out of 5 DLBCL) with no response to antiviral treatment. Of note, 2 of these DLBCL were derived from low-grade underlying lymphoma. Regarding IgH/BCL2 translocations, unexpected results in HCV-related NHL have been published so far. Hence, whereas the presence of IgH/BCL2 clones is frequent in HCV-infected patients, especially when associated to MC, the translocation was not shown to be any more frequent among HCV-infected NHL patients than among HCV-negative NHL patients. [55-57] This finding suggests that IgH/BCL2, which is present at a low frequency in healthy subjects, [58] may be amplified by a bystander effect due to a non specific stimulation of the immune system rather than being an additional oncogenic hit that leads to malignant transformation.
Response to treatment from our cohort of patients illustrated the hypothesis of additional oncogenic events driving oligoclonal proliferation in MC to monoclonal expansion in lymphoma. Accordingly, despite the efficacy of antiviral treatment in a majority of patients, leading to complete virological and hematological responses including the disappearance of detectable MC, the B-cell clones were still detectable in all patients.[16] Those results contrasts with previous findings in HCV-associated patients with MC [32,59] where B-cell clones were not detectable anymore in case of complete virological response. This may reflect differences in oncogenic potential between HCV-driven MC-producing clones and SLVL malignant B cells. In the latter, B-cell clones are therefore likely to have accumulated unknown additional oncogenic events, inducing a survival benefit for this expanded clonal population that remains however still dependent upon antigenic stimulation as demonstrated by response to antiviral treatment.
Aside from SLVL and other low-grade B-cell NHL, HCV is epidemiologically associated with DLBCL. [3,4] We have recently shown that DLBCL in HCV-positive patients were more frequently transformed from low-grade B-cell lymphomas than DLBCL in HCV-negative patients. [60] The finding that splenic involvement was more frequent in HCV-positive cases also supports a possible transformation from an underlying low-grade B-cell NHL. Furthermore, some differences exist in the clinical outcome of these transformed DLBCL. Surprisingly, patients in the HCV-positive group exhibited a longer event-free survival although the overall survival was significantly shorter in HCV positive patients. [60] These findings suggest a difference in the pathophysiological mechanisms underlying lymphomagenesis in HCV-associated DLBCL, and, by extension, a causal role for HCV in this lymphoma subtype. Therefore, DLBCL might be seen as the ultimate stage of lymphomagenesis in HCV-positive patients, ranging from indolent lymphoma responsive to therapy like SLVL to aggressive disease independent of antigenic stimulation like DLBCL.

Conclusions
Among
the many extrahepatic manifestations of HCV, the interactions of the
virus with B cells and their subsequent diseases are major consequences
of chronic HCV infection. In some cases, the restricted B-cell response
to HCV might undergo an oncogenic event giving survival advantage to a
subclonal population, which may ultimately lead to a frank
lymphoproliferative disorder. Evidence indicates a potential infection
of the B-cell compartment in HCV-positive patients but it is likely an
indirect transformation process that accounts for HCV-associated
lymphoproliferative disorders.
HCV associated MC, low-grade B-cell lymphomas and particularly SLVL and a subset of large B-cell lymphomas can fit in a continuum whereby chronic antigenic stimulation by persistent viral replication leads to progressive and antigen-driven B-cell transformation. These different stages could correspond to a step-by-step model of B-cell lymphomagenesis, ultimately leading to complete transformation and loss of antigen-dependence as seen in DLBCL. MC could thus be viewed as a marker of antigen-dependence of the lymphoproliferation. Several lines of evidence strongly suggest that antiviral therapy should be considered as first-line therapy in HCV-associated lymphomas, especially in the presence of MC. Antiviral therapy could also potentially benefit HCV patients with DLBCL by reducing liver injury inflicted by persistent HCV replication, and possibly by reducing the role of HCV as an oncogenic promoter.
Because of its clinical implications, HCV related low grade lymphoma should be classified as a special entity as it has been proposed in the World Health Organization lymphoma classification for T-cell lymphoproliferation related to HTLV-1.
Acknowledgments
Authors are indebted to Veronique Bachy for her assistance in reviewing the manuscript. This work is supported by ANRS Grants.
HCV associated MC, low-grade B-cell lymphomas and particularly SLVL and a subset of large B-cell lymphomas can fit in a continuum whereby chronic antigenic stimulation by persistent viral replication leads to progressive and antigen-driven B-cell transformation. These different stages could correspond to a step-by-step model of B-cell lymphomagenesis, ultimately leading to complete transformation and loss of antigen-dependence as seen in DLBCL. MC could thus be viewed as a marker of antigen-dependence of the lymphoproliferation. Several lines of evidence strongly suggest that antiviral therapy should be considered as first-line therapy in HCV-associated lymphomas, especially in the presence of MC. Antiviral therapy could also potentially benefit HCV patients with DLBCL by reducing liver injury inflicted by persistent HCV replication, and possibly by reducing the role of HCV as an oncogenic promoter.
Because of its clinical implications, HCV related low grade lymphoma should be classified as a special entity as it has been proposed in the World Health Organization lymphoma classification for T-cell lymphoproliferation related to HTLV-1.
Acknowledgments
Authors are indebted to Veronique Bachy for her assistance in reviewing the manuscript. This work is supported by ANRS Grants.
References
- Ferri C, Caracciolo F, Zignego AL, La
Civita L, Monti M, Longombardo G, Lombardini F, Greco F, Capochiani E,
Mazzoni A, et al. Hepatitis C virus infection in patients with
non-Hodgkin's lymphoma. Br J Haematol. 1994 Oct;88(2):392-4.

- Luppi M, Grazia Ferrari M, Bonaccorsi G,
Longo G, Narni F, Barozzi P, Marasca R, Mussini C, Torelli G. Hepatitis
C virus infection in subsets of neoplastic lymphoproliferations not
associated with cryoglobulinemia. Leukemia. 1996 Feb;10(2):351-5.

- Talamini R, Montella M, Crovatto M, et al.
Non-Hodgkin's lymphoma and hepatitis C virus: a case-control study from
northern and southern Italy. Int J Cancer. 2004;110:380-385.

- Zuckerman E, Zuckerman T, Levine AM, et al.
Hepatitis C virus infection in patients with B-cell non-Hodgkin
lymphoma. Ann Intern Med. 1997;127:423-428.

- Collier JD, Zanke B, Moore M, et al. No
association between hepatitis C and B-cell lymphoma. Hepatology.
1999;29:1259-1261.

- McColl MD, Singer IO, Tait RC, McNeil IR,
Cumming RL, Hogg RB. The role of hepatitis C virus in the aetiology of
non-Hodgkins lymphoma--a regional association? Leuk Lymphoma.
1997;26:127-130.

- Giordano TP, Henderson L, Landgren O, et
al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor
diseases in US veterans with hepatitis C virus. Jama.
2007;297:2010-2017.

- Nieters A, Kallinowski B, Brennan P, et al.
Hepatitis C and risk of lymphoma: results of the European multicenter
case-control study EPILYMPH. Gastroenterology. 2006;131:1879-1886.

- Dal Maso L, Franceschi S. Hepatitis C virus
and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of
epidemiologic studies. Cancer Epidemiol Biomarkers Prev.
2006;15:2078-2085.

- de Sanjose S, Benavente Y, Vajdic CM, et
al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269
controls from the International Lymphoma Epidemiology Consortium. Clin
Gastroenterol Hepatol. 2008;6:451-458.

- Matsuo K, Kusano A, Sugumar A, Nakamura S,
Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk
of non-Hodgkin's lymphoma: a meta-analysis of epidemiological studies.
Cancer Sci. 2004;95:745-752.

- Ambrosetti A, Zanotti R, Pattaro C, et al.
Most cases of primary salivary mucosa-associated lymphoid tissue
lymphoma are associated either with Sjoegren syndrome or hepatitis C
virus infection. Br J Haematol. 2004;126:43-49.

- Arcaini L, Paulli M, Boveri E, et al.
Splenic and nodal marginal zone lymphomas are indolent disorders at
high hepatitis C virus seroprevalence with distinct presenting features
but similar morphologic and phenotypic profiles. Cancer.
2004;100:107-115.

- Hermine O, Lefrere F, Bronowicki JP, et
al. Regression of splenic lymphoma with villous lymphocytes after
treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89-94.

- Troussard X, Valensi F, Duchayne E, et al.
Splenic lymphoma with villous lymphocytes: clinical presentation,
biology and prognostic factors in a series of 100 patients. Groupe
Francais d'Hematologie Cellulaire (GFHC). Br J Haematol.
1996;93:731-736.

- Saadoun D, Suarez F, Lefrere F, et al.
Splenic lymphoma with villous lymphocytes, associated with type II
cryoglobulinemia and HCV infection: a new entity? Blood. 2005;105:74-76.

- Kelaidi C, Rollot F, Park S, et al.
Response to antiviral treatment in hepatitis C virus-associated
marginal zone lymphomas. Leukemia. 2004;18:1711-1716.

- Vallisa D, Bernuzzi P, Arcaini L, et al.
Role of anti-hepatitis C virus (HCV) treatment in HCV-related,
low-grade, B-cell, non-Hodgkin's lymphoma: a multicenter Italian
experience. J Clin Oncol. 2005;23:468-473.

- Okano M. Haematological associations of
Epstein-Barr virus infection. Baillieres Best Pract Res Clin Haematol.
2000;13:199-214.

- Bazarbachi A, Ghez D, Lepelletier Y, et
al. New therapeutic approaches for adult T-cell leukaemia. Lancet
Oncol. 2004;5:664-672.

- Boshoff C, Weiss R. AIDS-related
malignancies. Nat Rev Cancer. 2002;2:373-382.

- Suarez F, Lortholary O, Hermine O, Lecuit
M. Infection-associated lymphomas derived from marginal zone B cells: a
model of antigen-driven lymphoproliferation. Blood. 2006;107:3034-3044.

- Roschke V, Kopantzev E, Dertzbaugh M,
Rudikoff S. Chromosomal translocations deregulating c-myc are
associated with normal immune responses. Oncogene. 1997;14:3011-3016.

- Ramiro AR, Jankovic M, Eisenreich T, et
al. AID is required for c-myc/IgH chromosome translocations in vivo.
Cell. 2004;118:431-438.

- Ramiro AR, Jankovic M, Callen E, et al.
Role of genomic instability and p53 in AID-induced c-myc-Igh
translocations. Nature. 2006;440:105-109.

- Pileri P, Uematsu Y, Campagnoli S, et al.
Binding of hepatitis C virus to CD81. Science. 1998;282:938-941.

- Machida K, Cheng KT, Sung VM, Lee KJ,
Levine AM, Lai MM. Hepatitis C virus infection activates the
immunologic (type II) isoform of nitric oxide synthase and thereby
enhances DNA damage and mutations of cellular genes. J Virol.
2004;78:8835-8843.

- Machida K, Cheng KT, Sung VM, et al.
Hepatitis C virus induces a mutator phenotype: enhanced mutations of
immunoglobulin and protooncogenes. Proc Natl Acad Sci U S A.
2004;101:4262-4267.

- Machida K, Liu JC, McNamara G, Levine A,
Duan L, Lai MM. Hepatitis C virus causes uncoupling of mitotic
checkpoint and chromosomal polyploidy through the Rb pathway. J Virol.
2009;83:12590-12600.

- Sansonno D, Tucci FA, Lauletta G, et al.
Hepatitis C virus productive infection in mononuclear cells from
patients with cryoglobulinaemia. Clin Exp Immunol. 2007;147:241-248.

- Levine AM, Shimodaira S, Lai MM. Treatment
of HCV-related mantle-cell lymphoma with ribavirin and pegylated
interferon Alfa. N Engl J Med. 2003;349:2078-2079.

- Mazzaro C, Franzin F, Tulissi P, et al.
Regression of monoclonal B-cell expansion in patients affected by mixed
cryoglobulinemia responsive to alpha-interferon therapy. Cancer.
1996;77:2604-2613.

- Casato M, Lagana B, Antonelli G, Dianzani
F, Bonomo L. Long-term results of therapy with interferon-alpha for
type II essential mixed cryoglobulinemia. Blood. 1991;78:3142-3147.

- Saadoun D, Cacoub P. Treatment with
Peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated
mixed cryoglobulinemia. J Hepatol. 2005;43:737; author reply 738.

- Saadoun D, Resche-Rigon M, Thibault V,
Piette JC, Cacoub P. Antiviral therapy for hepatitis C
virus--associated mixed cryoglobulinemia vasculitis: a long-term
followup study. Arthritis Rheum. 2006;54:3696-3706.

- Marasca R, Vaccari P, Luppi M, et al.
Immunoglobulin gene mutations and frequent use of VH1-69 and VH4-34
segments in hepatitis C virus-positive and hepatitis C virus-negative
nodal marginal zone B-cell lymphoma. Am J Pathol. 2001;159:253-261.

- Ivanovski M, Silvestri F, Pozzato G, et
al. Somatic hypermutation, clonal diversity, and preferential
expression of the VH 51p1/VL kv325 immunoglobulin gene combination in
hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433-2442.

- De Re V, Sansonno D, Simula MP, et al.
HCV-NS3 and IgG-Fc crossreactive IgM in patients with type II mixed
cryoglobulinemia and B-cell clonal proliferations. Leukemia.
2006;20:1145-1154.

- Quinn ER, Chan CH, Hadlock KG, Foung SK,
Flint M, Levy S. The B-cell receptor of a hepatitis C virus
(HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope
protein, implicating HCV in lymphomagenesis. Blood. 2001;98:3745-3749.

- Flint M, McKeating JA. The role of the
hepatitis C virus glycoproteins in infection. Rev Med Virol.
2000;10:101-117.

- Machida K, Cheng KT, Pavio N, Sung VM, Lai
MM. Hepatitis C virus E2-CD81 interaction induces hypermutation of the
immunoglobulin gene in B cells. J Virol. 2005;79:8079-8089.

- Monti G, Pioltelli P, Saccardo F, et al.
Incidence and characteristics of non-Hodgkin lymphomas in a multicenter
case file of patients with hepatitis C virus-related symptomatic mixed
cryoglobulinemias. Arch Intern Med. 2005;165:101-105.

- Batten M, Groom J, Cachero TG, et al. BAFF
mediates survival of peripheral immature B lymphocytes. J Exp Med.
2000;192:1453-1466.

- Do RK, Hatada E, Lee H, Tourigny MR,
Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B
lymphocyte stimulator enhancement of humoral immune response. J Exp
Med. 2000;192:953-964.

- Litinskiy MB, Nardelli B, Hilbert DM, et
al. DCs induce CD40-independent immunoglobulin class switching through
BLyS and APRIL. Nat Immunol. 2002;3:822-829.

- Doreau A, Belot A, Bastid J, et al.
Interleukin 17 acts in synergy with B cell-activating factor to
influence B cell biology and the pathophysiology of systemic lupus
erythematosus. Nat Immunol. 2009;10:778-785.

- Schneider P, MacKay F, Steiner V, et al.
BAFF, a novel ligand of the tumor necrosis factor family, stimulates B
cell growth. J Exp Med. 1999;189:1747-1756.

- Mackay F, Woodcock SA, Lawton P, et al.
Mice transgenic for BAFF develop lymphocytic disorders along with
autoimmune manifestations. J Exp Med. 1999;190:1697-1710.

- Fabris M, Quartuccio L, Sacco S, et al.
B-Lymphocyte stimulator (BLyS) up-regulation in mixed cryoglobulinaemia
syndrome and hepatitis-C virus infection. Rheumatology (Oxford).
2007;46:37-43.

- Sene D, Limal N, Ghillani-Dalbin P,
Saadoun D, Piette JC, Cacoub P. Hepatitis C virus-associated B-cell
proliferation--the role of serum B lymphocyte stimulator (BLyS/BAFF).
Rheumatology (Oxford). 2007;46:65-69.

- Mackay F, Tangye SG. The role of the
BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin
Pharmacol. 2004;4:347-354.

- Pene J, Chevalier S, Preisser L, et al.
Chronically inflamed human tissues are infiltrated by highly
differentiated Th17 lymphocytes. J Immunol. 2008;180:7423-7430.

- Rowan AG, Fletcher JM, Ryan EJ, et al.
Hepatitis C virus-specific Th17 cells are suppressed by virus-induced
TGF-beta. J Immunol. 2008;181:4485-4494.

- Matteucci C, Bracci M, Barba G, et al.
Different genomic imbalances in low- and high-grade HCV-related
lymphomas. Leukemia. 2008;22:219-222.

- Libra M, De Re V, De Vita S, et al. Low
frequency of bcl-2 rearrangement in HCV-associated non-Hodgkin's
lymphoma tissue. Leukemia. 2003;17:1433-1436.

- Zuckerman E, Zuckerman T, Sahar D, et al.
bcl-2 and immunoglobulin gene rearrangement in patients with hepatitis
C virus infection. Br J Haematol. 2001;112:364-369.

- Zignego AL, Ferri C, Giannelli F, et al.
Prevalence of bcl-2 rearrangement in patients with hepatitis C
virus-related mixed cryoglobulinemia with or without B-cell lymphomas.
Ann Intern Med. 2002;137:571-580.

- Limpens J, Stad R, Vos C, et al.
Lymphoma-associated translocation t(14;18) in blood B cells of normal
individuals. Blood. 1995;85:2528-2536.

- Zuckerman E, Zuckerman T, Sahar D, et al.
The effect of antiviral therapy on t(14;18) translocation and
immunoglobulin gene rearrangement in patients with chronic hepatitis C
virus infection. Blood. 2001;97:1555-1559.

- Besson C, Canioni D, Lepage E, et al.
Characteristics and outcome of diffuse large B-cell lymphoma in
hepatitis C virus-positive patients in LNH 93 and LNH 98 Groupe d'Etude
des Lymphomes de l'Adulte programs. J Clin Oncol. 2006;24:953-960.
