Toll-like Receptors in Chronic Lymphocytic Leukemia
Marta Muzio1, Eleonora Fonte1,2 and Federico Caligaris-Cappio1,3,4
1 Division of Molecular Oncology, Ospedale San Raffaele, Milano, Italy
2 UniversitÓ degli Studi di Pavia, Pavia, Italy
3 UniversitÓ Vita-Salute San Raffaele, Milano, Italy
4 Department of OncoHematology, Ospedale San Raffaele, Milano, Italy
2 UniversitÓ degli Studi di Pavia, Pavia, Italy
3 UniversitÓ Vita-Salute San Raffaele, Milano, Italy
4 Department of OncoHematology, Ospedale San Raffaele, Milano, Italy
Correspondence
to: Marta Muzio, Ospedale San Raffaele, Via Olgettina 58, 20132, Milano, Italy; E-mail: muzio.marta@hsr.it
Published: August 9, 2012
Received: June 28, 2012
Accepted: July 17, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012055, DOI 10.4084/MJHID.2012.055
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Toll-like
receptors belong to the pattern recognition receptors family present on
a variety of immune cells including normal and malignant B-cells. They
act as immediate molecular sentinels of innate immunity but also act as
a molecular bridge between the innate and the adaptive immune response;
distinct Toll-like receptors are able to bind specific pattern
molecules of bacteria, viruses and autoantigens. In this review we will
briefly introduce the Toll-like receptor family and their expression
pattern, signaling and function in the B cell context; following we
will summarize the published data on TLR in chronic lymphocytic
leukemia, and we will discuss their emerging role in the modulation of
leukemia pathobiology.
Introduction
Inflammation primarily defends the host organism against infections and is a self limiting process; however, its deregulation leads to chronic inflammatory processes that may favor the development of cancer.[1] Toll-like receptors (TLR) are well known for their key role regulating innate immunity; emerging evidence support their key regulatory role also in tumor biology. Among other microenvironmental elements, TLR may also play a role in hematologic malignancies, especially in chronic lymphoid malignancies.[2,3] First, we will briefly introduce the role of TLR in normal immune cells. Next we will describe available data on the expression and function of TLR in malignant B lymphocytes. On these bases we will discuss the pathobiology of TLR in Chronic Lymphocytic Leukemia (CLL).
Toll-like Receptors (TLR)
Innate immune cells express various pattern-recognition receptors (PRR) which recognize common signatures of molecules that are important components of bacteria and viruses and are called pathogen-associated molecular patterns (PAMP).[4] The response of the innate immune system plays a central role not only in eliminating infectious agents but also in developing pathogen-specific adaptive immunity mediated by B and T cells. Toll-like receptors (TLR) are PRR expressed by a variety of leukocytes as well as by non-immune cells present in particular sites of barrier function such as intestinal or airway epithelia. However, it has been demonstrated that they are activated not only by exogenous PAMP but also by endogenous ligands, so called “danger signals”. These danger-associated molecular patterns (DAMPs) are host-derived TLR ligands[5,6] (see Figure 1 for a schematic representation of TLR and ligands).
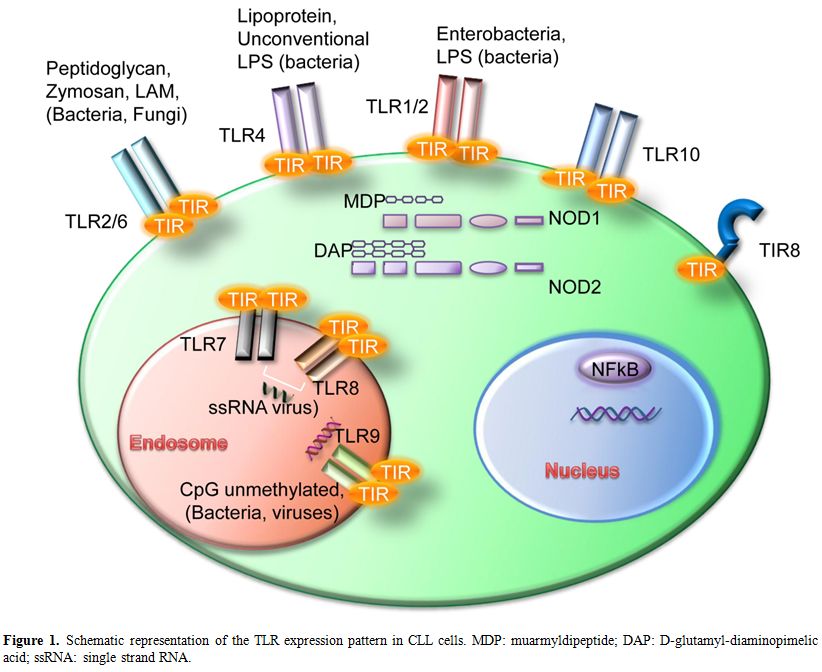
Figure 1. Schematic representation of the TLR expression pattern in CLL cells. MDP: muarmyldipeptide; DAP: D-glutamyl-diaminopimelic acid; ssRNA: single strand RNA.
TLR are grouped into the same family based on their sequence similarity and structural features; there are ten TLR in humans and twelve in mice where TLR10 pseudogene does not translate into a functional protein.[4] They are expressed within distinct cellular compartments: TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are present on the cell surface whereas TLR3, TLR7, TLR8 and TLR9 are localized into intracellular vesicles such as endosomes, lysosomes and ER. Intracellular TLR are transported into the vesicles via the transmembrane protein UNC93B1 which is localized in the ER of the cells.[7] Each TLR can sense specific PAMPs; in details, TLR1 can form heterodimers with TLR2 and bind tri-acetylated lipopeptides, which are coupled with peptidoglycan layer of bacteria. Another well characterized heterodimer is TLR2/TLR6 which recognizes di-acetylated lipopeptides and bacterial cell wall components such as lipoteichoic acid or peptidoglycans, mycobacterial cell wall components like lipomannans and the yeast cell wall zymosan. TLR3 binds to double stranded RNA from viral sources while TLR4 responds to lipopolysaccharide (LPS) mostly from Gram-negative bacteria; the only known ligand for TLR5 is flagellin. The intracellular TLR7 and TLR8 share the same ligand, the single stranded RNA from viruses, while TLR9 binds to DNA-containing unmethylated CpG motifs which are commonly found in bacterial DNA.[4,8]
Distinct TLR can also sense different DAMPs.[5] TLR2 and TLR4 are the best characterized in this respect; they have several endogenous ligands such as heat shock proteins including HSP70 and Gp96),[9,10] HMGB1,[11] extracellular matrix (ECM) molecules[12] and their fragments.[13] TLR1/TLR2 were shown to be activated by β-defensin-3;[14] TLR3 by self-nucleic acids.[15] TLR7 and TLR9 can also participate to autoantigen response together with the B cell receptor by recognizing RNA-associated autoantigens[16] and chromatin-IgG complexes[17] respectively.
The last discovered TLR10 is an orphan receptors; however, sequence analysis as well as chimeric receptors experiments suggested that human TLR10 and TLR1 share common mechanisms of innate immune sensing but not signaling.[18,19]
CD180 (also named RP105 or Ly64) is homologous to TLR4 but lacks the intracellular TLR-like domain.[20] RP105 is associated with MD-1 which is indispensable for its cell-surface expression.[20] RP105-deficient B cells are defective in response to TLR2 and TLR4 ligands.[21] However, it was also demonstrated that RP105 may prevent the interaction of LPS with TLR4 in macrophages and dendritic cells[22] suggesting different roles of RP105 within different cell types. Other studies demonstrated that CD180 has a role in B-cell activation (by up-regulating CD86) and proliferation.[23] Furthermore CD180 stimulation induces B cell proliferation and differentiation, causing increases in IgG, and integrates MyD88-dependent TLR signals to modulate proliferation, production of cytokines, and differentiation.[24]
NOD-like Receptors
Another family of PRRs, the NOD-like receptors family (NLRs), is composed of several cytosolic molecules including the first discovered NOD1 and NOD2 (also known as CARD4 and CARD15). NLRs show a variable modular structure and contain different domains: a CARD or pyrin domain at the N-terminal portion; a central nucleotide binding and oligomerization domain; Leucine Rich Repeats (LRR) at the C-terminal domain. While NODs contain a CARD domain, NALPs contain a pyrin domain NAIP being an exception as it contains different BIR domains.[25] NOD1 and NOD2 are well characterized intracellular molecules that recognize bacterial peptidoglycans. Their expression is wide and includes B-cells where a synergism between NOD-like receptors and Toll-like receptors was observed.[26] NOD1 binds iE-DAP dipeptide which is found in peptidoglycan of most Gram-negative bacteria while NOD2 responds to MDP (muarmyldipeptide) which is the minimal bioactive peptidoglycan motif common to all bacteria. NOD stimulation leads to inflammatory genes transcription through NF-kB and MAPK activation.[27,28]
TLR Signaling
TLR are type I integral membrane glycoproteins and have a modular structure. The extracellular N-Terminal domain consists of approximately 16-28 Leucin-Rich Repeats (LRRs) which mediate ligand binding specificity. The cytoplasmic domain is highly conserved and termed Toll-IL-1R (TIR) domain according to the high similarity shared with the Drosophila Toll and the mammalian IL-1R protein.[29] This domain acts as binding site for downstream adaptor molecules that mediate the signal to others proteins (see Figure 2 for a schematic representation of TLR signaling pathways). Two main adaptors are recruited to the TIR domain; MyD88, which is recruited to the TLR-TIR domain together with Mal (MyD88 adaptor-like) also called TIRAP (TIR-domain-containing adaptor protein), and TRIF (Toll-receptor-associated activator of interferon).[30] Throughout the first pathway, the induction of specific gene expression is mediated by the NF-κB (nuclear factor k B) transcription factor, AP-1 (activating protein 1) or IRF1, 5 and 7 (interferon-response factor); on the other hand, the second pathway is regulated by IRF3 and NF-κB.[31] Only TLR4 can trigger downstream signals through both pathways; all the others, except for TLR3, act via MyD88. The death domain of MyD88 recruits IRAK family members to the TLR signaling complex, activate them and transmit the signal to TRAF6 which allows the phosphorylation of IKK. The pathway flows with the activation of NF-κB and the recruitment of TAK1 that induces the MAPK pathways. These signaling cascades eventually induce the transcription of inflammatory cytokines, type I or II interferons and chemokines.[30]
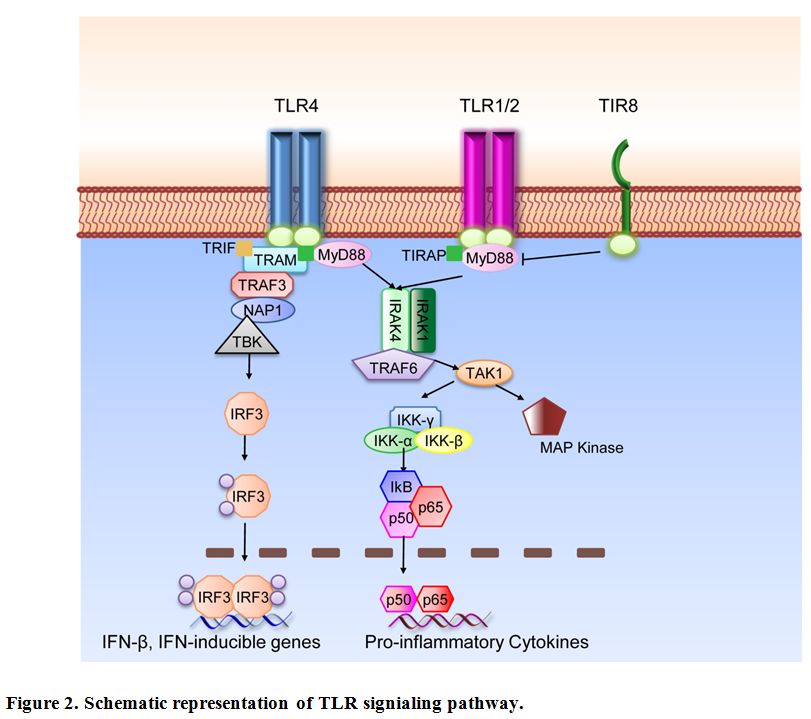
Figure 2. Schematic representation of TLR signaling pathway.
Fine tuning of TLR and IL-1R family (ILR) is regulated by the inhibitory receptor TIR8 (Toll IL-1R 8), also known as Single Ig IL-1 related receptor (SIGIRR); TIR8 acts as a decoy target for TLR and ILR (IL-1R, IL-18R, IL-33R/ST2) signaling molecules.[32,33] SIGIRR inhibits interleukin-1 receptor- and TLR-mediated signaling through different mechanisms. Both extracellular domain and the intracellular portion of TIR8 are involved; the intracellular TIR domain of TIR8 sequesters MyD88 and IRAK-1, while the extracellular domain interferes with heterodimerization of IL-1R1 and IL-1AcP.[34,35]
Other signaling molecules such as IRAK-M and SOCS-1 negatively regulate IRAK. IRAK-M expression has been shown to be restricted to monocytes and macrophages; it blocks the dissociation of IRAK1/4 and the following activation of TRAF6.[36] SOCS-1 knock-out mice show over-expression of different cytokines specifically after treatment with LPS.[37]
TLR in Normal B Lymphocytes
The TLR expression pattern is quite specific and unique for each cell type;[38] in normal human B-cells TLR1, TLR2, TLR6, TLR7, TLR8, TLR9 and TLR10 are prevalently expressed; in contrast TLR repertoire in mouse B cells includes high levels of TLR4 and no TLR10 protein.[39-41] In human cells, TLR expression is rapidly up-regulated by BCR triggering of na´ve B cells suggesting a synergism between BCR and TLR leading to B-cell proliferation and differentiation.[39,40] TLR expression is also specific for each B-cell subset.[42] B cells of the inflamed tonsils show abundant TLR expression.[43]
In general terms TLR stimulation can trigger activation, proliferation and differentiation of B cells; nevertheless, TLR ligation with specific ligands can induce specific responses in the B cell subsets analyzed either in mouse models or human system (see specific reviews on this topic).[44-46] In human cells It was shown that maintenance of serological memory can be achieved by polyclonal activation, and memory B cells can be activated by CpG and cytokines without need for BCR triggering.[47] However, it has been proposed a model in which the costimulation of three different signals derived from BCR, CD40 and TLR is required to induce full activation, proliferation and differentiation of naive B-cells.[48] Recent studies also showed that a specific culture system using CpG together with sequential steps for T-cell-independent activation of naive human B cells can also induce plasma-cell differentiation.[49]
Expression Pattern of TLR in CLL Cells
Already before the discovery of TLR9 as the cognate receptor for unmethylated-CpG-oligonucleotides,[50] these immunostimulatory agents have been used to stimulate leukemic CLL cells for immunotherapeutic strategies.[51] Therefore, it was assumed, and soon confirmed, that CLL cells express functional TLR9, similar to normal B lymphocytes.[52,53] However, only recently the full expression pattern of TLR was assessed in CLL samples by different groups.[54-56]
The expression of TLR in CLL is quite heterogeneous between patients but most cases express TLR1, TLR2, TLR6, TLR10 on the cell surface, and TLR7, TLR8, TLR9 within endosomes[55] thus resembling normal mature B lymphocytes.[39,40,43] Recently, we have studied the full expression profile of mRNA for TLR and signaling molecules in a large group of chronic lymphocytic leukemia (CLL) patients to search for potential differences in specific subsets of patients. At cohort levels, CLL cells show high expression of TLR7, intermediate expression of TLR1, TLR6, TLR10 and low expression of TLR2, TLR4, TLR8 and TLR9. As for TLR4 and TLR8 a significant variation was observed among different samples.[57] Comparison in subgroups of cases carrying mutated or unmutated IGHV genes revealed few significant differences in TLR signaling molecules; up-regulation of TLR8 mRNA and down-regulation of TLR4 were observed in the unmutated subgroup.[57]
The TLR related molecule RP105, also called CD180 or Ly64 was shown to be variably expressed by leukemic cells.[58,59] Significantly higher levels of CD180 were expressed by CLL cells with mutated IGVH genes as compared to unmutated CLL.[58]
Further, expression of NOD1 (CARD4) and NOD2 (CARD15) mRNA was studied in a group of CLL patients in comparison with MEC1 cell line and normal B-lymphocytes. Both NOD molecules, including two different isoforms of NOD1, were expressed in all B-cell types analyzed.[55]
We have previously shown that both normal and leukemic B cells express detectable levels of TIR8 mRNA;[55] however, by PCR array analysis, malignant B cells appear to have very low levels of mRNA.[60] Since mRNA and protein levels of TIR8 have been shown to be differentially regulated,[61] it will be important to analyze TIR8 protein expression on the cell surface of normal and leukemic B lymphocytes.
Finally, our group recently analyzed the mRNA expression pattern of the molecules regulating Toll-like receptor signaling pathway in a large cohort of CLL patients; different members of the NFKB, JNK/p38, NF/IL6 and IRF pathways are intermediately-to-highly expressed, while inhibitors of TLR activity are generally low-to-undetectable, indicating that the TLR signaling framework is competent in CLL cells.[57]
To note, all these studies analyzed leukemic cells isolated from the peripheral blood of patients; it will be of interest to compare TLR expression pattern within lymphoid tissues where one would expect TLR ligation to occur. In fact, it was reported that, in addition to BCR, TLR signaling pathways could contribute to NF-kB activation specifically in the lymph node microenvironment.[62]
Gene Polymorphisms and Mutations of the TLR Pathway in CLL
Functional polymorphisms in TLR genes were analyzed by different groups to determine if they influenced lymphoma susceptibility. TLR6 variants were found to be important in different B cell lymphomas including CLL;[63] the TLR2-16933T>A variant was associated with a decreased risk of CLL;[64] two TLR10-TLR1-TLR6 variants in moderate linkage disequilibrium were significantly associated with Non Hodgkin Lymphoma including CLL cases.[65]
More recently, MyD88 oncogenic mutations were described in different B cell malignancies; in details, recurrent single point mutations were found in 29% of ABC type Diffuse Large B Cell Lymphoma cases,[66] 13% of Splenic Marginal Zone Lymphoma cases,[67] 36% of Primary Central Nervous System Lymphoma cases,[68] and 3-10% of CLL cases.[69-71] Since MyD88 is a signaling molecule specific for TLR and IL-1R family, this mutation may affect specific signaling pathways in leukemic cells which may be considered as novel therapeutic targets. Indeed, inhibition of the MyD88 downstream kinases IRAK1/4 with small molecule inhibitors was shown to effectively block TLR signaling cascade in vitro and to induce cell death of lymphoma cells bearing specific MyD88 mutations.[66]
TLR and Activation of Leukemic Cells
Several reports (mainly focused onto TLR9) showed that CpG immunostimulatory oligonucleotides shape an immunogenic phenotype in CLL cells.[51,72] Several surface antigens have been investigated in CLL cells before and after CpG addition to the cell culture; among these CD25, CD40, CD54, CD80, CD86 CD95, MHCI, MHCII;[51,73] the expression of these molecules contribute to increase of the immunogenicity of the leukemic cells that are per se weakly immunogenic and may escape the control exerted by tumor-reactive T cells.[74-76] Stimulation of CLL cells with different agonists of TLR also increases the number of CD25, CD80 and CD86 positive cells, and this was demonstrated for PAM3CSK4 (palmitoyl-3-cysteine-serine-lysine-4 binding to TLR1/2 heterodimer), MALP-2 (Mycoplasmal Macrophage-activating Lipopeptide-2 binding to TLR2/6 heterodimer) and MDP (Muramildipeptide binding to NOD2).[56,77] TLR7 stimulation is also able to increase the expression of costimulatory molecules on leukemic cells (CD25, CD80, CD86) and the production of inflammatory cytokines including TNF and IL-6.[78,79] Moreover, the TLR9 ligand CpG induces the production of TNFα, IL-10 and to a lesser extent of IL6.[51,56,73,80] Given the ability of CpG to induce CD25 expression, TLR9 ligands were also tested in vitro in combination with a specific anti-CD25 immunotoxin to treat CLL cells.[81] Both TLR7 and TLR9 agonists have been studied for immunotherapy approaches in preclinical models of CLL in vitro,[73,82,83] and are currently under clinical investigation[84-86] (see reference[87] for a recent review on this topic).
More recently, our group showed differences in the induction of co-stimulatory molecules and/or apoptosis in mutated vs. unmutated CLL. Different responses were also identified in subsets with stereotyped BCR.[79] The distinct patterns of TLR/NOD2 functional activity in cells from CLL subgroups defined by the molecular features of the BCR might prove relevant for elucidating the immune mechanisms underlying the natural history of CLL and for defining subgroups of patients who might benefit from treatment with specific TLR ligands.
TLR, Proliferation and Apoptosis of Leukemic Cells
Previous reports showed that CpG immunostimulatory oligonucleotides induce proliferation of CLL cells, either alone or in combination with IL-2.[51,72] However, it was also reported that TLR9 signaling by CpG-B oligonucleotides may induce an apoptotic pathway in CLL.[56,73] Further, several groups described a heterogeneous response to TLR9 stimulation in terms of proliferation and apoptosis within different groups of patients;[56,88] in details, it was reported that CpG induced apoptosis in mutated, and proliferation in unmutated patient samples.[53,89] It is relevant to note in this context that phosphorothioate oligonucleotides may induce apoptosis of CLL cells independently of their CpG motifs, indicating that the presence of a chemical backbone and nucleotide composition may contribute to the observed cell death.[90]
As for additional TLR, we previously showed that TLR1/2 and TLR2/6 heterodimers can activate and protect leukemic cells from spontaneous apoptosis in vitro.[55,79] Again, different groups showed heterogeneity among patients samples and apoptosis induction was reported in a proportion of cases;[56] nevertheless, it was recently shown that distinct innate immunity pathways can be activated in subgroups of CLL with distinct immunoglobulin receptors.[79]
Dissecting the Role of TLR in CLL Pathobiology
CLL patients are often associated with an increased frequency and severity of infections which is a characteristic feature of the disease.[91] In addition, autoimmune complications can occur in up to a quarter of CLL patients.[92] It was also suggested that common infections may play a role in CLL etiology;[93-95] this may be due to underlying immune disturbance in CLL patients, and/or to a direct effect of microbial antigens on the leukemic clone. Given all this, one could hypothesize that inflammation or autoimmunity mediated by distinct TLR may also play a role in regulating the development, progression and/or accumulation of CLL. Indeed, in mouse models of CLL the lack of the inhibitory receptor TIR8, which allows an unabated TLR-mediated stimulation, triggers leukemia progression in vivo.[96] However, these findings do not allow to understand whether TLR contribute (and to what extent) to early or late or both/any phase of disease progression.
Since TLR can improve immune response but may also be involved in modulating tumor cell proliferation and apoptosis, the possibility that TLR activity may shuttle between defense from and promotion of leukemic growth has to be taken into account. Future studies combining in vitro and in vivo approaches will help to identify the specific role of TLR within specific subsets of patients. Finally, kinetic studies will help elucidating the distinct role of distinct TLR in different phases of disease initiation, accumulation and/or progression.
Inflammation primarily defends the host organism against infections and is a self limiting process; however, its deregulation leads to chronic inflammatory processes that may favor the development of cancer.[1] Toll-like receptors (TLR) are well known for their key role regulating innate immunity; emerging evidence support their key regulatory role also in tumor biology. Among other microenvironmental elements, TLR may also play a role in hematologic malignancies, especially in chronic lymphoid malignancies.[2,3] First, we will briefly introduce the role of TLR in normal immune cells. Next we will describe available data on the expression and function of TLR in malignant B lymphocytes. On these bases we will discuss the pathobiology of TLR in Chronic Lymphocytic Leukemia (CLL).
Toll-like Receptors (TLR)
Innate immune cells express various pattern-recognition receptors (PRR) which recognize common signatures of molecules that are important components of bacteria and viruses and are called pathogen-associated molecular patterns (PAMP).[4] The response of the innate immune system plays a central role not only in eliminating infectious agents but also in developing pathogen-specific adaptive immunity mediated by B and T cells. Toll-like receptors (TLR) are PRR expressed by a variety of leukocytes as well as by non-immune cells present in particular sites of barrier function such as intestinal or airway epithelia. However, it has been demonstrated that they are activated not only by exogenous PAMP but also by endogenous ligands, so called “danger signals”. These danger-associated molecular patterns (DAMPs) are host-derived TLR ligands[5,6] (see Figure 1 for a schematic representation of TLR and ligands).

Figure 1. Schematic representation of the TLR expression pattern in CLL cells. MDP: muarmyldipeptide; DAP: D-glutamyl-diaminopimelic acid; ssRNA: single strand RNA.
TLR are grouped into the same family based on their sequence similarity and structural features; there are ten TLR in humans and twelve in mice where TLR10 pseudogene does not translate into a functional protein.[4] They are expressed within distinct cellular compartments: TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are present on the cell surface whereas TLR3, TLR7, TLR8 and TLR9 are localized into intracellular vesicles such as endosomes, lysosomes and ER. Intracellular TLR are transported into the vesicles via the transmembrane protein UNC93B1 which is localized in the ER of the cells.[7] Each TLR can sense specific PAMPs; in details, TLR1 can form heterodimers with TLR2 and bind tri-acetylated lipopeptides, which are coupled with peptidoglycan layer of bacteria. Another well characterized heterodimer is TLR2/TLR6 which recognizes di-acetylated lipopeptides and bacterial cell wall components such as lipoteichoic acid or peptidoglycans, mycobacterial cell wall components like lipomannans and the yeast cell wall zymosan. TLR3 binds to double stranded RNA from viral sources while TLR4 responds to lipopolysaccharide (LPS) mostly from Gram-negative bacteria; the only known ligand for TLR5 is flagellin. The intracellular TLR7 and TLR8 share the same ligand, the single stranded RNA from viruses, while TLR9 binds to DNA-containing unmethylated CpG motifs which are commonly found in bacterial DNA.[4,8]
Distinct TLR can also sense different DAMPs.[5] TLR2 and TLR4 are the best characterized in this respect; they have several endogenous ligands such as heat shock proteins including HSP70 and Gp96),[9,10] HMGB1,[11] extracellular matrix (ECM) molecules[12] and their fragments.[13] TLR1/TLR2 were shown to be activated by β-defensin-3;[14] TLR3 by self-nucleic acids.[15] TLR7 and TLR9 can also participate to autoantigen response together with the B cell receptor by recognizing RNA-associated autoantigens[16] and chromatin-IgG complexes[17] respectively.
The last discovered TLR10 is an orphan receptors; however, sequence analysis as well as chimeric receptors experiments suggested that human TLR10 and TLR1 share common mechanisms of innate immune sensing but not signaling.[18,19]
CD180 (also named RP105 or Ly64) is homologous to TLR4 but lacks the intracellular TLR-like domain.[20] RP105 is associated with MD-1 which is indispensable for its cell-surface expression.[20] RP105-deficient B cells are defective in response to TLR2 and TLR4 ligands.[21] However, it was also demonstrated that RP105 may prevent the interaction of LPS with TLR4 in macrophages and dendritic cells[22] suggesting different roles of RP105 within different cell types. Other studies demonstrated that CD180 has a role in B-cell activation (by up-regulating CD86) and proliferation.[23] Furthermore CD180 stimulation induces B cell proliferation and differentiation, causing increases in IgG, and integrates MyD88-dependent TLR signals to modulate proliferation, production of cytokines, and differentiation.[24]
NOD-like Receptors
Another family of PRRs, the NOD-like receptors family (NLRs), is composed of several cytosolic molecules including the first discovered NOD1 and NOD2 (also known as CARD4 and CARD15). NLRs show a variable modular structure and contain different domains: a CARD or pyrin domain at the N-terminal portion; a central nucleotide binding and oligomerization domain; Leucine Rich Repeats (LRR) at the C-terminal domain. While NODs contain a CARD domain, NALPs contain a pyrin domain NAIP being an exception as it contains different BIR domains.[25] NOD1 and NOD2 are well characterized intracellular molecules that recognize bacterial peptidoglycans. Their expression is wide and includes B-cells where a synergism between NOD-like receptors and Toll-like receptors was observed.[26] NOD1 binds iE-DAP dipeptide which is found in peptidoglycan of most Gram-negative bacteria while NOD2 responds to MDP (muarmyldipeptide) which is the minimal bioactive peptidoglycan motif common to all bacteria. NOD stimulation leads to inflammatory genes transcription through NF-kB and MAPK activation.[27,28]
TLR Signaling
TLR are type I integral membrane glycoproteins and have a modular structure. The extracellular N-Terminal domain consists of approximately 16-28 Leucin-Rich Repeats (LRRs) which mediate ligand binding specificity. The cytoplasmic domain is highly conserved and termed Toll-IL-1R (TIR) domain according to the high similarity shared with the Drosophila Toll and the mammalian IL-1R protein.[29] This domain acts as binding site for downstream adaptor molecules that mediate the signal to others proteins (see Figure 2 for a schematic representation of TLR signaling pathways). Two main adaptors are recruited to the TIR domain; MyD88, which is recruited to the TLR-TIR domain together with Mal (MyD88 adaptor-like) also called TIRAP (TIR-domain-containing adaptor protein), and TRIF (Toll-receptor-associated activator of interferon).[30] Throughout the first pathway, the induction of specific gene expression is mediated by the NF-κB (nuclear factor k B) transcription factor, AP-1 (activating protein 1) or IRF1, 5 and 7 (interferon-response factor); on the other hand, the second pathway is regulated by IRF3 and NF-κB.[31] Only TLR4 can trigger downstream signals through both pathways; all the others, except for TLR3, act via MyD88. The death domain of MyD88 recruits IRAK family members to the TLR signaling complex, activate them and transmit the signal to TRAF6 which allows the phosphorylation of IKK. The pathway flows with the activation of NF-κB and the recruitment of TAK1 that induces the MAPK pathways. These signaling cascades eventually induce the transcription of inflammatory cytokines, type I or II interferons and chemokines.[30]

Figure 2. Schematic representation of TLR signaling pathway.
Fine tuning of TLR and IL-1R family (ILR) is regulated by the inhibitory receptor TIR8 (Toll IL-1R 8), also known as Single Ig IL-1 related receptor (SIGIRR); TIR8 acts as a decoy target for TLR and ILR (IL-1R, IL-18R, IL-33R/ST2) signaling molecules.[32,33] SIGIRR inhibits interleukin-1 receptor- and TLR-mediated signaling through different mechanisms. Both extracellular domain and the intracellular portion of TIR8 are involved; the intracellular TIR domain of TIR8 sequesters MyD88 and IRAK-1, while the extracellular domain interferes with heterodimerization of IL-1R1 and IL-1AcP.[34,35]
Other signaling molecules such as IRAK-M and SOCS-1 negatively regulate IRAK. IRAK-M expression has been shown to be restricted to monocytes and macrophages; it blocks the dissociation of IRAK1/4 and the following activation of TRAF6.[36] SOCS-1 knock-out mice show over-expression of different cytokines specifically after treatment with LPS.[37]
TLR in Normal B Lymphocytes
The TLR expression pattern is quite specific and unique for each cell type;[38] in normal human B-cells TLR1, TLR2, TLR6, TLR7, TLR8, TLR9 and TLR10 are prevalently expressed; in contrast TLR repertoire in mouse B cells includes high levels of TLR4 and no TLR10 protein.[39-41] In human cells, TLR expression is rapidly up-regulated by BCR triggering of na´ve B cells suggesting a synergism between BCR and TLR leading to B-cell proliferation and differentiation.[39,40] TLR expression is also specific for each B-cell subset.[42] B cells of the inflamed tonsils show abundant TLR expression.[43]
In general terms TLR stimulation can trigger activation, proliferation and differentiation of B cells; nevertheless, TLR ligation with specific ligands can induce specific responses in the B cell subsets analyzed either in mouse models or human system (see specific reviews on this topic).[44-46] In human cells It was shown that maintenance of serological memory can be achieved by polyclonal activation, and memory B cells can be activated by CpG and cytokines without need for BCR triggering.[47] However, it has been proposed a model in which the costimulation of three different signals derived from BCR, CD40 and TLR is required to induce full activation, proliferation and differentiation of naive B-cells.[48] Recent studies also showed that a specific culture system using CpG together with sequential steps for T-cell-independent activation of naive human B cells can also induce plasma-cell differentiation.[49]
Expression Pattern of TLR in CLL Cells
Already before the discovery of TLR9 as the cognate receptor for unmethylated-CpG-oligonucleotides,[50] these immunostimulatory agents have been used to stimulate leukemic CLL cells for immunotherapeutic strategies.[51] Therefore, it was assumed, and soon confirmed, that CLL cells express functional TLR9, similar to normal B lymphocytes.[52,53] However, only recently the full expression pattern of TLR was assessed in CLL samples by different groups.[54-56]
The expression of TLR in CLL is quite heterogeneous between patients but most cases express TLR1, TLR2, TLR6, TLR10 on the cell surface, and TLR7, TLR8, TLR9 within endosomes[55] thus resembling normal mature B lymphocytes.[39,40,43] Recently, we have studied the full expression profile of mRNA for TLR and signaling molecules in a large group of chronic lymphocytic leukemia (CLL) patients to search for potential differences in specific subsets of patients. At cohort levels, CLL cells show high expression of TLR7, intermediate expression of TLR1, TLR6, TLR10 and low expression of TLR2, TLR4, TLR8 and TLR9. As for TLR4 and TLR8 a significant variation was observed among different samples.[57] Comparison in subgroups of cases carrying mutated or unmutated IGHV genes revealed few significant differences in TLR signaling molecules; up-regulation of TLR8 mRNA and down-regulation of TLR4 were observed in the unmutated subgroup.[57]
The TLR related molecule RP105, also called CD180 or Ly64 was shown to be variably expressed by leukemic cells.[58,59] Significantly higher levels of CD180 were expressed by CLL cells with mutated IGVH genes as compared to unmutated CLL.[58]
Further, expression of NOD1 (CARD4) and NOD2 (CARD15) mRNA was studied in a group of CLL patients in comparison with MEC1 cell line and normal B-lymphocytes. Both NOD molecules, including two different isoforms of NOD1, were expressed in all B-cell types analyzed.[55]
We have previously shown that both normal and leukemic B cells express detectable levels of TIR8 mRNA;[55] however, by PCR array analysis, malignant B cells appear to have very low levels of mRNA.[60] Since mRNA and protein levels of TIR8 have been shown to be differentially regulated,[61] it will be important to analyze TIR8 protein expression on the cell surface of normal and leukemic B lymphocytes.
Finally, our group recently analyzed the mRNA expression pattern of the molecules regulating Toll-like receptor signaling pathway in a large cohort of CLL patients; different members of the NFKB, JNK/p38, NF/IL6 and IRF pathways are intermediately-to-highly expressed, while inhibitors of TLR activity are generally low-to-undetectable, indicating that the TLR signaling framework is competent in CLL cells.[57]
To note, all these studies analyzed leukemic cells isolated from the peripheral blood of patients; it will be of interest to compare TLR expression pattern within lymphoid tissues where one would expect TLR ligation to occur. In fact, it was reported that, in addition to BCR, TLR signaling pathways could contribute to NF-kB activation specifically in the lymph node microenvironment.[62]
Gene Polymorphisms and Mutations of the TLR Pathway in CLL
Functional polymorphisms in TLR genes were analyzed by different groups to determine if they influenced lymphoma susceptibility. TLR6 variants were found to be important in different B cell lymphomas including CLL;[63] the TLR2-16933T>A variant was associated with a decreased risk of CLL;[64] two TLR10-TLR1-TLR6 variants in moderate linkage disequilibrium were significantly associated with Non Hodgkin Lymphoma including CLL cases.[65]
More recently, MyD88 oncogenic mutations were described in different B cell malignancies; in details, recurrent single point mutations were found in 29% of ABC type Diffuse Large B Cell Lymphoma cases,[66] 13% of Splenic Marginal Zone Lymphoma cases,[67] 36% of Primary Central Nervous System Lymphoma cases,[68] and 3-10% of CLL cases.[69-71] Since MyD88 is a signaling molecule specific for TLR and IL-1R family, this mutation may affect specific signaling pathways in leukemic cells which may be considered as novel therapeutic targets. Indeed, inhibition of the MyD88 downstream kinases IRAK1/4 with small molecule inhibitors was shown to effectively block TLR signaling cascade in vitro and to induce cell death of lymphoma cells bearing specific MyD88 mutations.[66]
TLR and Activation of Leukemic Cells
Several reports (mainly focused onto TLR9) showed that CpG immunostimulatory oligonucleotides shape an immunogenic phenotype in CLL cells.[51,72] Several surface antigens have been investigated in CLL cells before and after CpG addition to the cell culture; among these CD25, CD40, CD54, CD80, CD86 CD95, MHCI, MHCII;[51,73] the expression of these molecules contribute to increase of the immunogenicity of the leukemic cells that are per se weakly immunogenic and may escape the control exerted by tumor-reactive T cells.[74-76] Stimulation of CLL cells with different agonists of TLR also increases the number of CD25, CD80 and CD86 positive cells, and this was demonstrated for PAM3CSK4 (palmitoyl-3-cysteine-serine-lysine-4 binding to TLR1/2 heterodimer), MALP-2 (Mycoplasmal Macrophage-activating Lipopeptide-2 binding to TLR2/6 heterodimer) and MDP (Muramildipeptide binding to NOD2).[56,77] TLR7 stimulation is also able to increase the expression of costimulatory molecules on leukemic cells (CD25, CD80, CD86) and the production of inflammatory cytokines including TNF and IL-6.[78,79] Moreover, the TLR9 ligand CpG induces the production of TNFα, IL-10 and to a lesser extent of IL6.[51,56,73,80] Given the ability of CpG to induce CD25 expression, TLR9 ligands were also tested in vitro in combination with a specific anti-CD25 immunotoxin to treat CLL cells.[81] Both TLR7 and TLR9 agonists have been studied for immunotherapy approaches in preclinical models of CLL in vitro,[73,82,83] and are currently under clinical investigation[84-86] (see reference[87] for a recent review on this topic).
More recently, our group showed differences in the induction of co-stimulatory molecules and/or apoptosis in mutated vs. unmutated CLL. Different responses were also identified in subsets with stereotyped BCR.[79] The distinct patterns of TLR/NOD2 functional activity in cells from CLL subgroups defined by the molecular features of the BCR might prove relevant for elucidating the immune mechanisms underlying the natural history of CLL and for defining subgroups of patients who might benefit from treatment with specific TLR ligands.
TLR, Proliferation and Apoptosis of Leukemic Cells
Previous reports showed that CpG immunostimulatory oligonucleotides induce proliferation of CLL cells, either alone or in combination with IL-2.[51,72] However, it was also reported that TLR9 signaling by CpG-B oligonucleotides may induce an apoptotic pathway in CLL.[56,73] Further, several groups described a heterogeneous response to TLR9 stimulation in terms of proliferation and apoptosis within different groups of patients;[56,88] in details, it was reported that CpG induced apoptosis in mutated, and proliferation in unmutated patient samples.[53,89] It is relevant to note in this context that phosphorothioate oligonucleotides may induce apoptosis of CLL cells independently of their CpG motifs, indicating that the presence of a chemical backbone and nucleotide composition may contribute to the observed cell death.[90]
As for additional TLR, we previously showed that TLR1/2 and TLR2/6 heterodimers can activate and protect leukemic cells from spontaneous apoptosis in vitro.[55,79] Again, different groups showed heterogeneity among patients samples and apoptosis induction was reported in a proportion of cases;[56] nevertheless, it was recently shown that distinct innate immunity pathways can be activated in subgroups of CLL with distinct immunoglobulin receptors.[79]
Dissecting the Role of TLR in CLL Pathobiology
CLL patients are often associated with an increased frequency and severity of infections which is a characteristic feature of the disease.[91] In addition, autoimmune complications can occur in up to a quarter of CLL patients.[92] It was also suggested that common infections may play a role in CLL etiology;[93-95] this may be due to underlying immune disturbance in CLL patients, and/or to a direct effect of microbial antigens on the leukemic clone. Given all this, one could hypothesize that inflammation or autoimmunity mediated by distinct TLR may also play a role in regulating the development, progression and/or accumulation of CLL. Indeed, in mouse models of CLL the lack of the inhibitory receptor TIR8, which allows an unabated TLR-mediated stimulation, triggers leukemia progression in vivo.[96] However, these findings do not allow to understand whether TLR contribute (and to what extent) to early or late or both/any phase of disease progression.
Since TLR can improve immune response but may also be involved in modulating tumor cell proliferation and apoptosis, the possibility that TLR activity may shuttle between defense from and promotion of leukemic growth has to be taken into account. Future studies combining in vitro and in vivo approaches will help to identify the specific role of TLR within specific subsets of patients. Finally, kinetic studies will help elucidating the distinct role of distinct TLR in different phases of disease initiation, accumulation and/or progression.
References
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008 Jul 24;454(7203):436-444. http://dx.doi.org/10.1038/nature07205 PMid:18650914

- Mantovani A, Garlanda C. Inflammation and multiple myeloma: the Toll connection. Leukemia. 2006 Jun;20(6):937-938. http://dx.doi.org/10.1038/sj.leu.2404229 PMid:16721383

- Ferreri AJ, Zucca E. Marginal-zone lymphoma. Crit Rev Oncol Hematol. 2007 Sep;63(3):245-256. http://dx.doi.org/10.1016/j.critrevonc.2007.04.009 PMid:17583528

- Kawai T, Akira S. Toll-like receptors and
their crosstalk with other innate receptors in infection and immunity.
Immunity. 2011 May 27;34(5):637-650. http://dx.doi.org/10.1016/j.immuni.2011.05.006 PMid:21616434

- Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010.

- Sims GP, Rowe DC, Rietdijk ST, Herbst R,
Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol.
2010;28:367-388. http://dx.doi.org/10.1146/annurev.immunol.021908.132603 PMid:20192808

- Kim YM, Brinkmann MM, Paquet ME, Ploegh HL.
UNC93B1 delivers nucleotide-sensing toll-like receptors to
endolysosomes. Nature. 2008 Mar 13;452(7184):234-238. http://dx.doi.org/10.1038/nature06726 PMid:18305481

- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009 Oct 30;388(4):621-625. http://dx.doi.org/10.1016/j.bbrc.2009.08.062 PMid:19686699

- Asea A, Rehli M, Kabingu E, Boch JA, Bare
O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction
pathway utilized by extracellular HSP70: role of toll-like receptor
(TLR) 2 and TLR4. J Biol Chem. 2002 Apr 26;277(17):15028-15034. http://dx.doi.org/10.1074/jbc.M200497200 PMid:11836257

- Vabulas RM, Wagner H, Schild H. Heat shock
proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol.
2002;270:169-184. http://dx.doi.org/10.1007/978-3-642-59430-4_11

- Park JS, Svetkauskaite D, He Q, Kim JY,
Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors
2 and 4 in cellular activation by high mobility group box 1 protein. J
Biol Chem. 2004 Feb 27;279(9):7370-7377. http://dx.doi.org/10.1074/jbc.M306793200 PMid:14660645

- Schaefer L, Babelova A, Kiss E, Hausser
HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M,
Malle E, Schaefer RM, Grone HJ. The matrix component biglycan is
proinflammatory and signals through Toll-like receptors 4 and 2 in
macrophages. J Clin Invest. 2005 Aug;115(8):2223-2233. http://dx.doi.org/10.1172/JCI23755 PMid:16025156 PMCid:1174916

- Termeer C, Benedix F, Sleeman J, Fieber C,
Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC.
Oligosaccharides of Hyaluronan activate dendritic cells via toll-like
receptor 4. J Exp Med. 2002 Jan 7;195(1):99-111. http://dx.doi.org/10.1084/jem.20001858 PMid:11781369 PMCid:2196009

- Funderburg N, Lederman MM, Feng Z, Drage
MG, Jadlowsky J, Harding CV, Weinberg A, Sieg SF. Human -defensin-3
activates professional antigen-presenting cells via Toll-like receptors
1 and 2. Proc Natl Acad Sci U S A. 2007 Nov 20;104(47):18631-18635. http://dx.doi.org/10.1073/pnas.0702130104 PMid:18006661 PMCid:2141828

- Kariko K, Ni H, Capodici J, Lamphier M,
Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J
Biol Chem. 2004 Mar 26;279(13):12542-12550. http://dx.doi.org/10.1074/jbc.M310175200 PMid:14729660

- Lau CM, Broughton C, Tabor AS, Akira S,
Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA,
Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B
cells by combined B cell antigen receptor/Toll-like receptor 7
engagement. J Exp Med. 2005 Nov 7;202(9):1171-1177. http://dx.doi.org/10.1084/jem.20050630 PMid:16260486 PMCid:2213226

- Leadbetter EA, Rifkin IR, Hohlbaum AM,
Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG
complexes activate B cells by dual engagement of IgM and Toll-like
receptors. Nature. 2002 Apr 11;416(6881):603-607. http://dx.doi.org/10.1038/416603a PMid:11948342

- Govindaraj RG, Manavalan B, Lee G, Choi S.
Molecular modeling-based evaluation of hTLR10 and identification of
potential ligands in Toll-like receptor signaling. PLoS
One.5(9):e12713. http://dx.doi.org/10.1371/journal.pone.0012713 PMid:20877634 PMCid:2943521

- Guan Y, Ranoa DR, Jiang S, Mutha SK, Li X,
Baudry J, Tapping RI. Human TLRs 10 and 1 share common mechanisms of
innate immune sensing but not signaling. J Immunol. 2010 May
1;184(9):5094-5103. http://dx.doi.org/10.4049/jimmunol.0901888 PMid:20348427

- Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008 Aug;20(4):420-425. http://dx.doi.org/10.1016/j.coi.2008.07.001 PMid:18625310

- Nagai Y, Kobayashi T, Motoi Y, Ishiguro K,
Akashi S, Saitoh S, Kusumoto Y, Kaisho T, Akira S, Matsumoto M, Takatsu
K, Miyake K. The radioprotective 105/MD-1 complex links TLR2 and
TLR4/MD-2 in antibody response to microbial membranes. J Immunol. 2005
Jun 1;174(11):7043-7049. PMid:15905547

- Divanovic S, Trompette A, Atabani SF,
Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN,
Belkaid Y, Kurt-Jones EA, Karp CL. Negative regulation of Toll-like
receptor 4 signaling by the Toll-like receptor homolog RP105. Nat
Immunol. 2005 Jun;6(6):571-578. http://dx.doi.org/10.1038/ni1198 PMid:15852007 PMCid:2144914

- Miura Y, Shimazu R, Miyake K, Akashi S,
Ogata H, Yamashita Y, Narisawa Y, Kimoto M. RP105 is associated with
MD-1 and transmits an activation signal in human B cells. Blood. 1998
Oct 15;92(8):2815-2822. PMid:9763566

- Chaplin JW, Kasahara S, Clark EA,
Ledbetter JA. Anti-CD180 (RP105) activates B cells to rapidly produce
polyclonal Ig via a T cell and MyD88-independent pathway. J Immunol.
2011 Oct 15;187(8):4199-4209. http://dx.doi.org/10.4049/jimmunol.1100198 PMid:21918197

- Rodriguez A, Martinez N, Camacho FI,
Ruiz-Ballesteros E, Algara P, Garcia JF, Menarguez J, Alvaro T, Fresno
MF, Solano F, Mollejo M, Martin C, Piris MA. Variability in the degree
of expression of phosphorylated IkappaBalpha in chronic lymphocytic
leukemia cases with nodal involvement. Clin Cancer Res. 2004 Oct
15;10(20):6796-6806. http://dx.doi.org/10.1158/1078-0432.CCR-04-0753 PMid:15501956

- Petterson T, Jendholm J, Mansson A,
Bjartell A, Riesbeck K, Cardell LO. Effects of NOD-like receptors in
human B lymphocytes and crosstalk between NOD1/NOD2 and Toll-like
receptors. J Leukoc Biol. Feb;89(2):177-187.

- Inohara, Chamaillard, McDonald C, Nunez G.
NOD-LRR proteins: role in host-microbial interactions and inflammatory
disease. Annu Rev Biochem. 2005;74:355-383. http://dx.doi.org/10.1146/annurev.biochem.74.082803.133347 PMid:15952891

- Fritz JH, Ferrero RL, Philpott DJ,
Girardin SE. Nod-like proteins in immunity, inflammation and disease.
Nat Immunol. 2006 Dec;7(12):1250-1257. http://dx.doi.org/10.1038/ni1412 PMid:17110941

- Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991 May 30;351(6325):355-356. http://dx.doi.org/10.1038/351355b0 PMid:1851964

- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1-16. http://dx.doi.org/10.1007/3-540-32636-7_1

- Honda K, Taniguchi T. IRFs: master
regulators of signalling by Toll-like receptors and cytosolic
pattern-recognition receptors. Nat Rev Immunol. 2006 Sep;6(9):644-658. http://dx.doi.org/10.1038/nri1900 PMid:16932750

- Garlanda C, Riva F, Polentarutti N,
Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E,
Vecchi A, Hirsch E, Mantovani A. Intestinal inflammation in mice
deficient in Tir8, an inhibitory member of the IL-1 receptor family.
Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3522-3526. http://dx.doi.org/10.1073/pnas.0308680101 PMid:14993616 PMCid:373495

- Wald D, Qin J, Zhao Z, Qian Y, Naramura M,
Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator
of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol.
2003 Sep;4(9):920-927. http://dx.doi.org/10.1038/ni968 PMid:12925853

- Qin J, Qian Y, Yao J, Grace C, Li X.
SIGIRR inhibits interleukin-1 receptor- and toll-like receptor
4-mediated signaling through different mechanisms. J Biol Chem. 2005
Jul 1;280(26):25233-25241. http://dx.doi.org/10.1074/jbc.M501363200 PMid:15866876

- Drexler SK, Wales J, Andreakos E, Kong P,
Davis A, Garlanda C, Mantovani A, Hussell T, Feldmann M, Foxwell BM.
Evidence for a DC-specific inhibitory mechanism that depends on MyD88
and SIGIRR. Scand J Immunol. 2010 Jun;71(6):393-402. http://dx.doi.org/10.1111/j.1365-3083.2010.02392.x PMid:20500691 PMCid:2878274

- Kobayashi K, Hernandez LD, Galan JE,
Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative
regulator of Toll-like receptor signaling. Cell. 2002 Jul
26;110(2):191-202. http://dx.doi.org/10.1016/S0092-8674(02)00827-9

- Nakagawa R, Naka T, Tsutsui H, Fujimoto M,
Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S,
Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. SOCS-1 participates in
negative regulation of LPS responses. Immunity. 2002 Nov;17(5):677-687.
http://dx.doi.org/10.1016/S1074-7613(02)00449-1

- Muzio M, Bosisio D, Polentarutti N,
D'Amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G,
Ruco LP, Allavena P, Mantovani A. Differential expression and
regulation of toll-like receptors (TLR) in human leukocytes: selective
expression of TLR3 in dendritic cells. J Immunol. 2000 Jun
1;164(11):5998-6004. PMid:10820283

- Bourke E, Bosisio D, Golay J, Polentarutti
N, Mantovani A. The toll-like receptor repertoire of human B
lymphocytes: inducible and selective expression of TLR9 and TLR10 in
normal and transformed cells. Blood. 2003 Aug 1;102(3):956-963. http://dx.doi.org/10.1182/blood-2002-11-3355 PMid:12689944

- Bernasconi NL, Onai N, Lanzavecchia A. A
role for Toll-like receptors in acquired immunity: up-regulation of
TLR9 by BCR triggering in naive B cells and constitutive expression in
memory B cells. Blood. 2003 Jun 1;101(11):4500-4504. http://dx.doi.org/10.1182/blood-2002-11-3569 PMid:12560217

- Hornung V, Rothenfusser S, Britsch S, Krug
A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative
expression of toll-like receptor 1-10 mRNA in cellular subsets of human
peripheral blood mononuclear cells and sensitivity to CpG
oligodeoxynucleotides. J Immunol. 2002 May 1;168(9):4531-4537.
PMid:11970999

- Dorner M, Brandt S, Tinguely M, Zucol F,
Bourquin JP, Zauner L, Berger C, Bernasconi M, Speck RF, Nadal D.
Plasma cell toll-like receptor (TLR) expression differs from that of B
cells, and plasma cell TLR triggering enhances immunoglobulin
production. Immunology. 2009 Dec;128(4):573-579. http://dx.doi.org/10.1111/j.1365-2567.2009.03143.x PMid:19950420 PMCid:2792141

- Mansson A, Adner M, Hockerfelt U, Cardell
LO. A distinct Toll-like receptor repertoire in human tonsillar B
cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation.
Immunology. 2006 Aug;118(4):539-548. PMid:16780564 PMCid:1782320

- Rawlings DJ, Schwartz MA, Jackson SW,
Meyer-Bahlburg A. Integration of B cell responses through Toll-like
receptors and antigen receptors. Nat Rev Immunol. 2012
Apr;12(4):282-294. http://dx.doi.org/10.1038/nri3190 PMid:22421786

- Bekeredjian-Ding I, Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009 Nov;128(3):311-323. http://dx.doi.org/10.1111/j.1365-2567.2009.03173.x PMid:20067531 PMCid:2770679

- Lanzavecchia A, Sallusto F. Toll-like
receptors and innate immunity in B-cell activation and antibody
responses. Curr Opin Immunol. 2007 Jun;19(3):268-274. http://dx.doi.org/10.1016/j.coi.2007.04.002 PMid:17433875

- Bernasconi NL, Traggiai E, Lanzavecchia A.
Maintenance of serological memory by polyclonal activation of human
memory B cells. Science. 2002 Dec 13;298(5601):2199-2202. http://dx.doi.org/10.1126/science.1076071 PMid:12481138

- Ruprecht CR, Lanzavecchia A. Toll-like
receptor stimulation as a third signal required for activation of human
naive B cells. Eur J Immunol. 2006 Apr;36(4):810-816. http://dx.doi.org/10.1002/eji.200535744 PMid:16541472

- Huggins J, Pellegrin T, Felgar RE, Wei C,
Brown M, Zheng B, Milner EC, Bernstein SH, Sanz I, Zand MS. CpG DNA
activation and plasma-cell differentiation of CD27- naive human B
cells. Blood. 2007 Feb 15;109(4):1611-1619. http://dx.doi.org/10.1182/blood-2006-03-008441 PMid:17032927 PMCid:1794051

- Hemmi H, Takeuchi O, Kawai T, Kaisho T,
Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A
Toll-like receptor recognizes bacterial DNA. Nature. 2000 Dec
7;408(6813):740-745. http://dx.doi.org/10.1038/35047123 PMid:11130078

- Decker T, Schneller F, Sparwasser T,
Tretter T, Lipford GB, Wagner H, Peschel C. Immunostimulatory
CpG-oligonucleotides cause proliferation, cytokine production, and an
immunogenic phenotype in chronic lymphocytic leukemia B cells. Blood.
2000 Feb 1;95(3):999-1006.PMid:10648415

- Jahrsdorfer B, Muhlenhoff L, Blackwell SE,
Wagner M, Poeck H, Hartmann E, Jox R, Giese T, Emmerich B, Endres S,
Weiner GJ, Hartmann G. B-cell lymphomas differ in their responsiveness
to CpG oligodeoxynucleotides. Clin Cancer Res. 2005 Feb
15;11(4):1490-1499. http://dx.doi.org/10.1158/1078-0432.CCR-04-1890 PMid:15746051

- Longo PG, Laurenti L, Gobessi S,
Petlickovski A, Pelosi M, Chiusolo P, Sica S, Leone G, Efremov DG. The
Akt signaling pathway determines the different proliferative capacity
of chronic lymphocytic leukemia B-cells from patients with progressive
and stable disease. Leukemia. 2007 Jan;21(1):110-120. http://dx.doi.org/10.1038/sj.leu.2404417 PMid:17024114

- Grandjenette C, Kennel A, Faure GC, Bene
MC, Feugier P. Expression of functional toll-like receptors by
B-chronic lymphocytic leukemia cells. Haematologica. 2007
Sep;92(9):1279-1281. http://dx.doi.org/10.3324/haematol.10975 PMid:17768129

- Muzio M, Scielzo C, Bertilaccio MT,
Frenquelli M, Ghia P, Caligaris-Cappio F. Expression and function of
toll like receptors in chronic lymphocytic leukaemia cells. Br J
Haematol. 2009 Feb;144(4):507-516. http://dx.doi.org/10.1111/j.1365-2141.2008.07475.x PMid:19036098

- Rozkova D, Novotna L, Pytlik R, Hochova I,
Kozak T, Bartunkova J, Spisek R. Toll-like receptors on B-CLL cells:
expression and functional consequences of their stimulation. Int J
Cancer. 2010 Mar 1;126(5):1132-1143. PMid:19685493

- Arvaniti E, Ntoufa S, Papakonstantinou N,
Touloumenidou T, Laoutaris N, Anagnostopoulos A, Lamnissou K,
Caligaris-Cappio F, Stamatopoulos K, Ghia P, Muzio M, Belessi C.
Toll-like receptor signaling pathway in chronic lymphocytic leukemia:
distinct gene expression profiles of potential pathogenetic
significance in specific subsets of patients. Haematologica. 2011 Jul
12. http://dx.doi.org/10.3324/haematol.2011.044792 PMid:21750087 PMCid:3208682

- Porakishvili N, Kulikova N, Jewell AP,
Youinou PY, Yong K, Nathwani A, Heelan B, Duke V, Hamblin TJ, Wallace
P, Ely P, Clark EA, Lydyard PM. Differential expression of CD 180 and
IgM by B-cell chronic lymphocytic leukaemia cells using mutated and
unmutated immunoglobulin VH genes. Br J Haematol. 2005
Nov;131(3):313-319. http://dx.doi.org/10.1111/j.1365-2141.2005.05775.x PMid:16225650

- Antosz H, Sajewicz J, Marzec-Kotarska B,
Kocki J, Dmoszynska A. Different expression of CD180, CD284 and CD14
receptors on the CD19+ subpopulation of normal and B-CLL lymphocytes.
Folia Histochem Cytobiol. 2009;47(4):593-598. http://dx.doi.org/10.2478/v10042-009-0112-1 PMid:20430725

- Arvaniti E, Ntoufa S, Papakonstantinou N,
Touloumenidou T, Laoutaris N, Anagnostopoulos A, Lamnissou K,
Caligaris-Cappio F, Stamatopoulos K, Ghia P, Muzio M, Belessi C.
Toll-like receptor signaling pathway in chronic lymphocytic leukemia:
distinct gene expression profiles of potential pathogenic significance
in specific subsets of patients. Haematologica. 2011
Nov;96(11):1644-1652. http://dx.doi.org/10.3324/haematol.2011.044792 PMid:21750087 PMCid:3208682

- Veliz Rodriguez T, Moalli F, Polentarutti
N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S,
Bragonzi A, Mantovani A, Riva F, Garlanda C. Role of Toll interleukin-1
receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor
signaling, in resistance to acute Pseudomonas aeruginosa lung
infection. Infect Immun. 2012 Jan;80(1):100-109. http://dx.doi.org/10.1128/IAI.05695-11 PMid:22025515 PMCid:3255652

- Herishanu Y, Perez-Galan P, Liu D,
Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E,
Stennett L, Raghavachari N, Liu P, McCoy JP, Raffeld M,
Stetler-Stevenson M, Yuan C, Sherry R, Arthur DC, Maric I, White T,
Marti GE, Munson P, Wilson WH, Wiestner A. The lymph node
microenvironment promotes B-cell receptor signaling, NF-kappaB
activation, and tumor proliferation in chronic lymphocytic leukemia.
Blood. 2011 Jan 13;117(2):563-574. http://dx.doi.org/10.1182/blood-2010-05-284984 PMid:20940416 PMCid:3031480

- Cerhan JR, Ansell SM, Fredericksen ZS, Kay
NE, Liebow M, Call TG, Dogan A, Cunningham JM, Wang AH, Liu-Mares W,
Macon WR, Jelinek D, Witzig TE, Habermann TM, Slager SL. Genetic
variation in 1253 immune and inflammation genes and risk of non-Hodgkin
lymphoma. Blood. 2007 Dec 15;110(13):4455-4463. http://dx.doi.org/10.1182/blood-2007-05-088682 PMid:17827388 PMCid:2234796

- Nieters A, Beckmann L, Deeg E, Becker N.
Gene polymorphisms in Toll-like receptors, interleukin-10, and
interleukin-10 receptor alpha and lymphoma risk. Genes Immun. 2006
Dec;7(8):615-624. http://dx.doi.org/10.1038/sj.gene.6364337 PMid:16971956

- Purdue MP, Lan Q, Wang SS, Kricker A,
Menashe I, Zheng TZ, Hartge P, Grulich AE, Zhang Y, Morton LM, Vajdic
CM, Holford TR, Severson RK, Leaderer BP, Cerhan JR, Yeager M, Cozen W,
Jacobs K, Davis S, Rothman N, Chanock SJ, Chatterjee N, Armstrong BK. A
pooled investigation of Toll-like receptor gene variants and risk of
non-Hodgkin lymphoma. Carcinogenesis. 2009 Feb;30(2):275-281. http://dx.doi.org/10.1093/carcin/bgn262 PMid:19029192 PMCid:2639046

- Ngo VN, Young RM, Schmitz R, Jhavar S,
Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL,
Romesser P, Wright G, Powell J, Rosenwald A, Muller-Hermelink HK, Ott
G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J,
Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD,
Chan WC, Staudt LM. Oncogenically active MYD88 mutations in human
lymphoma. Nature. 2010 Feb 3;470(7332):115-119. http://dx.doi.org/10.1038/nature09671 PMid:21179087

- Yan Y, Huang Y, Watkins AJ, Kocialkowski
S, Zeng N, Hamoudi RA, Isaacson PG, de Leval L, Wotherspoon A, Du MQ.
BCR and TLR signalling pathways are recurrently targeted by genetic
changes in splenic marginal zone lymphomas. Haematologica. 2012 Nov 18.
http://dx.doi.org/10.3324/haematol.2011.054080 PMid:22102703 PMCid:3347666

- Montesinos-Rongen M, Godlewska E, Brunn A,
Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the
MYD88 gene are common in primary central nervous system lymphoma. Acta
Neuropathol. 2011 Dec;122(6):791-792. http://dx.doi.org/10.1007/s00401-011-0891-2 PMid:22020631

- Puente XS, Pinyol M, Quesada V, Conde L,
Ordonez GR, Villamor N, Escaramis G, Jares P, Bea S, Gonzalez-Diaz M,
Bassaganyas L, Baumann T, Juan M, Lopez-Guerra M, Colomer D, Tubio JM,
Lopez C, Navarro A, Tornador C, Aymerich M, Rozman M, Hernandez JM,
Puente DA, Freije JM, Velasco G, Gutierrez-Fernandez A, Costa D, Carrio
A, Guijarro S, Enjuanes A, Hernandez L, Yague J, Nicolas P,
Romeo-Casabona CM, Himmelbauer H, Castillo E, Dohm JC, de Sanjose S,
Piris MA, de Alava E, San Miguel J, Royo R, Gelpi JL, Torrents D,
Orozco M, Pisano DG, Valencia A, Guigo R, Bayes M, Heath S, Gut M,
Klatt P, Marshall J, Raine K, Stebbings LA, Futreal PA, Stratton MR,
Campbell PJ, Gut I, Lopez-Guillermo A, Estivill X, Montserrat E,
Lopez-Otin C, Campo E. Whole-genome sequencing identifies recurrent
mutations in chronic lymphocytic leukaemia. Nature. 2011 Jul
7;475(7354):101-105. http://dx.doi.org/10.1038/nature10113 PMid:21642962 PMCid:3322590

- Wang L, Lawrence MS, Wan Y, Stojanov P,
Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L,
Zhang W, Vartanov AR, Fernandes SM, Goldstein NR, Folco EG, Cibulskis
K, Tesar B, Sievers QL, Shefler E, Gabriel S, Hacohen N, Reed R,
Meyerson M, Golub TR, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ.
SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N
Engl J Med. 2011 Dec 29;365(26):2497-2506. http://dx.doi.org/10.1056/NEJMoa1109016 PMid:22150006

- Fabbri G, Rasi S, Rossi D, Trifonov V,
Khiabanian H, Ma J, Grunn A, Fangazio M, Capello D, Monti S, Cresta S,
Gargiulo E, Forconi F, Guarini A, Arcaini L, Paulli M, Laurenti L,
Larocca LM, Marasca R, Gattei V, Oscier D, Bertoni F, Mullighan CG, Foa
R, Pasqualucci L, Rabadan R, Dalla-Favera R, Gaidano G. Analysis of the
chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational
activation. J Exp Med. 2011 Jul 4;208(7):1389-1401. http://dx.doi.org/10.1084/jem.20110921 PMid:21670202 PMCid:3135373

- Jahrsdorfer B, Hartmann G, Racila E,
Jackson W, Muhlenhoff L, Meinhardt G, Endres S, Link BK, Krieg AM,
Weiner GJ. CpG DNA increases primary malignant B cell expression of
costimulatory molecules and target antigens. J Leukoc Biol. 2001
Jan;69(1):81-88. PMid:11200072

- Liang X, Moseman EA, Farrar MA, Bachanova
V, Weisdorf DJ, Blazar BR, Chen W. Toll-like receptor 9 signaling by
CpG-B oligodeoxynucleotides induces an apoptotic pathway in human
chronic lymphocytic leukemia B cells. Blood. 2010 Jun
17;115(24):5041-5052. http://dx.doi.org/10.1182/blood-2009-03-213363 PMid:20339095 PMCid:2890142

- Spaner DE, Hammond C, Mena J, Foden C,
Deabreu A. A phase I/II trial of oxidized autologous tumor vaccines
during the "watch and wait" phase of chronic lymphocytic leukemia.
Cancer Immunol Immunother. 2005 Jul;54(7):635-646. http://dx.doi.org/10.1007/s00262-004-0626-5 PMid:15918075

- Gitelson E, Hammond C, Mena J, Lorenzo M,
Buckstein R, Berinstein NL, Imrie K, Spaner DE. Chronic lymphocytic
leukemia-reactive T cells during disease progression and after
autologous tumor cell vaccines. Clin Cancer Res. 2003
May;9(5):1656-1665. PMid:12738718

- Gribben JG, Ramsay AG, Riches JC. Immune
Dysfunction in Chronic Lymphocytic Leukemia: The Role for
Immunotherapy. Curr Pharm Des. 2012 May 10.

- Muzio M, Bertilaccio MT, Simonetti G,
Frenquelli M, Caligaris-Cappio F. The role of Toll-like receptors in
chronic B cell malignancies. Leuk Lymphoma. 2009;50(10):1573-1580. http://dx.doi.org/10.1080/10428190903115410

- Spaner DE, Shi Y, White D, Mena J, Hammond
C, Tomic J, He L, Tomai MA, Miller RL, Booth J, Radvanyi L.
Immunomodulatory effects of Toll-like receptor-7 activation on chronic
lymphocytic leukemia cells. Leukemia. 2005 Feb;20(2):286-295. http://dx.doi.org/10.1038/sj.leu.2404061 PMid:16341037

- Ntoufa S, Vardi A, Papakonstantinou N,
Anagnostopoulos A, Aleporou-Marinou V, Belessi C, Ghia P,
Caligaris-Cappio F, Muzio M, Stamatopoulos K. Distinct Innate Immunity
Pathways to Activation and Tolerance in Subgroups of Chronic
Lymphocytic Leukemia with Distinct Immunoglobulin Receptors. Mol Med.
2012 Mar 19.

- Decker T, Peschel C. Effect of
immunostimulatory CpG-oligonucleotides in chronic lymphocytic leukemia
B cells. Leuk Lymphoma. 2001 Jul;42(3):301-307. http://dx.doi.org/10.3109/10428190109064586

- Decker T, Hipp S, Kreitman RJ, Pastan I,
Peschel C, Licht T. Sensitization of B-cell chronic lymphocytic
leukemia cells to recombinant immunotoxin by immunostimulatory
phosphorothioate oligodeoxynucleotides. Blood. 2002 Feb
15;99(4):1320-1326. PMid:11830482

- Spaner DE, Shi Y, White D, Mena J, Hammond
C, Tomic J, He L, Tomai MA, Miller RL, Booth J, Radvanyi L.
Immunomodulatory effects of Toll-like receptor-7 activation on chronic
lymphocytic leukemia cells. Leukemia. 2006 Feb;20(2):286-295. http://dx.doi.org/10.1038/sj.leu.2404061 PMid:16341037

- Hoogendoorn M, Wolbers JO, Smit WM,
Schaafsma MR, Barge RM, Willemze R, Falkenburg JH. Generation of B-cell
chronic lymphocytic leukemia (B-CLL)-reactive T-cell lines and clones
from HLA class I-matched donors using modified B-CLL cells as
stimulators: implications for adoptive immunotherapy. Leukemia. 2004
Jul;18(7):1278-1287. http://dx.doi.org/10.1038/sj.leu.2403358 PMid:15116118

- Spaner DE, Shi Y, White D, Shaha S, He L,
Masellis A, Wong K, Gorczynski R. A phase I/II trial of TLR-7 agonist
immunotherapy in chronic lymphocytic leukemia. Leukemia. 2009
Jan;24(1):222-226. http://dx.doi.org/10.1038/leu.2009.195 PMid:19759558

- Zent CS, Smith BJ, Ballas ZK, Wooldridge
JE, Link BK, Call TG, Shanafelt TD, Bowen DA, Kay NE, Witzig TE, Weiner
GJ. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in
patients with previously treated chronic lymphocytic leukemia. Leuk
Lymphoma. 2011 Feb;53(2):211-217. http://dx.doi.org/10.3109/10428194.2011.608451

- Jahrsdorfer B, Blackwell S, Weiner G. The
effects of CpG ODN on CLL proliferation, apoptosis or phenotype could
have an impact on its clinical utility. Leukemia. 2007
Nov;21(11):2354-2355; author reply 2355-2356. http://dx.doi.org/10.1038/sj.leu.2404870 PMid:17657215

- Spaner DE, Masellis A. Toll-like receptor
agonists in the treatment of chronic lymphocytic leukemia. Leukemia.
2007 Jan;21(1):53-60. http://dx.doi.org/10.1038/sj.leu.2404456 PMid:17066089

- Jahrsdorfer B, Jox R, Muhlenhoff L,
Tschoep K, Krug A, Rothenfusser S, Meinhardt G, Emmerich B, Endres S,
Hartmann G. Modulation of malignant B cell activation and apoptosis by
bcl-2 antisense ODN and immunostimulatory CpG ODN. J Leukoc Biol. 2002
Jul;72(1):83-92. PMid:12101266

- Tromp JM, Tonino SH, Elias JA, Jaspers A,
Luijks DM, Kater AP, van Lier RA, van Oers MH, Eldering E. Dichotomy in
NF-kappaB signaling and chemoresistance in immunoglobulin variable
heavy-chain-mutated versus unmutated CLL cells upon CD40/TLR9
triggering. Oncogene. 2010 Sep 9;29(36):5071-5082. http://dx.doi.org/10.1038/onc.2010.248 PMid:20581863

- Castro JE, Prada CE, Aguillon RA, Kitada
S, Fukuda T, Motta M, Wu C, Dicker F, Sun G, Wang JY, Carson DA, Reed
JC, Kipps TJ. Thymidine-phosphorothioate oligonucleotides induce
activation and apoptosis of CLL cells independently of CpG motifs or
BCL-2 gene interference. Leukemia. 2006 Apr;20(4):680-688. http://dx.doi.org/10.1038/sj.leu.2404144 PMid:16498393

- Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006 Apr;33(2):240-249. http://dx.doi.org/10.1053/j.seminoncol.2005.12.013 PMid:16616071

- Ghia P, Scielzo C, Frenquelli M, Muzio M,
Caligaris-Cappio F. From normal to clonal B cells: Chronic lymphocytic
leukemia (CLL) at the crossroad between neoplasia and autoimmunity.
Autoimmun Rev. 2007 Dec;7(2):127-131. http://dx.doi.org/10.1016/j.autrev.2007.02.014 PMid:18035322

- Anderson LA, Landgren O, Engels EA. Common
community acquired infections and subsequent risk of chronic
lymphocytic leukaemia. Br J Haematol. 2009 Sep 1. http://dx.doi.org/10.1111/j.1365-2141.2009.07849.x PMid:19725826 PMCid:2777688

- Landgren O, Rapkin JS, Caporaso NE,
Mellemkjaer L, Gridley G, Goldin LR, Engels EA. Respiratory tract
infections and subsequent risk of chronic lymphocytic leukemia. Blood.
2007 Mar 1;109(5):2198-2201. http://dx.doi.org/10.1182/blood-2006-08-044008 PMid:17082317 PMCid:1801057

- Landgren O, Gridley G, Check D, Caporaso
NE, Morris Brown L. Acquired immune-related and inflammatory conditions
and subsequent chronic lymphocytic leukaemia. Br J Haematol. 2007
Dec;139(5):791-798. http://dx.doi.org/10.1111/j.1365-2141.2007.06859.x PMid:17941950

- Bertilaccio MT, Simonetti G, Dagklis A,
Rocchi M, Veliz Rodriguez T, Apollonio B, Mantovani A, Ponzoni M, Ghia
P, Garlanda C, Caligaris-Cappio F, Muzio M. Lack of TIR8/SIGIRR
triggers progression of chronic lymphocytic leukemia in mouse models.
Blood. 2011 Jul 21;118(3):660-669. http://dx.doi.org/10.1182/blood-2011-01-329870 PMid:21652674
